Abstract
Dysfunction of β-cells is a major characteristic in the pathogenesis of type 2 diabetes mellitus (T2DM). The combination of obesity and T2DM is associated with elevated plasma free fatty acids (FFAs). However, molecular mechanisms linking FFAs to β-cell dysfunction remain poorly understood. In the present study, we identified that the major endoplasmic reticulum stress (ERS) marker, Grp78 and ERS-induced apoptotic factor, CHOP, were time-dependently increased by exposure of β-TC3 cells to FFA. The expression of ATF6 and the phosphorylation levels of PERK and IRE1, which trigger ERS signaling, markedly increased after FFA treatments. FFA treatments increased cell apoptosis by inducing ERS in β-TC3 cells. We also found that FFA-induced ERS was mediated by the store-operated Ca2+ entry through promoting the association of STIM1 and Orai1. Moreover, calpain-2 was required for FFA-induced expression of CHOP and activation of caspase-12 and caspase-3, thus promoting cell apoptosis in β-TC3 cells. Together, these results reveal pivotal roles for Ca2+/calpain-2 pathways in modulating FFA-induced β-TC3 cell ERS and apoptosis.
Introduction
Type 2 diabetes mellitus (T2DM) is a multifactorial disease induced by genetic and many other environmental factors. Dysfunction of β-cells is a major characteristic in the pathogenesis of T2DM [1]. The prevalence of obesity in modern society has increased dramatically over the past few years and has reached epidemic proportions. Obesity, if sustained, may result in dyslipidemia, hypertension, glucose intolerance, insulin resistance and inflammation [2]. The combination of obesity and T2DM, is associated with excessive release of fatty acids from the expanded adipose tissue mass, leading to elevated plasma free fatty acids (FFAs) [3].
Dysfunction of β-cells is induced by several molecules including glucose, FFAs, and certain cytokines such as TNF-α [4]. Elevated plasma FFAs levels, which are accompanied by obesity, may play a causal role in β-cell dysfunction. It has been reported that acute FFA exposure stimulates insulin secretion, while prolonged FFA exposure decreases glucose-stimulated insulin secretion (GSIS) [5], [6]. However, molecular mechanisms linking FFA to β-cell dysfunction remain poorly understood.
The endoplasmic reticulum (ER) is responsible for protein folding and assembling into newly synthesized secretory proteins. When its function is disturbed by various physiological and pathological conditions such as misfolded protein accumulation, hypoxia, Ca2+ depletion, or microbial infection, endoplasmic reticulum stress (ERS) develops. ERS can regulate processes such as cell survival and cell death. Diets rich in saturated fats cause obesity. Concomitantly, cells are exposed to increased levels of FFAs of dietary origin or released by adipose tissues. Chronic exposure to high FFAs concentrations causes ERS which may contribute to cell apoptosis [2]. Growing evidence suggests that ERS signaling has been associated with Parkinson’s disease (PD), T2DM, and many other human diseases [7].
Unregulated Ca2+ influx might be a mediator of β-cell dysfunction and apoptosis in T2DM. In nonexcitable cells, such as β-cells, store-operated Ca2+ entry is the predominant Ca2+ influx mechanism [8]. Recent studies have identified that STIM1 and Orai1 are responsible for store-operated Ca2+ entry [9], [10]. Upon depletion of internal Ca2+ stores, STIM1, the endoplasmic reticulum-resident Ca2+ store sensor, translocate to areas near the plasma membrane to signal the activation of store-operated Ca2+ channels encoded by Orai1 proteins. Association of STIM1 with Orai1 is sufficient to reconstitute the store-operated Ca2+ channel function [9], [10]. Dysregulation of store-operated Ca2+ entry has been identified predominant incentive resulting in Ca2+ overload [11]. It is well recognized that cytoplasmic Ca2+ overload is a ubiquitous cause of cell death in neurons, cardiomyocytes, and insulin-producing β-cells [12], [13]. Effectors or executors of Ca2+ overload include calpains, kinases/phosphatases, calmodulin, and calcineurin [14]. Ca2+-dependent calpains belong to the cysteine protease family, and sustained hyperactivation of calpain is provoked in many pathological processes, including T2DM, ischemia, traumatic injury, and neurodegenerative disorders such as Alzheimer’s disease [13], [15], [16]. β-cells express several calpains, including calpain-10, calpain-1, and calpain-2. Polymorphisms in calpain-10 are associated with the risk of developing T2DM in some ethnic groups [17].
In this study, we found that by promoting store-operated Ca2+ entry through STIM1-Orai1 pathway, FFA treatments increase calpain-2 activity to induce expression of CHOP and activation of caspase-12 and caspase-3, thus enhancing cell apoptosis in β-TC3 cells.
Results
FFA Treatments Induce ERS in β-TC3 Cells and Increase Cell Apoptosis
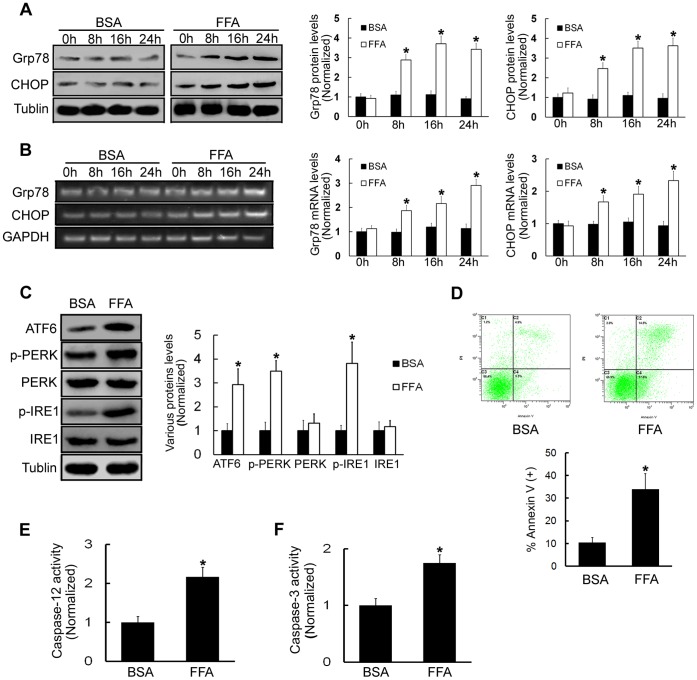
To examine whether FFA triggers ERS in β-TC3 cells, we examined the expression patterns of several molecular indicators of ERS. The ER chaperon glucose regulated 78-kDa protein (Grp78), also named Bip, and the transcription factor CHOP are central regulators of ERS. Mouse pancreatic β-TC3 cells were treated with FFA (0.5 mM) for different times (0, 8, 16, or 24 h). Bovine serum albumin (BSA) was used as a negative control. The results showed that Grp78 and CHOP protein levels were time-dependently increased in cells treated with FFA compared with corresponding BSA treated cells (P<0.05, Figure 1A). The mRNA levels of Grp78 and CHOP were also increased in a time-dependently manner in cells treated with FFA compared with corresponding BSA treated cells (P<0.05, Figure 1B). In order to further assess whether FFA treatments could induce ERS, we examined the expression and phosphorylation levels of ATF6, PERK, and IRE1, the three key factors that trigger ERS signaling in β-TC3 cells. We observed that the expression of ATF6 and the phosphorylation levels of PERK and IRE1 markedly increased after FFA treatments for 16 h (293.4%±65.2%, 349.3%±43.7% and 381.6%±89.5%, respectively, P<0.05, Figure 1C). All these data demonstrated that ERS can be activated by FFA treatments in β-TC3 cells.
Figure 1. FFA treatments induce ERS in β-TC3 cells and increase cell apoptosis.
(A) Cells were treated with 0.5 mM FFA or BSA for 0, 8, 16, or 24 h. Western blot was used to examine Grp78 and CHOP protein levels. (B) RT-PCR was used to test Grp78 and CHOP mRNA levels. (C) Cells were treated with 0.5 mM FFA or BSA for 16 h. Western blot was used to examine the expression levels of ATF6, p-PERK, PERK, p-IRE1 and IRE1. (D) Cells were treated with 0.5 mM FFA or BSA for 16 h. Cell death was quantified by annexin V/PI double staining. (E) Caspase-12 activity was detected after cells were treated with 0.5 mM FFA or BSA for 16 h. (F) Caspase-3 activity was detected after cells were treated with 0.5 mM FFA or BSA for 16 h. BSA-treated cells were used as a negative control. Bars represent each sample performed in triplicate, and the error bars represent the standard deviations. *P<0.05, by the Student’s t-test.
ERS might be a mediator of cell death, thus β-TC3 cell death was examined by annexin V/propidium iodide (PI) double staining after FFA treatments. The results demonstrated that FFA treatments caused more cell apoptosis than BSA control treatments (33.9%±6.91% and 10.42%±2.15%, respectively, P<0.05, Figure 1D). It is known that activation of murine caspase-12 is associated with ERS-induced cell apoptosis [18]. Thus, we detected the activation of caspase-12. Compared with the activation in BSA-treated β-TC3 cells, caspase-12 activation in FFA-treated β-TC3 cells was significantly increased (216.7%±24.3%, P<0.05, Figure 1E). Caspase-3 (also named CPP32, apopain, and YAMA) has been identified as a key mediator of apoptosis in mammalian cells. We further assessed the activation of caspase-3 (cleaved caspase-3), and the results showed that FFA treatments increased caspase-3 activation (175.4%±14.2%, P<0.05, Figure 1F). From the above results, we concluded that FFA treatments could induce ERS and increase apoptosis in β-TC3 cells.
FFA Treatments Promote Store-operated Ca2+ Entry to Induce ERS in β-TC3 Cells
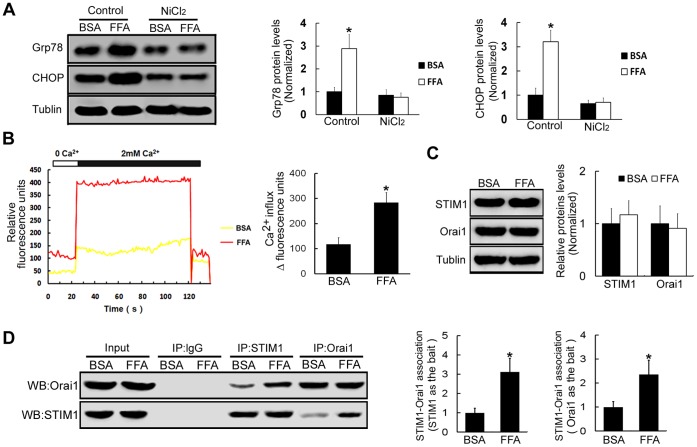
It is well known that the disruption of intracellular Ca2+ homeostasis can disturb ER function and induce ERS. In nonexcitable cells, such as pancreatic β-cells, Ca2+ influx is the predominant mechanism to maintain intracellular Ca2+ homeostasis [19]. To assess whether FFA treatments-induced ERS is mediated by Ca2+ influx changes in β-TC3 cells, NiCl2, a Ca2+ channel blocker, was used. The results showed that FFA treatments were not able to increase Grp78 and CHOP levels in the condition that Ca2+ influx was blocked with NiCl2 treatments (P>0.05, Figure 2A). The results suggested that FFA treatments induced-ERS is mediated by the increase of Ca2+ influx. One of the major routes for Ca2+ influx into cells is through store-operated Ca2+ entry channels [8]. To further assess whether FFA treatments may induce store-operated Ca2+ entry in β-TC3 cells, store-operated Ca2+ entry was examined after FFA treatments. The results demonstrated that FFA treatments resulted in more than a 2-fold increase of store-operated Ca2+ entry in β-TC3 cells compared with BSA treatments (P<0.05, Figure 2B). Recent studies have identified that STIM1 and Orai1 are responsible for store-operated Ca2+ entry. We found that the expression levels of STIM1 and Orai1 in cells treated with FFA was similar to cells treated with BSA (P>0.05, Figure 2C). However, immunoprecipitation with anti-STIM1 antibody followed by western blot with anti-Orai1 antibody revealed that the association of STIM1 with Orai1 in samples from FFA-treated cells, was much more than the association observed in BSA-treated cells (312.5%±71.4%, P<0.05, Figure 2D). The results were further strengthened with Orai1 as the bait (236.2%±59.1%, P<0.05, Figure 2D). The results suggested that FFA enhanced the association of STIM1 with Orai1. Therefore, these results indicated that FFA treatments induced-ERS is mediated by the store-operated Ca2+ entry which is regulated by the association of STIM1 with Orai1.
Figure 2. FFA treatments increase Ca2+ influx to induce ERS in β-TC3 cells.
(A) β-TC3 cells were incubated with FFA or BSA for 16 h, western blot was used to examine the protein expression levels of Grp78 and CHOP following the treatment with Ca2+ channel blocker NiCl2. (B) β-TC3 cells were incubated with FFA or BSA for 16 h, and then stimulated with 4 µM thapsigargin for 20 min to activate store-operated Ca2+ entry. Fluorescence densities of Ca2+ change were monitored in Fluo-8/AM-loaded β-TC3 cells after FFA or BSA treatments. (C) The protein expression levels of STIM1 and Orai1 were tested by western blot following treatments with FFA or BSA for 16 h in β-TC3 cells. (D) β-TC3 cells were incubated with FFA or BSA for 16 h, and then stimulated with 4 µM thapsigargin for 20 min. Cell lysates were immunoprecipitated with anti-STIM1 antibody followed by western blot using anti-Orai1 antibody and with anti-Orai1 antibody followed by western blot using anti-STIM1 antibody. Immunoprecipitated with anti-IgG antibody was used as the negative control. Bars represent each sample performed in triplicate, and the error bars represent the standard deviations. *P<0.05, by the Student’s t-test.
By Increasing Calpain-2 Activity, FFA Treatments Promote CHOP Induction in β-TC3 Cells
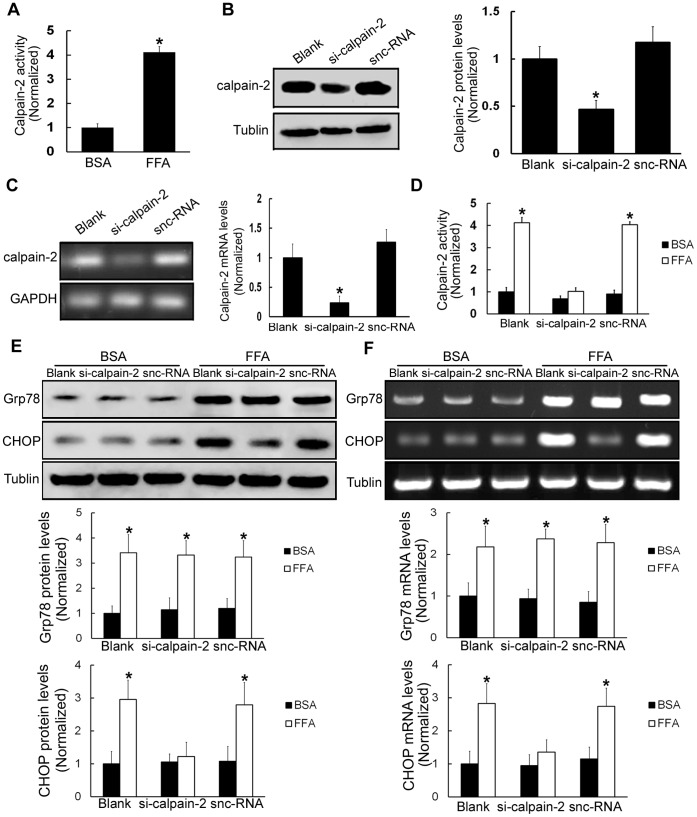
Calpain-2, a neutral Ca2+-dependent protease, mediates a variety of physiological functions such as cytoskeleton remodeling, vesicle trafficking, and membrane fusion [20]. To assess whether FFA treatments may influence calpain-2 activity in β-TC3 cells, calpain-2 activity was examined after FFA treatments. The results demonstrated that after FFA treatments, calpain-2 activity was dramatically enhanced in β-TC3 cells (411.4%±23.5%, P<0.05, Figure 3A). To examine whether FFA-induced ERS is dependent on calpain-2, we used small interfering RNA (siRNA) to knock down calpain-2 in β-TC3 cells. Calpain-2 siRNA led to a marked reduction of calpain-2 protein and mRNA levels (46.7%±9.5% and 23.4%±11.6%, respectively, P<0.05, Figure 3B and Figure 3C). When the cells were treated with calpain-2 siRNA, FFA treatments did not increase calpain-2 activity compared with corresponding BSA treatments in β-TC3 cells (P>0.05, Figure 3D). To further validate whether calpain-2 is involve in FFA-triggered ERS in β-TC3 cells, we examined the protein and mRNA levels of Grp78 and CHOP in the condition that calpain-2 activity was inhibited with calpain-2 siRNA. The results showed that FFA treatments increased Grp78 protein and mRNA levels following calpain-2 siRNA treatments (P<0.05, Figure 3E and Figure 3F). However, FFA treatments were not able to increase CHOP protein and mRNA levels following calpain-2 siRNA treatments (P>0.05, Figure 3E and Figure 3F). These results suggested that calpain-2 is involved in FFA-induced CHOP expression, but not involved in FFA-induced Grp78 expression.
Figure 3. FFA treatments increase calpain-2 activity, thus inducing ERS in β-TC3 cells.
(A) The activity of calpain-2 was tested in β-TC3 cells treated with 0.5 mM FFA or BSA for 16 h. (B) β-TC3 cells were treated with calpain-2 siRNA for 24 h. Western blot was performed to examine calpain-2 protein levels. (C) RT-PCR was used to analyze calpain-2 mRNA levels. (D) Calpain Activity Assay Kit was used to analyze calpain-2 activity. Silencer negative control siRNA (snc-RNA)-treated cells were used as a negative control. (E) β-TC3 cells were treated with calpain-2 siRNA for 24 h and then stimulated with FFA for 16 h, and western blot was used to examine the protein expression levels of Grp78 and CHOP. (F) RT-PCR was used to test Grp78 and CHOP mRNA levels. BSA-treated β-TC3 cells were used as a negative control. Bars represent each sample performed in triplicate, and the error bars represent the standard deviations. *P<0.05, by the Student’s t-test.
Calpain-2 is Required for FFA-induced β-TC3 Cell Apoptosis
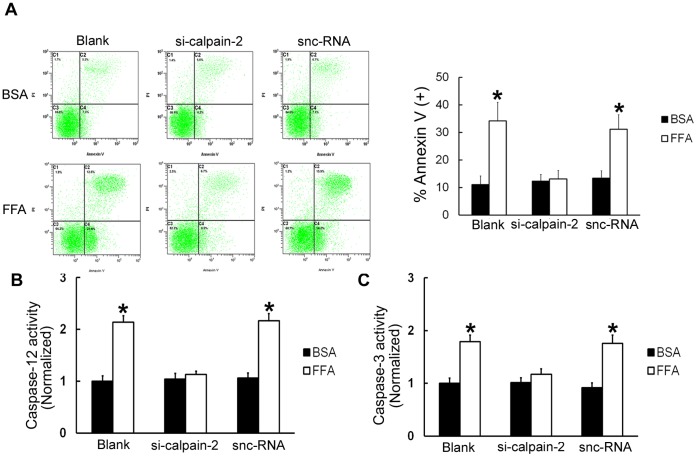
The above results demonstrated that FFA treatments can increase apoptosis in β-TC3 cells. To further confirm that calpain-2 is involved in this process, calpain-2 siRNA was used. Annexin V/PI double staining demonstrated that FFA treatments were not able to increase the percentage of apoptotic cells compared with corresponding BSA treatments following calpain-2 siRNA treatments (P>0.05, Figure 4A). To corroborate these results, the activation of caspase-12 and caspase-3 were detected. The results showed that FFA treatments were not able to increase caspase-12 and caspase-3 activation compared with corresponding BSA treatments following calpain-2 depletion (P>0.05, Figure 4B and Figure 4C). Therefore, we concluded that calpain-2 is required for FFA-induced β-TC3 cell apoptosis.
Figure 4. Calpain-2 is required for FFA treatment-induced β-TC3 cell apoptosis.
The cells were treated with calpain-2 siRNA, incubated for 24 h, and then stimulated with FFA or BSA for 16 h. (A) Cell death was quantified by annexin V/PI double staining. (B) Caspase-12 activity was detected after cells were treated with calpain-2 siRNA. (C) Caspase-3 activity was detected after cells were treated with calpain-2 siRNA. snc-RNA-treated cells were used as a negative control. Bars represent each sample performed in triplicate, and the error bars represent the standard deviations. *P<0.05, by one-way ANOVA.
Discussion
This study was designed to identify the molecular mechanisms of β-cell dysfunction induced by FFA. The underlying concept is that once the primary pathogenesis of diabetes is established, hyperglycemia and very commonly hyperlipidemia ensue, and thereafter they exert additional damaging or toxic effects on β-cells [21]. It has been suggested that insulin resistance precedes the development of T2DM. A common feature of insulin resistant states is high plasma FFA content [22]. In addition to reducing insulin sensitivity in peripheral tissues, elevated plasma FFA levels may also contribute to the deterioration of β-cell function. Many studies, using insulin-secreting cells and isolated islets, have attempted to identify the mechanisms of lipotoxicity in β-cell dysfunction. In vitro, prolonged exposure of isolated islets or insulin-secreting cells to elevated FFA levels is associated with inhibition of GSIS [23], [24], which has also been observed in vivo in rats [25] and humans [26]; impairment of insulin gene expression [27]–[32]; and induction of cell apoptosis [33], [34]. In vitro, prolonged exposure of isolated islets or insulin-secreting cells to elevated FFA causes β-cell apoptosis which may contribute to the loss of β-cell mass in T2DM [23], [24]. Moreover, ERS has been reported to link FFA–induced β-cell apoptosis with insufficient insulin secretion in rodents. Previous researches indicated that excessive ERS can trigger cellular apoptosis through the activation of caspase-12 [29]–[32]. Furthermore, CHOP, a crucial transcription factor in ERS induced apoptosis, mediates cell death through the induction of various genes including GADD34 and ERO1, which may tip the balance towards apoptosis [33], [34]. However, the detailed mechanism that FFA-triggered β-cell apoptosis and eventually enhanced loss of β-cell mass in T2DM has not been determined. Our data indicated that FFA could induce ERS through enhancing Ca2+ influx which is mediated by promoting the association of STIM1 and Orai1. Furthermore, we showed that FFA could activate calpain-2 to induce CHOP expression and β-cells apoptosis, thus enhancing loss of β-cell mass in T2DM.
We demonstrated that the expression levels of molecular indicators of ERS, Grp78 and CHOP, are time-dependently increased by exposure of β-cells to FFA. The expression of ATF6 and the phosphorylation levels of PERK and IRE1, which trigger ERS signaling, markedly increase after FFA treatments. These results demonstrated that ERS could be activated by FFA treatments in β-cells. ERS has been demonstrated to be involved in apoptosis induction during pathophysiological processes, including diabetes [35]. Grp78, the major ER-resident chaperone and the most abundant glycoprotein in the ER, plays critical roles in protein folding and ER Ca2+ binding, and it is widely used as a biomarker of ERS [36]. Moreover, other ERS-related chaperones, such as CHOP, are also components of the apoptosis pathway mediated by ERS [37]. In mammalian cells, upon ERS, the ER transmembrane kinase IRE1 initiates the splice processing of XBP1 mRNA and results in activation of XBP1, which can then bind ERS response elements and activate the transcriptional set of ER chaperones, such as Grp78 and CHOP [38]. Several studies have shown that the upregulation of these ERS-related chaperones is believed to induce ERS, leading to cell apoptosis by toxic insults [39].
Aberrantly increased cytoplasmic Ca2+ has been shown to mediate cell dysfunction and cell death in neurodegenerative diseases [40]. Store-operated Ca2+ entry (SOCE) is a common and ubiquitous mechanism of regulating Ca2+ influx into cells [10]. Two genes, STIM1 and Orai1, are responsible for store-operated Ca2+ entry activation. Once endoplasmic reticulum Ca2+ depleted, STIM1 proteins aggregate into multiple puncta and translocate to the plasma membranes. Orai1 molecule, an essential component of the SOCE channel, translocates to the same STIM1-containing structures to form association with STIM1 during store depletion and opens to mediate Ca2+ entry [9], [10]. Our studies implied that the enhancement of store-operated Ca2+ entry which is regulated by the association of STIM1 with Orai1 contributed to the cytotoxicity of FFA. Moreover, these data suggested that dysfunction and increased apoptosis of β-cells are mediated, at least partially, through FFA induced Ca2+ overload which caused by association of STIM1 with Orai1.
The calpains are a family of Ca2+-dependent, non-lysosomal, neutral cysteine endopeptidases [41]. They are known to play important roles in several Ca2+ regulated physiological processes, including cytoskeletal remodeling, cellular signaling, cell proliferation, cell cycle progression, apoptosis, and cell survival [42]. Two isoforms in particular, calpain-1 (μ-calpain) and calpain-2 (m-calpain), have been extensively studied. Both isoforms function as heterodimeric enzymes and are composed of a distinct, large catalytic subunit (80 kDa) associated with a common, small regulatory subunit (30 kDa) that helps maintain calpain activity [43], [44]. Calpain activity is regulated by multiple mechanisms, including Ca2+ modulation, autoproteolysis, phosphorylation, intracellular distribution, and inhibition by calpastatin. The best studied mechanism is Ca2+ activation, because calpains contain Ca2+ binding EF-hand motifs in domains IV and VI [41]. The two isoforms differ not only in their amino acid sequence of the large catalytic subunits, but also in their Ca2+ requirements for activation in vitro. Calpain-2 requires millimolar concentrations of Ca2+, whereas calpain-1 requires micromolar concentrations. In general, however, these concentrations of Ca2+ are far greater than that can be achieved intracellularly, suggesting that additional factors, such as phospholipids or activation proteins, are required for activation of both isoforms in vivo [45]. In the study, we found that calpain-2 is markedly activated and plays critical roles in FFA induced cell apoptosis. CHOP, known as growth arrest and DNA damage inducible gene, is a member of the C/EBP transcription factor family. It has been reported that CHOP induction, which occurs during several responses to cellular stress, is involved in the ERS-induced apoptosis pathway [37]. In particular, CHOP is the first molecule identified to mediate ERS-induced apoptosis. CHOP inhibits expression of Bcl-2, depletes glutathione, facilitates translocation of proapoptotic protein Bax, and thereby induces apoptosis [46]. We observed that inhibition of calpain-2 decreased CHOP expression after FFA treatments. Thus, calpain-2-induced CHOP expression may result in FFA treatments-induced β-cells apoptosis. It is reported that transcription factor ATF2 may bind to CHOP promoter to promote CHOP expression. The transactivating capacity of ATF2 is depending on phosphorylation of N-terminal residues Thr69, Thr71, and Ser90 [47], [48]. We suspect that by phosphorylation of ATF2, calpain-2 affects the expression of CHOP. The study is underway in our lab.
Most ERS-related pro-apoptotic signals ultimately lead to caspase activation. Caspase-12 and caspases-3, which have been proposed as specific mediators of ERS-induced apoptosis in rodent cells [49], were observed activated after FFA treatments in our study. Furthermore, we found that calpain-2 is required for FFA treatments-induced cell apoptosis and activation of caspase-12 and caspase-3 in β-cells, which may account for cell apoptosis in hyperlipidemia induced T2DM. Therefore, the inhibition of calpain-2 may decrease hyperlipidemia-induced β-cells apoptosis in T2DM. Given the nature of in vitro study, our work has some limitations. The extensive interactions among cells and tissues cannot be completely duplicated in vitro study. Our results should be further demonstrated in animal models, and the study is underway in our lab.
The contribution of Ca2+ to FFA treatments-induced ERS in β-cells has been established for many years, but as Ca2+ is a widely existed second messenger,which has been shown to play roles in the regulation of proliferation, invasion, and metastatic potential, the side effect of inhibition Ca2+ level is major challenges to its clinical application. As we demonstrated that calpain-2 is required for FFA treatments-induced ERS in β-cells. The development of new therapeutic approaches, including small molecules or antibodies that can specifically block calpain-2 expression and/or its activity, is clinically feasible in the future. In this regard, drugs that target calpain-2 may represent a novel approach to T2DM treatment.
Materials and Methods
Cell Culture
Mouse β-TC3 cell line, which was kindly provided by Novo Nordisk, Copenhagen, Denmark [50], was cultured in RPMI 1640 medium (Gibco, Grand Island, USA), supplemented with 10% FBS, 1% penicillin/streptomycin, and 2% L-glutamine at 37°C in a humidified atmosphere of 5% CO2.
Treatment of Cells with FFA
β-TC3 cells were treated with 0.5 mM palmitic acid (Sigma, St. Louis, MO, USA) for 0, 8, 16, and 24 h. Equivalent amounts of fatty acid-free BSA were added to the control group. Palmitic acid solution was prepared as described previously [51].
Gene Silencing
The sense sequence for calpain-2 siRNA was 5′-GCGAGGACATGCACACCAT-3′ (GenePharma, China). The cells were transfected with siRNA using the LipofectAMINE 2000 reagent (Invitrogen, CA, USA) according to the manufacturer’s instructions. The sense sequence for silencer negative control siRNA (snc-RNA) was 5′-AGTCGACGTCAGCTGAAGGC-3′ (GenePharma, China), which was used as a negative control under similar conditions.
Western Blot Analysis
Cells were lysed in 1% OG buffer (20 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1% OG, 1 mM EDTA, 10 µg/ml leupeptin, 2 µg/ml aprotinin, and 1 mM PMSF). BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA) was then used to determine the total protein density. Then equal amounts of protein were separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) microporous membrane (Millipore Billerica, MA, USA). After being blocked with 5% non-fat milk, the membrane was incubated for 2 h at room temperature with the designated antibody. A Western-Light chemiluminescent detection system (Applied Biosystems, Foster City, CA, USA) was used for immunodetection.
Immunoprecipitation Analysis
β-TC3 cells were preincubated with 0.5 mM FFA or fatty acid-free BSA for 16 h before treated with 4 µM thapsigargin (Invitrogen, CA, USA) for 20 min to activate store-operated Ca2+ entry. Cells were then washed and resuspended in NPBS. Cells were lysed in 1% OG buffer. BCA Protein Assay Kit (Pierce Biotechnology, Rockford, IL, USA) was then used to determine the total protein density. Fraction of the lysates was saved as “input”. Then, aliquots of lysates (1 mL) were immunoprecipitated by 25 µL of protein A-agarose that was pre-bound with 2 µg of anti-IgG antibody, anti-STIM1 antibody or anti-Orai1 antibody overnight at 4°C. Bound proteins were then eluted from protein A-agarose with elution buffer, and aliquots of the eluent were submitted to western blot using anti-Orai1 antibody diluted 1∶250 in TBST or anti-STIM1 antibody diluted 1∶300 in TBST for 2 h at room temperature. A Western-Light chemiluminescent detection system (Applied Biosystems, Foster City, CA, USA) was used for immunodetection.
Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from the cells with TRIzol reagents (Invitrogen) and reversely transcribed into cDNA with the ReverTra Ace-a kit (Toyobo). The primers were synthesized by Shanghai Sangon Co. as follows: Grp78, forward primer 5′-CACAGACGGGTCATTCC-3′, reverse primer 5′-CCTATGTCGCCTTCACT-3′; CHOP, forward primer 5′-GACGCTTCACTACTCTTGACCCTGCG-3′, reverse primer 5′-GGATGTGCGTGTGACCTCTGT-3′; calpain-2, forward primer 5′-GCAGCCATTGCCTCCCTCAC-3′, reverse primer 5′-ACCTCCACCCACTCGCCGTA-3′; GAPDH, forward primer 5′-AATGTCACCGTTGTCCAGTTGC-3′, reverse primer 5′-CACCATCTTAGGAGGAGGAGTAGC-3′. GAPDH mRNA was used as an internal control. The PCR conditions were 1 cycle of 94°C for 5 min; 35 cycles of 94°C for 60 s, 57°C for 30 s, and 72°C for 30 s; and finally 72°C for 5 min. PCR products were electrophoresed on 1% agarose gels.
Measurement of Store-operated Ca2+ Entry
Store-operated Ca2+ entry was measured using Fluo-8 acetoxymethyl ester(Fluo-8/AM)(Abcam, Cambridge, UK). β-TC3 cells were preincubated with FFA or BSA for 16 h before loading with the dye by incubation with 0.1 mg/ml Fluo-8/AM for 45 min in the dark at 37°C. Cells were then washed and resuspended in NPBS. To start the experiment, cells were pretreated with 4 µM thapsigargin (Invitrogen, CA, USA) for 20 min to deplete internal Ca2+ stores. After washing off extracellular thapsigargin, Ca2+-free phosphate-buffered saline (PBS) containing 3 mM EGTA was then added to the cells. To record Ca2+ influx, 2 mM Ca2+ was added after approximately 20 s. Ca2+ influx levels were recorded from Fluo-8 loaded cells excited at wavelengths of 340 nm and 380 nm and imaged with 510 nm filters. The fluorescence signal was monitored and recorded by an FV300 laser scanning confocal microscope (Olympus).
Measurement of Calpain-2 Activity
Calpain-2 activity was determined using the Calpain Activity Assay Kit (Abcam, Cambridge, UK) following the manufacturer’s instructions. In brief, cell lysates were incubated at 37°C with Ac-LLY-AFC, a calpain-2 substrate, for 1 h. The fluorescence of the cleaved substrate was measured by a spectrofluorometer (Molecular devices, CA, USA).
Annexin V/PI Double Staining
The number of dead cells was determined by annexin V/PI double staining. Cells were exposed to 0.5 mM FFA or BSA for 16 h and then incubated with FITC-conjugated annexin V in binding buffer (0.01 µM HEPES, 0.14 µM NaCl, and 2.5 mM CaCl2, pH 7.4) for 20 min at 37°C in the dark. After incubation, the cells were washed and resuspended in 200 µl PBS with 1% FCS and additionally incubated with 10 µl of 1 mg/ml PI solution. The annexin V-positive cells were detected using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA), and the results were analyzed using CellQuest software (BD Biosciences, San Jose, CA, USA). Annexin V-FITC conjugates were detected with the FL1 channel of the FACSCalibur machine. PI was read on the FL2 channel.
Detection of Caspase Activity
Caspase-3 activity was measured spectrophotometrically via the detection of pNA cleavage by caspase-3-specific substrates. These experiments were completed using a Caspase-3 Assay Kit (Beyotime, Shanghai, China). After the cell lysates were incubated with Ac-DEVD-pNA for 2 h at 37°C, the samples were read at 405 nm. Caspase-12 activity was measured spectrophotometrically via the detection of free AFC cleavage by caspase-12-specific substrates. These experiments were completed using a Caspase-12 Assay Kit (Biovision, San Francisco, CA, USA). After the cell lysates were incubated with ATAD-AFC for 2 h at 37°C, the samples were read at 505 nm.
Statistical Analysis
Statistical analysis was performed using SPSS 13.0 statistical software. The results were expressed as mean values ± SD. And the Student’s t-test or one-way ANOVA were used to evaluate the statistical significance in the groups. The differences were considered significant when P<0.05.
Acknowledgments
We are grateful to Chuan Qin for excellent assistance.
Funding Statement
This work is supported by National Natural Science Foundation of China (30870948). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Guillausseau PJ, Meas T, Virally M, Laloi-Michelin M, Médeau V, et al. (2008) Abnormalities in insulin secretion in type 2 diabetes mellitus. Diabetes Metab 34 Suppl 2 S43–48. [DOI] [PubMed] [Google Scholar]
- 2. Ceylan-Isik AF, Sreejayan N, Ren J (2011) Endoplasmic reticulum chaperon tauroursodeoxycholic acid alleviates obesity-induced myocardial contractile dysfunction. J Mol Cell Cardiol 50: 107–116. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3. Bikopoulos G, da Silva Pimenta A, Lee SC, Lakey JR, Der SD, et al. (2008) Ex vivo transcriptional profiling of human pancreatic islets following chronic exposure to monounsaturated fatty acids. J Endocrinol 196: 455–464. [DOI] [PubMed] [Google Scholar]
- 4. Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, et al. (2010) Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes 59: 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou YP, Grill VE (1994) Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest 93: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou YP, Grill VE (1995) Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab 80: 1584–1590. [DOI] [PubMed] [Google Scholar]
- 7. Tang J, Guo YS, Zhang Y, Yu XL, Li L, et al. (2012) CD147 induces UPR to inhibit apoptosis and chemosensitivity by increasing the transcription of Bip in hepatocellular carcinoma. Cell Death Differ 19: 1779–1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, et al. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441: 179–185. [DOI] [PubMed] [Google Scholar]
- 9. Park CY, Hoover PJ, Mullins FM, Bachhawat P, Covington ED, et al. (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136: 876–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Muik M, Fahrner M, Derler I, Schindl R, Bergsmann J, et al. (2009) A cytosolic homomerization and a modulatory domain within STIM1 C-terminus determine coupling to ORAI1 channels. J Biol Chem 284: 8421–8426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palty R, Raveh A, Kaminsky I, Meller R, Reuveny E (2012) SARAF inactivates the store operated calcium entry machinery to prevent excess calcium refilling. Cell 149: 425–438. [DOI] [PubMed] [Google Scholar]
- 12. Saito K, Elce JS, Hamos JE, Nixon RA (1993) Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A 90: 2628–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bevers MB, Neumar RW (2008) Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 28: 655–673. [DOI] [PubMed] [Google Scholar]
- 14. Demuro A, Mina E, Kayed R, Milton SC, Parker I, et al. (2005) Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem 280: 17294–17300. [DOI] [PubMed] [Google Scholar]
- 15. Kuwako K, Nishimura I, Uetsuki T, Saido TC, Yoshikawa K (2002) Activation of calpain in cultured neurons overexpressing Alzheimer amyloid precursor protein. Brain Res Mol Brain Res 107: 166–175. [DOI] [PubMed] [Google Scholar]
- 16. Norberg E, Gogvadze V, Ott M, Horn M, Uhlén P, et al. (2008) An increase in intracellular Ca2+ is required for the activation of mitochondrial calpain to release AIF during cell death. Cell Death Differ 15: 1857–1864. [DOI] [PubMed] [Google Scholar]
- 17. Song Y, You NC, Hsu YH, Sul J, Wang L, et al. (2007) Common genetic variation in calpain-10 gene (CAPN10) and diabetes risk in a multi-ethnic cohort of American postmenopausal women. Hum Mol Genet 16: 2960–2971. [DOI] [PubMed] [Google Scholar]
- 18. Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, et al. (2001) Activation of caspase-12, an endoplasmic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276: 13935–13940. [DOI] [PubMed] [Google Scholar]
- 19. Sammels E, Parys JB, Missiaen L, De Smedt H, Bultynck G (2010) Intracellular Ca2+ storage in health and disease: a dynamic equilibrium. Cell Calcium 47: 297–314. [DOI] [PubMed] [Google Scholar]
- 20. Wang CF, Huang YS (2012) Calpain 2 activated through N-Methyl-D-Aspartic acid receptor signaling cleaves CPEB3 and abrogates CPEB3-repressed translation in neurons. Mol Cell Biol 32: 3321–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poitout V, Robertson RP (2008) Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev 29: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Randle PJ, Garland PB, Hales CN, Newsholme EA (1963) The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet 1: 785–789. [DOI] [PubMed] [Google Scholar]
- 23. Sako Y, Grill VE (1990) A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology 127: 1580–1589. [DOI] [PubMed] [Google Scholar]
- 24. Elks ML (1993) Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology 133: 208–214. [DOI] [PubMed] [Google Scholar]
- 25. Mason TM, Goh T, Tchipashvili V, Sandhu H, Gupta N, et al. (1999) Prolonged elevation of plasma free fatty acids desensitizes the insulin secretory response to glucose in vivo in rats. Diabetes 48: 524–530. [DOI] [PubMed] [Google Scholar]
- 26. Paolisso G, Gambardella A, Amato L, Tortoriello R, D’Amore A, et al. (1995) Opposite effects of short- and long-term fatty acid infusion on insulin secretion in healthy subjects. Diabetologia 38: 1295–1299. [DOI] [PubMed] [Google Scholar]
- 27. Gremlich S, Bonny C, Waeber G, Thorens B (1997) Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J Biol Chem 272: 30261–30269. [DOI] [PubMed] [Google Scholar]
- 28. Ritz-Laser B, Meda P, Constant I, Klages N, Charollais A, et al. (1999) Glucose-induced preproinsulin gene expression is inhibited by the free fatty acid palmitate. Endocrinology 140: 4005–4014. [DOI] [PubMed] [Google Scholar]
- 29. Jacqueminet S, Briaud I, Rouault C, Reach G, Poitout V (2000) Inhibition of insulin gene expression by long-term exposure of pancreatic beta cells to palmitate is dependent on the presence of a stimulatory glucose concentration. Metabolism 49: 532–536. [DOI] [PubMed] [Google Scholar]
- 30. Briaud I, Harmon JS, Kelpe CL, Segu VB, Poitout V (2001) Lipotoxicity of the pancreatic beta-cell is associated with glucose-dependent esterification of fatty acids into neutral lipids. Diabetes 50: 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kelpe CL, Moore PC, Parazzoli SD, Wicksteed B, Rhodes CJ, et al. (2003) Palmitate inhibition of insulin gene expression is mediated at the transcriptional level via ceramide synthesis. J Biol Chem 278: 30015–30021. [DOI] [PubMed] [Google Scholar]
- 32. Hagman DK, Hays LB, Parazzoli SD, Poitout V (2005) Palmitate inhibits insulin gene expression by altering PDX-1 nuclear localization and reducing MafA expression in isolated rat islets of Langerhans. J Biol Chem 280: 32413–32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lupi R, Dotta F, Marselli L, Del Guerra S, Masini M, et al. (2002) Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51: 1437–1442. [DOI] [PubMed] [Google Scholar]
- 34. El-Assaad W, Buteau J, Peyot ML, Nolan C, Roduit R, et al. (2003) Saturated fatty acids synergize with elevated glucose to cause pancreatic beta-cell death. Endocrinology 144: 4154–4163. [DOI] [PubMed] [Google Scholar]
- 35. Fonseca SG, Burcin M, Gromada J, Urano F (2009) Endoplasmic reticulum stress in beta-cells and development of diabetes. Curr Opin Pharmacol 9: 763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26: 504–510. [DOI] [PubMed] [Google Scholar]
- 37. Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, et al. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12: 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi Y, Wei Y, Qu S, Wang Y, Li Y, et al. (2010) Arsenic induces apoptosis of human umbilical vein endothelial cells through mitochondrial pathways. Cardiovasc Toxicol 10: 153–160. [DOI] [PubMed] [Google Scholar]
- 40. Bevers MB, Neumar RW (2008) Mechanistic role of calpains in postischemic neurodegeneration. J Cereb Blood Flow Metab 28: 655–673. [DOI] [PubMed] [Google Scholar]
- 41. Goll DE, Thompson VF, Li H, Wei W, Cong J (2003) The calpain system. Physiol Rev 83: 731–801. [DOI] [PubMed] [Google Scholar]
- 42. Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG (2011) The calpain system and cancer. Nat Rev Cancer 11: 364–374. [DOI] [PubMed] [Google Scholar]
- 43. Sorimachi H, Suzuki K (2001) The structure of calpain. J Biochem 129: 653–664. [DOI] [PubMed] [Google Scholar]
- 44. Ma J, Cui W, He SM, Duan YH, Heng LJ, et al. (2012) Human U87 astrocytoma cell invasion induced by interaction of βig-h3 with integrin α5β1 involves calpain-2. Plos One 7: e37297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu LJ, Deng XM (2006) Suppression of cancer cell migration and invasion by protein phosphatase 2A through dephosphorylation of μ- and m-Calpains. J Biol Chem 281: 35567–35575. [DOI] [PubMed] [Google Scholar]
- 46. Gotoh T, Terada K, Oyadomari S, Mori M (2004) Hsp70-DnaJ chaperone pair prevent snitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ 11: 390–402. [DOI] [PubMed] [Google Scholar]
- 47. Ubeda M, Habener JF (2000) CHOP gene expression in response to endoplasmic-reticular stress requires NFY interaction with different domains of a conserved DNA-binding element. Nucleic Acids Res 28: 4987–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michiel HM, Henriet M, Martin H, Bernd HJ, Arie BV, et al. (2004) Induction of CCAAT/enhancer-binding protein (C/EBP)-homologous protein/growth arrest and DNA damage-inducible protein 153 expression during inhibition of phosphatidylcholine synthesis is mediated via activation of a C/EBP-activating transcription factor-responsive element. J Biol Chem 279: 52007–52015. [DOI] [PubMed] [Google Scholar]
- 49. Rao RV, Ellerby H, Bredesen DE (2004) Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11: 372–380. [DOI] [PubMed] [Google Scholar]
- 50. Gromada J, Dissing S, Kofod H, Frøkjaer-Jensen J (1995) Effects of the hypoglycaemic drugs repaglinide and glibenclamide on ATP-sensitive potassium-channels and cytosolic calcium levels in beta TC3 cells and rat pancreatic beta cells. Diabetologia 38: 1025–1032. [DOI] [PubMed] [Google Scholar]
- 51. Wang XL, Zhang L, Youker K, Zhang MX, Wang J, et al. (2006) Free fatty acids inhibit insulin signaling-stimulated endothelial nitric oxide synthase activation through upregulating PTEN or inhibiting Akt kinase. Diabetes 55: 2301–2310. [DOI] [PubMed] [Google Scholar]