Abstract
Snake bite is an important cause of mortality and morbidity in India with an estimated 35 000–50 000 fatal bites occurring annually. Neurological deficits following vasculotoxic snake bite are either due to intracranial haemorrhage or subarachnoid bleed as a result of consumption coagulopathy. However, ischaemic strokes and acute disseminated encephalomyelitis have been reported occasionally. We hereby report a case of snake bite leading to leucoencephalopathy.
Background
Snake bites are very common in India especially in the rainy season (June–September). Depending on their fang marks poisonous snakes are classified into five families. In India only three families of snakes namely Viperidae (Vipers), Elapidae (Cobra and Krait) and Hydrophidae (Sea snakes) are commonly found. Viperidae, Russell's viper (Daboia russelli) and saw scaled viper (Echis carinatus), are the leading cause of fatal snake bite in India. The common clinical characteristics of viper bite include local cellulitis, systemic haemorrhagic manifestations due to disseminated intravascular coagulation with consumption of clotting factors and renal failure. Neurological deficits following a viper bite are not uncommon and are usually due to an intracerebral or subarachnoid bleed.1 Ischaemic infarction involving different arterial territories,2–4 including brain stem infarction5 and acute disseminated encephalomyelitis (ADEM)6 following viper envenomation have been described. Asymmetric leucoencephalopathic changes after viper bite are a rare phenomenon and data in this regard is sparse.
Case presentation
A 40-year-old, previously healthy woman was bitten by a snake in the evening hours while she was working in the fields in her village. She became unconscious within half an hour of the bite and was taken to a nearby primary health centre where fang marks were noticed over the right foot. On the basis of observer account, epidemiology and examination she was transfused eight vials of polyvalent antisnake venom (ASV) with local site infiltration and referred to our centre for further management. At the time of presentation to our medical emergency she was in altered sensorium with Glasgow Coma Scale (GCS) score of 7 (E1M4V2). She had cellulitis involving the right lower limb. She was afebrile with a pulse rate of 94 beats/min, the blood pressure was measured at 100/70 mm Hg in right arm supine position and the respiratory rate was 18 breaths/min. Pupils were bilaterally equal but sluggishly reacting to light. Deep tendon reflexes were diminished and the plantar response was flexor bilaterally. Cardiovascular and respiratory system examinations were normal and she was passing adequate urine.
Investigations
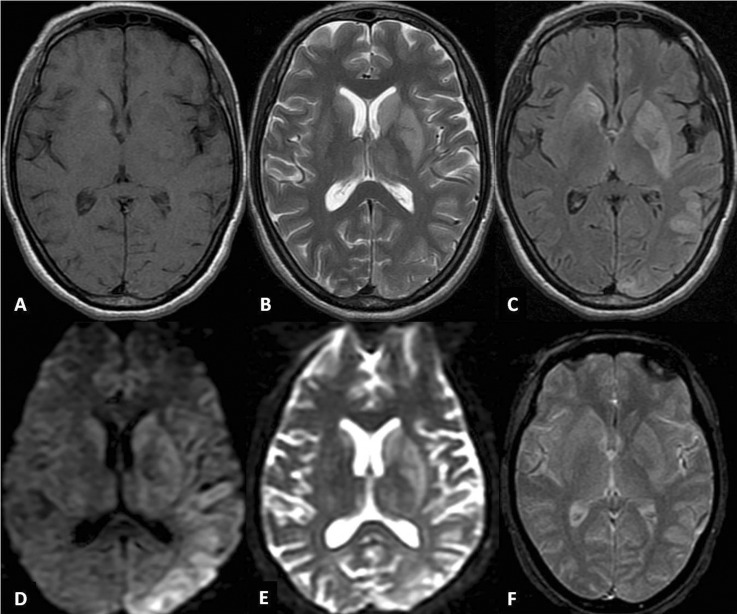
Investigations revealed haemoglobin of 10.4 g%, total leucocyte count 12 800/mm3 differential leucocyte count P75L22E2M1 and platelet count 180 000/mm.3 Twenty minutes whole blood clot test was positive. Random blood sugar was 173 mg/dl. Urine routine examination revealed 1–2 pus cells and 10–15 RBC's/HPF. Her total serum bilirubin was 0.6 mg/dl, serum alanine aminotransferase 168 U/l, serum aspartate aminotransferase 95 U/l, serum alkaline phosphatase 74 U/l, total serum protein 5.7 g/dl (albumin 2.4 g/dl), prothrombin time 24 s (International Randomised Ratio (INR) 1.71), blood urea 27.7 mg/dl, serum creatine 0.8 mg/dl, serum sodium 148 mEq/l and serum potassium was 3.8 mEq/l. Malarial parasite was negative. Hepatitis B surface antigen, hepatitis C virus antibody and ELISA for HIV were non-reactive. An MRI of the brain revealed signal intensity alteration in the caudate nuclei, lenticular nuclei and the thalami, along with involvement of the cortical rim suggestive of an asymmetrical leucoencephalopathy (figure 1). Diffusion-weighted imaging and the apparent diffusion coefficient were not suggestive of stroke. Cerebrospinal fluid (CSF) examination was normal (total cells 5, protein 45.6 mg/dl, sugar 75.5 mg/dl with a corresponding blood sugar of 158.2 mg/dl). The CSF and serum were negative for viral markers, especially herpes simplex, Japanese encephalitis and dengue.
Figure 1.
MRI of the brain depicts hyperintense signal changes involving the caudate nuclei, lenticular nuclei and the thalami (left > right) on T2-weighted (B) and fluid-attenuated inversion recovery (C) images; focal hyperintensity is noted in the right caudate nuclei on T1-weighted (A) sequence. Cortical rim hyperintensity involving the posterior half of the left hemisphere is evident on T2 weighted (B), fluid-attenuated inversion recovery (C), diffusion-weighted (D) and apparent-diffusion coefficient (E) images. There is no suggestion of haemorrhage or any such transformation on gradient-recall echo (F) image.
Differential diagnosis
Cerebrovascular accident
Viral encephalitis
Posterior reversible leucoencephalopathy syndrome
Treatment
Polyvalent ASV was given at a dose of 10 vials initially, followed by 5 vials 6 hourly for 2 days (total 50 vials); mannitol 100 ml intravenous infusion 8 hourly for 5 days, and ceftriaxone 1 g intravenous infusion 12 hourly for 7 days. Barring administration of ASV, the treatment was essentially supportive.
Outcome and follow-up
On day 2 of the treatment her clot test became negative and she showed some improvement in her level of consciousness. By the fourth day of treatment her GCS score had improved to 10 (E4M5V1) but she was in an akinetic-mute state. She was discharged on the 14th day and continued to improve till follow-up at 6 weeks. Her verbal output increased along with an improvement in motor abilities. The akinetic state had now transformed to a less disabling bradykinetic state. At this time she was initiated on a combination of levodopa and carbidopa (100 mg+25 mg) titrated to 300 mg equivalents of levodopa. She exhibited excellent response to this treatment and at the last follow-up at 10 weeks had minimal evidence of parkinsonism. She continues to be on a levodopa combination.
Discussion
Neurological signs and symptoms after a venomous snake bite are most often related to the toxic effects of venom, that is, anticoagulant/procoagulant activity or neurotoxicity. Some patients develop neurological complications related to cerebral hypoxia, which, in turn, are related to hypotensive shock that may accompany some snake bite envenomations. Neuromuscular disorders, that is, damage of the peripheral nervous system occurs most often after the bite of elapids, but may also occur following a viper bite. The effect of neurotoxins may start from minutes to a few hours after the inoculation of venom, causing weakness related to a blockage of synaptic transmission, at either presynaptic or postsynaptic levels.7
Common neurological manifestations in decreasing order of frequency include ptosis (85.7%), ophthalmoplegia (75%), limb weakness (26.8%), respiratory failure (17.9%), palatal weakness (10.7%) and neck muscle weakness (7.1%). A large prospective clinical study in Sri Lanka showed alteration in level of consciousness in 71%, autonomic disturbance in 66%, anterograde memory loss in 40% and delayed neuropathy in 22%.8 It is postulated that delayed neuropathy could be either due to direct neurotoxicity or a reaction to antivenom. Adverse reactions to antivenom appear in two forms; early and late. Early reactions tend to occur within 10–180 min after treatment and range from urticaria to anaphylactic shock. Late reactions are immune complex diseases and present in the form of serum sickness syndrome usually 5–24 days after antivenom administration. Both central and peripheral nervous system manifestations are seen in association with serum sickness.9 Generalised myokymia, syndrome of continuous and spontaneous muscular activity, resembling fasciculations following snake bite has also been reported.7
Cerebrovascular complications in the form of ischaemic strokes in various arterial territories, haemorrhagic stroke including multiple lobar haemorrhages with or without ventricular extension, haemorrhages in subarachnoid and subdural spaces, cerebellar haemorrhage, epidural haematoma, optic neuritis, delayed cerebellar ataxia and disseminated encephalomyelitis have been reported. The cause of ischaemic stroke in snake bite victims is controversial. The proposed etiopathogenesis involves the presence of venom toxins causing hypercoagulability and endothelial damage, immune-mediated vasculitis and systemic hypotension. Infarctions are most likely related to the prothrombotic effects of venom and to the presence of endothelial damage; this is supported by the finding of multiple cerebral infarctions in more than 60% of cases suffering from snake bite. Intracranial haemorrhages are related to abnormalities in haemostatic factors ranging from decreased platelets to a severe consumption coagulopathy. A number of pathogenetic mechanisms have been postulated to explain optic neuritis, including direct toxic effects of venom, vasoconstriction of optic nerve vascular supply, retinal or systemic haemorrhages and hypersensitivity reaction to antivenom. However, delayed cerebellar ataxia or disseminated encephalomyelitis is most probably related to an immune-mediated damage triggered by antivenin administration.7
Neurological features of viperine bite include drowsiness, confusion, fainting, dizziness, blurred vision, loss of muscle coordination and convulsions.1 The most common and serious central nervous system complication following vasculotoxic snake bite is intracranial haemorrhage. Ischaemic strokes involving various arterial territories2–4 of the brain, including brain stem5 and fatal ADEM6 have also been described. In most of the cases, infarction is multifactorial. Occasionally, cerebral infarction may be unrelated to bite and could be due to underlying medical illness.1 Altered sensorium following an hour after the bite is probably related to direct arterial endothelial injuries caused by the venom itself. The diffuse cerebral disturbances may be caused by toxic encephalopathy due to toxins in venom. To the best of our knowledge asymmetrical leucoencephalopathy involving the basal ganglia and thalami (deep nuclei) as well as the cortical rim following snake bite has not been reported. Although the pathogenesis of leucoencephalopathy is not clear, we feel that it could be due to the direct toxic effect of venom as she presented to us within hours of snake bite. Diffuse cortical changes and involvement of the deep nuclei make the probability of posterior reversible leucoencephalopathy syndrome unlikely in our case; in the same vein, extrapyramidal dysfunction of this grade has neither been described nor is expected in similar circumstances.
ASV remains the mainstay of therapy and suspected snake envenomation should be treated empirically with intravenous polyvalent ASV as early as possible. It may reverse systemic envenoming even when this has persisted for several days or, in the case of haemostatic abnormalities, for two or more weeks. However, when there are signs of local envenoming, without systemic envenoming, antivenom will be effective only if it can be given within the first few hours after the bite. Neurotoxic envenoming of the postsynaptic type (cobra bites) may begin to improve as early as 30 mins after antivenom, but usually takes several hours. Envenoming with presynaptic toxins (kraits and sea snakes) is unlikely to respond to ASV.10
Common causes of death in snake bite are respiratory paralysis, complications of mechanical ventilation, shock, intracerebral haemorrhage, ischaemic stroke, disseminated intravascular coagulation, wound complications, tetanus, cortical venous thrombosis, renal failure and hypoxic brain damage.8 Respiratory failure is the most common cause of mortality and morbidity in victims bitten by snakes. A death rate of 7.6% has been observed in patients on intensive care management. A prompt recognition of respiratory failure and timely mechanical ventilation can decrease morbidity and mortality.
Learning points.
Twenty minutes whole blood clotting test is an important simple measure for identification of a vasculotoxic snake bite.
Abnormal presentations in the form of leucoencephalopathy occurring secondary to a probable neurotoxic injury are rare and need to be differentiated from stroke and encephalitis.
The temporal relationship of the events and high index of suspicion helps in making the correct diagnosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Gouda S, Pandit V, Seshadri S, et al. Posterior circulation ischemic stroke following Russell's viper envenomation. Ann Indian Acad Neurol 2011;14:301–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narang SK, Paleti S, Azeez Asad MA, et al. Acute ischemic infarct in the middle cerebral artery territory following a Russell's viper bite. Neurol India 2009;57:479–80 [DOI] [PubMed] [Google Scholar]
- 3.Mugundhan K, Thruvarutchelvan K, Sivakumar S. Posterior circulation stroke in a young male following snake bite. J Assoc Physician India 2008;56:713–14 [PubMed] [Google Scholar]
- 4.Hoskote SS, Iyer VR, Kothari VM, et al. Bilateral anterior cerebral artery infarction following viper bite. J Assoc Physician India 2009;57:67–9 [PubMed] [Google Scholar]
- 5.Lee BC, Hwang SH, Bae JC, et al. Brainstem infarction following Korean viper bite. Neurology 2001;56:1244–5 [DOI] [PubMed] [Google Scholar]
- 6.Malhotra P, Sharma N, Awasthi A, et al. Fatal acute disseminated encephalomyelitis following treated snake bite in India. Emerg Med J 2005;22:308–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Brotto OH, Del Brotto VJ. Neurological complications of venomous snake bites: a review. Acta Neurol Scand 2012;125:363–72 [DOI] [PubMed] [Google Scholar]
- 8.Awasthi R, Shiva Narang S, Chowdhury PP. Cerebellar Ataxia following Snake Bite. J Assoc Physician India 2009;57:67–9 [PubMed] [Google Scholar]
- 9.Seneviratne U, Dissanayake S. Neurological manifestations of snake bite in Sri Lanka. J Postgrad Med 2002;48:275–9 [PubMed] [Google Scholar]
- 10.Warrell DA. Guidelines for the clinical management of snake bites in the South-East Asia region. Asian J Trop Med Public Health 1999;30(Suppl 1):1–67 [PubMed] [Google Scholar]