Abstract
We report three patients with ophthalmic herpes zoster (HZ) manifestations on the background diagnosis of multiple myeloma (MM). It seems that immunocompromised status has caused reactivation of the varicella zoster virus (VZV) producing a well-characterised neurological syndrome and subsequent postherpetic neuralgia in two patients. One patient experienced lymphocytic leptomeningitis resulting in unilateral optic neuritis. All patients received similar myeloma disease-specific treatment prior to HZ reactivation. All patients were treated with thalidomide and steroids, and they thereafter underwent autologous stem cell transplantation. Prior to HZ reactivation they received new immunomodulatory drugs in the form of thalidomide in addition to bortezomib (2 patients) and lenalidomide (1 patient). Immediate specific antiviral therapy was successfully applied with intravenous acyclovir for 10 days, followed by long-term oral famciclovir maintenance. Two patients progressed to have chronic HZ ophthalmicus and postherpetic neuralgia requiring ongoing antiviral therapy and neuroepileptic medications for the neuropathic pain.
Background
There is an increased risk of viral reactivation in conjunction with multiple myeloma (MM), autologous stem cell transplantation (ASCT) and new immunomodulatory therapies for MM such as thalidomide, bortezomib and lenalidomide.
A link between an increased incidence of varicella zoster virus (VZV) infections in patients with MM receiving bortezomib has been found. Although herpes zoster ophthalmicus (HZO) is considered as a relatively rare disease, we recorded three unusual cases of HZO in MM patients who were treated with standard thalidomide therapy and subsequent ASCT in addition to the new immunomodulatory therapy in the last 24 months.
Patients with MM are considered immunocompromised for different reasons; first, immune paresis because of secondary hypogammaglobulinaemia; second, application of treatment including high-dose chemotherapy ASCT; third, neutropenia associated with the treatment; and last, the increasing use of immunomodulatory therapy affecting the immune response of the host via different mechanisms. Furthermore, another potential risk to MM patients is the reduction of immunosuppressive natural killer (NK) cell activity that may increase the susceptibility to infection. A combination of these factors is probably contributory to the incidence of HZO in our cohort of patients.
There is no data available regarding the prevalence of HZO in myeloma patients, in particular, as in our cohort of patients, after ASCT and new immunomodulatory therapy.
In this case series we explore the association between immune therapy, ASCT and reactivation of VZV. In particular, we have highlighted a possible increased risk for VZV in MM patients who have received ASCT and were treated with the new immunomodulatory agents. This could serve as a reminder to medical practitioners to be vigilant for early clinical recognition of similar cranial nerve symptoms in this subgroup and may act as a template for larger studies to determine the true incidence of such infections.
Case presentation
Case 1
The first patient is a 72-year-old Caucasian woman who was diagnosed with κ IgG MM in October 2007. She had an IgG of 56 g/l with normal serum-free light chains at time of diagnosis. She was treated with thalidomide (100 mg daily orally) and tandem ASCT in 2008 with thalidomide maintenance therapy (50 mg daily until September 2010). Because of the patient not being able to tolerate thalidomide in late 2010, treatment was changed to ongoing lenalidomide therapy with an achievement of MM-disease control.
In June 2010, she developed a right-sided headache. She was diagnosed with herpetic neuralgia involving the ophthalmic branch of the right trigeminal nerve. At diagnosis, she had a positive Hutchinson's sign, normal distance vision, a mild follicular conjunctivitis and corneal pseudodendrites. Her anterior chamber was quiet, her fundus was normal and importantly no signs of optic neuropathy were noted. She was treated initially with intravenous acyclovir 10 mg/kg body weight (BW) three times daily for 10 days followed by oral famciclovir, 500 mg twice a day for 3 months. Local treatments with chloramphenicol eye drops and lubricant eye gels were applied. At the time, she had normal full blood count (FBC). She remained in continuous complete remission of the disease with undetectable paraprotein levels.
Case 2
The second case was a Caucasian female patient diagnosed in May 2010 at the age of 50 years with λ IgG MM. At the time of diagnosis, she had 90 g/l IgG-paraprotein level with 3000 mg/l λ FLC. She was treated with thalidomide, 100 mg daily, and dexamethasone for 6 months, with achievement of good remission of the disease. In April 2011, her disease progressed with a significant increase of IgG paraprotein level (60 g/l). At this point, she was started on bortezomib (2.6 mg/m2) at days 1, 4, 8 and 11 in a 21-day cycle for 11 consecutive cycles. In February 2012, she received ASCT after conditioning with high-dose melphalan (200 mg/m2). Three months later, she developed a sudden profound, acute loss of central vision with mild periorbital pain on the left side. Vision was reduced to finger counting at 1 metre in the affected eye. There was reduced colour saturation, a relative afferent pupil defect, but normal appearing optic disc, which suggested a retrobulbar optic neuritis. An MRI scan showed mild enlargement of the left optic nerve which was consistent with optic neuritis (figure 1). Her neutrophil count at that time was 1.8/nl with other FBC parameters being normal. IgG level was normal at 10 g/l and FLC was normal. A lumbar puncture was performed which revealed a lymphocytic pleocytosis in the cerebrospinal fluid (CSF). General Herpes MultiPlex PCR of the CSF showed no viral DNA detected. The viruses screened with this PCR were: herpes simplex virus (HSV)-1, HSV-2, cytomegalovirus and VZV. Furthermore, there was evidence of a lymphocytic leptomeningitis, producing optic neuritis. Despite PCR negativity, the overall clinical picture was consistent with herpes zoster (HZ) viral reactivation. Therefore, a viral aetiology was presumed and she was treated initially with intravenous acyclovir 10 mg/kg BW three times a day for 10 days and then with long-term oral famciclovir, 500 mg twice a day. Her vision recovered to normal after 2 weeks of treatment. Retrospectively, she was noted to have a previous history of an HZ infection at the age of 30 years.
Figure 1.
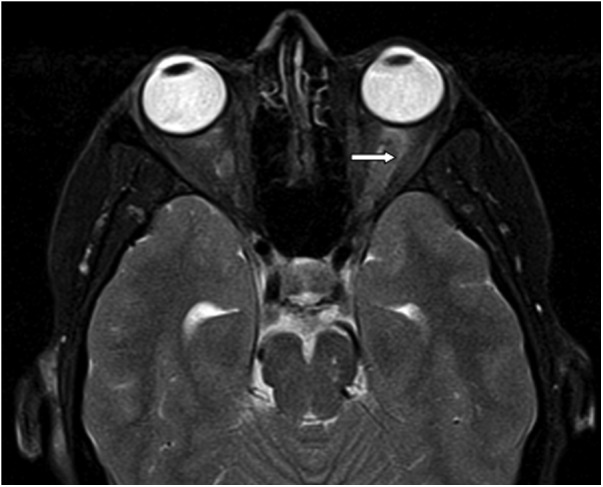
MRI scan in case 2—T2-weighted image showing mild hyperintensity of the left optic nerve (arrow), suggesting optic neuritis.
Case 3
The third case was a Caucasian male patient diagnosed with MM in April 2006 at the age of 68 years. This was on the background of a long standing history of multiple plasmacytosis which required multiple radiotherapy treatments. At the time of diagnosis, he had a positive Bence Jones proteinuria (BJP) and κ-free light chain of 4490 mg/l. At this time, his BJP excretion increased from 0.7 (at the time of diagnosis) to 1.7 g/l. He was treated initially with thalidomide, 100 mg daily and intermittent doses of 20 mg dexamethasone, orally. However, his disease progressed and therefore he underwent a tandem ASCT in late 2006 and early 2007 with excellent control of his disease. He received maintenance therapy with thalidomide.
In May 2010, he had disease progression with rise of κ-free light chain from a steady level below 500 to over 2100 mg/l. Therefore, he was started on bortezomib which was given on days 1, 4, 8 and 11 in a 21-day cycle for 11 cycles.
In August 2010, he developed swelling and pain in his right eye and pain in the right nostril which was diagnosed as HZO reactivation involving the ophthalmic division of the right trigeminal nerve. He was treated as an inpatient with intravenous acyclovir, 10 mg/kg BW three times a day, and then oral famciclovir, 500 mg twice daily for 3 months, with a maintenance dose of 500 mg twice daily. At this time, he had a low IgG level with κ-free light chains of 316 mg/l. His FBC was normal.
In January 2011, he suffered from another episode of HZO, which was treated with famciclovir, 500 mg three times a day for 2 weeks, with a maintenance dose of 500 mg twice daily. He had κ-FLC of 563 mg/l at this time with neutrophils of 5.5/nl.
Investigations
An MRI scan showed enhancement of the left optic nerve consistent with optic neuritis (figure 1) in case 2.
Differential diagnosis
Patients with other immune deficiency syndromes secondary to HIV infections reportedly have a higher rate of HZO. As a matter of fact, HZO is considered as one of the common manifestations of HIV infection. It is worth noting that all the patients in our cohort were HIV negative.
Treatment
Treatment of HZ reactivation in immunocompromised patients:
All patients received intravenous acyclovir, 10 mg/kg BW three times a day, in the inpatient setting. Thereafter, the patients received famciclovir, 500 mg three times a day, as an outpatient. All patients continued with famciclovir, 500 mg twice daily, as maintenance therapy. For postherpetic neuralgia, both patients were treated with neuroepileptics (gabapentin) along with amitriptyline in one patient.
Outcome and follow-up
Case 1
After 3 months of treatment with famciclovir at the prophylactic dose of 250 mg twice daily, she experienced what appears to be a progression of some chronic manifestations of HZO relapse of the right-side in January 2012 that required doubling the dose of famciclovir. Ophthalmological examination showed evidence of a chronic anterior uveitus with keratic precipitates. She also had an element of neurotropic cornea with diffuse punctate staining and reduced corneal sensation. These are hallmarks of chronic disease. Her vision is 6/7.5 in the right eye. Currently, she is still experiencing chronic pain that requires ongoing neuropathic pain medication with gabapentin.
Case 2
She regained her normal vision and continues with the treatment.
Case 3
He has postherpetic neuralgia requiring 300 mg of gabapentin twice daily and amitriptyline daily.
Because of secondary hypogammaglobulinaemia, all patients were receiving maintenance therapy with a monthly intravenous immunoglobulin of 0.4 g/kg BW from the time of diagnosis.
All patients received ongoing famciclovir, 500 mg twice daily, as part of the maintenance therapy. Two patients required neuropathic pain medications to control postherpetic neuralgia.
Discussion
MM is an incurable haematological cancer with a median survival of 3–7 years. This is despite advances in new therapies.1–3 Bortezomib, a proteasome inhibitor, has been shown to increase survival in relapsed multiple myeloma.2 3 This is achieved by inhibition of proteasome, which disrupts cell homeostasis, causing apoptosis. However, there is some evidence to suggest that bortezomib treatment in patients with MM increases the risk of HZ infection.4–7
Patients with MM also receive high-dose dexamethasone. Dexamethasone is associated with immunosuppression and subsequently higher rates of infection.7 8 However, patients receiving bortezomib have higher rates of infection with HZ than patients receiving high-dose dexamethasone alone.4 Furthermore, bone marrow transplantation is considered as an independent risk factor for infections including VZV infection.8–10
Some data suggest that the new immunomodulatory agents from the immune-mediated inflammatory disease class (thalidomide and lenalidomide) have an effect on T cells with inhibition of the production of interleukin (IL)-6 and activates T-cells to produce IL-2 which alters the number and function of NK cells. Furthermore, thalidomide has been shown to have antitumour necrosis factor α effects, which increases the risk of opportunistic infection.10 11 Lenalidomide has a different toxicity profile that includes neutropenia, an additional risk factor for infection.12 The cumulative effect of the addition of dexamethasone therapy reportedly increases the risk of infection to 11.3% using lenalidomide plus dexamethasone, versus 6.2% in the dexamethasone-only treated group.13 Bortezomib is a proteasome inhibitor with an inhibitory effect on T-cell apoptosis and interferes with the production of Th1 cytokines.3 4 11 The possible effect of bortezomib on cell-mediated immunity is likely responsible for the increased incidence of HZ infections found in MM patients.3 4 Bortezomib's possible interference with cell-mediated immunity could potentially be a cause for the increased rates of HZ in patients with MM.4
MM causes deficiency in the humoral immune system but has a secondary effect on cell-mediated immunity owing to the effect of therapy, typically stem cell transplantation, especially in conjunction with immunomodulatory agents. It is thought that the reactivation of dormant viral infections in the immunocompromised patient is related to defects in the cell-mediated immune system and less so in the humoral immune system.
ASCT patients also have a higher incidence of HZ infections.10 This is not only experienced during the neutropenia stage of transplant but also once the neutrophil count recovers. This is secondary to persistent abnormalities in the immune system. HZ is an infection related to the postengraftment phase because there is depression in cell-mediated immunity.11 12 Therefore, it is not unreasonable to expect a higher rate of HZ infections in MM patients with ASCT receiving bortezomib, compared with MM patients receiving bortezomib alone. On the other hand, there may also be a cumulative higher rate of HZ infections in MM patients who received bortezomib with ASCT compared to patients receiving ASCT alone.
In summary, HZV reactivation is caused by the recrudescence of the latent VZV from the dorsal root of cranial nerve ganglia after the primary infection with varicella. HZO accounts for 10% of HZ infections.12–15 Thirty per cent of patients with HZO develop a chronic disease.14 15 The causes of chronic HZO disease include: vasculitis, perivasculitis, neuritis and perineuritis.14
Although there are no specific recommendations in different guidelines for protection from HZO in such a cohort of patients, it is generally recommended to start, for a variable length of time ranging from 3 to 12 months, an antiviral prophylaxis following autologous stem cell transplant (SCT), more critically in allogeneic SCT patients because of a lower immunity.9 10 This is mainly to prevent HZ reactivation in general without addressing the additional risk of HZO. Another independent factor that may play a role in virus reactivation is that the present cases were over 50 years of the age and this may have attributed to the increased susceptibility to infection. It is worth noting that without any treatment of MM patients, there is a decrease in NK cell activity that can be additionally altered during immunosuppressive therapy.16 17
Furthermore, some reports suggest that the use of new immunomodulatory agents, especially bortezomib, in the treatment of MM may be associated with an increased risk of HZ reactivation and hence, recommend an extended viral prophylaxis in such patients.4–6 8 However, there is a lack of guidelines specifically addressing this issue, especially regarding HZO-risk in this cohort of patients. Perhaps the new guidelines should highlight this issue and identify that the use of these therapies in MM, particularly in conjunction with SCT, warrant an extended viral prophylaxis.
Learning points.
Patients receiving immunomodulatory therapy for multiple myeloma should be monitored closely for cranial nerve symptoms, especially herpes zoster (HZ) infections. It may also be prudent to place patients on a prophylactic dose of antiviral treatment when they are started on these therapies in conjunction with autologous stem cell transplantation;
Patients who describe headaches, facial rashes, visual changes or other cranial nerve dysfunction need to be reviewed by an appropriate specialist, an emergent cerebrospinal fluid (CSF) study and viral PCR studies in CSF to be performed, with a low threshold for starting intravenous acyclovir followed by oral famciclovir.
Patients with HZ ophthalmicus need to be reviewed regularly by an ophthalmologist to check for chronic disease.
Acknowledgments
The authors thank Dr Muhaijr Mohamed, Launceston General Hospital for his invaluable input regarding management of these difficult cases and also Mrs Mary Sexton, Haematology Research Coordinator for her help in collecting the data.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mahindra A, Laubach J, Raje N, et al. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol 2012;9:135–43 [DOI] [PubMed] [Google Scholar]
- 2.Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 2005;352:2487–98 [DOI] [PubMed] [Google Scholar]
- 3.Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): a novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control 2003;10:361–9 [DOI] [PubMed] [Google Scholar]
- 4.Chanan-Khan A, Sonneveld P, Schuster MW, et al. Analysis of herpes zoster events among bortezomib-treated patients in the phase III APEX study. J Clin Oncol 2008;29:4784–9 [DOI] [PubMed] [Google Scholar]
- 5.Kim SJ, Kim K, Kim BS, et al. Bortezomib and the increased incidence of herpes zoster in patients with multiple myeloma. Clin Lymphoma Leukaemia Myeloma 2008;8:237.40. [DOI] [PubMed] [Google Scholar]
- 6.Chanan-Khan AA, Sonneveld P, Schuster MW, et al. Analysis of varicella zoster virus reactivation among bortezomib-treated patients in the APEX study. Blood 2006;108:1009 [Google Scholar]
- 7.Morison WL. Herpes simplex and herpes zoster in neoplasia. Lancet 1974;1:1293. [DOI] [PubMed] [Google Scholar]
- 8.Khalafallah A, Maiwald M, Cox A, et al. Effect of immunoglobulin therapy on the rate of infections in multiple myeloma patients undergoing autologous stem cell transplantation or treated with immunomodulatory agents. Medit J Hemat Infect Dis 2009;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuchter LM, Wingard JR, Piantadosi S, et al. Herpes zoster infection after autologous bone marrow transplantation. Blood J 1989;74:1424–7 [PubMed] [Google Scholar]
- 10.Nucci1 M, Anaissie E. Infections in patients with multiple myeloma in the era of high-dose therapy and novel agents. Clin Infect Dis 2009;49:1121. [DOI] [PubMed] [Google Scholar]
- 11.Blanco B, Pérez-Simón JA, Sánchez-Abarca LI, et al. Bortezomib induces selective depletion of alloreactive T lymphocytes and decreases the production of Th1 cytokines. Blood 2006;107:3575–83 [DOI] [PubMed] [Google Scholar]
- 12.Thomas J, Liesegang MD. Herpes zoster ophthalmicus: natural history, risk factors, clinical presentation, and morbidity. Ophthalmology 2008;115:2–5 [DOI] [PubMed] [Google Scholar]
- 13.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med 2007;357:2123–32 [DOI] [PubMed] [Google Scholar]
- 14.Liesegang TJ. Varicella-zoster virus eye disease. Cornea 1999;18:511–31 [PubMed] [Google Scholar]
- 15.Insinga RP, Itzler RF, Pellissier JM, et al. The incidence of herpes zoster in a United States administrative database. J Gen Intern Med 2005;20:748–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurisic V, Colovic N, Konjevic G, et al. An aggressive extramedullary cutaneous plasmacytoma associated with extreme alterations in the innate immune system. Onkologie 2010;33:113–15 [DOI] [PubMed] [Google Scholar]
- 17.Jurisic V, Srdic T, Konjevic G, et al. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med Oncol 2007;24:312–17 [DOI] [PubMed] [Google Scholar]


