Abstract
Background
Fearful experiences can produce long-lasting and debilitating memories. Extinction of conditioned fear requires consolidation of new memories that compete with fearful associations. In human subjects, as well as rats, posttraining stimulation of the vagus nerve enhances memory consolidation. Subjects with posttraumatic stress disorder (PTSD) show impaired extinction of conditioned fear. The objective of this study was to determine whether vagus nerve stimulation (VNS) can enhance the consolidation of extinction of conditioned fear.
Methods
Male Sprague-Dawley rats were trained on an auditory fear conditioning task followed by 1–10 days of extinction training. Treatment with vagus nerve or sham stimulation was administered concurrently with exposure to the fear conditioned stimulus. Another group was given VNS and extinction training but the VNS was not paired with exposure to conditioned cues. Retention of fear conditioning was tested 24 hours after each treatment.
Results
VNS paired with exposure to conditioned cues enhanced the extinction of conditioned fear. After a single extinction trial, rats given VNS stimulation demonstrated a significantly lower level of freezing, compared to that of sham controls. When extinction trials were extended to 10 days, paired VNS accelerated extinction of the conditioned response.
Conclusions
Extinction paired with VNS is more rapid than extinction paired with sham stimulation. As it is currently approved by the Federal Food and Drug Administration for depression and seizure prevention, VNS is a readily-available and promising adjunct to exposure therapy for the treatment of severe anxiety disorders.
Keywords: Anxiety, PTSD, stress, noradrenaline, norepinephrine, exposure therapy
Introduction
Conditioned fear develops when a neutral cue is associated with an aversive stimulus or event. This association leads to heightened fear and anxiety in the presence of the formerly neutral cue. The process of replacing fearful associations with neutral associations is called extinction. Extinction learning does not erase initial learning, but the new learning competes with the old, eventually leading to a new situation-appropriate response (1). Development of posttraumatic stress disorder (PTSD) occurs in approximately 28–50% of trauma-exposed individuals (2). PTSD sufferers show an impaired ability to extinguish conditioned fear in controlled laboratory studies (3) and PTSD is characterized by fear that is resistant to extinction (4). This evidence suggests that the inability to extinguish conditioned fear enables the strengthening of traumatic memories over time, contributing to the development of PTSD. A method to facilitate extinction would therefore be beneficial in the treatment of PTSD.
The present set of experiments tests the hypothesis that extinction can be enhanced with concurrent exposure to conditioned cues and stimulation of the vagus nerve. Electrical stimulation of the vagus nerve (VNS) has been approved by the US Food and Drug Administration for prevention of seizures since 1997. The role of the vagus nerve in the stress/arousal pathway and the temporal specificity of electrical stimulation allow VNS to facilitate memory consolidation and synaptic plasticity. Posttraining administration of the stress hormone epinephrine enhances memory and produces vagal responses that lead to elevated norepinephrine levels in the brain (5–8). Like systemic administration of epinephrine, VNS immediately after training enhances memory consolidation in rats and in humans (9, 10). VNS coupled with precise timing of a tone promotes specific frequency map plasticity in primary auditory cortex (11) and VNS paired with movement produces an expansion of the region of primary motor cortex that generates the movement (12). Thus, delivery of VNS that is coupled with a sensory or motor event is capable of enhancing memory consolidation and generating specific plasticity in various brain regions, raising the possibility that VNS can be used to artificially modulate neural plasticity underlying memory.
A primary tool in the treatment of stress disorders such as PTSD, phobia, obsessive compulsive disorder, and addiction is exposure therapy, a treatment that uses exposure to desensitize patients to the cues that cause anxiety or compulsive behaviors. Prolonged exposure therapy is currently considered the most effective treatment for PTSD (13). Some of the most encouraging results of this research indicate that cognitive enhancers can facilitate extinction of conditioned fear and the effectiveness of exposure-based psychotherapy (14–16). To reduce the risk of reinforcing the aversion to fear-conditioned cues, optimal adjunct therapy should possess a rare combination of memory-enhancing and anxiety-reducing qualities. Unfortunately, most anxiolytic drugs that are currently used to treat the symptoms of anxiety disorders (benzodiazepines and β-adrenoceptor antagonists) also impair memory consolidation (17, 18), potentially interfering with progress in exposure therapy. As an adjunct to exposure therapy, VNS holds promise because it produces both memory enhancing and anxiolytic effects (19, 20).
Inspired by initial observations of mood improvement in patients given VNS to prevent epileptic seizures, studies investigated the effect of VNS on depression and anxiety. In an open-label clinical trial, George and colleagues (19) reported lasting improvements in patients with treatment-resistant anxiety disorders when VNS was administered as prescribed for epilepsy. Similarly, repeated VNS produces anxiolytic-like effects in rats (20). This evidence indicates that VNS has anxiety-reducing effects in addition to its effects on brain plasticity and memory. The present set of experiments examines the potential for VNS, paired with therapy, to enhance the consolidation of extinction of conditioned fear. To determine whether VNS facilitates the extinction process, VNS was administered concurrently with extinction training in fear conditioned rats.
Methods and Materials
Animals
Male Sprague-Dawley rats (Charles River) weighing 250–300g on arrival were housed in a standard animal care facility on a 12 hour light/dark cycle (lights on at 7:00 am) with access to food and water ad libitum. All procedures were approved by the Institutional Animal Care and Use Committee, University of Texas at Dallas.
Surgery
The VNS surgical procedure has been described in detail elsewhere (11). Briefly, platinum-iridium wire electrodes and biocompatible micro-renathane cuffs (0.04 in. i.d., 0.08 in. o.d., 4 mm long) were assembled in the laboratory at The University of Texas at Dallas. Isoflurane anesthesia (1% in O2 Western Medical Supply) was administered through inhalation and the vagus nerve was accessed at the cervical level through an incision in the skin along the ventral midline. The muscle layers were separated, exposing the vagus nerve and carotid artery and the electrode was wrapped around the nerve. Transient cessation of breathing was observed in anesthetized rats when VNS (0.2 mA, 60 Hz, 10 sec) was given through the implanted electrode, confirming that the cuff electrode was appropriately positioned to stimulate the vagus nerve. To ensure that the cuff electrode position was maintained throughout the experiment, transient cessation of breath was again observed upon completion of the study. Sham animals were subjected to the same surgical procedure however the circuit was designed to short at the level of the head implant. All rats were allowed to recover for 1 week after surgery. During the recovery period, rats were handled 5 min/day for 5 days in order to habituate them to being picked up by the experimenter.
Extinction of Auditory Fear Conditioning with Single Treatment
Auditory Fear Conditioning
All auditory fear conditioning and extinction trials were performed in two identical sound-attenuating boxes. The grid floor was cleaned with 20% ethanol before training each animal. To insure that rats were not inherently afraid of tone presentations, 5 tones were presented on the first day of conditioning (9 kHz, 85 dB SPL, 30 sec duration). Rats were then presented 8 tones (9 kHz, 85 dB SPL) overlapping with a 1 sec footshock (0.5 mA). To prevent the development of a specific temporal association with the footshock, a single 1-sec footshock was administered at a randomized time during each 30 sec tone presentation. To produce a robust conditioned fear, rats were again given 8 tones paired with footshock on a second conditioning day 24 hours later. The inter-stimulus-interval (ISI) varied between 3 and 5 min, averaging 4 min for every tone presentation (see Figure 1a for training and extinction timeline).
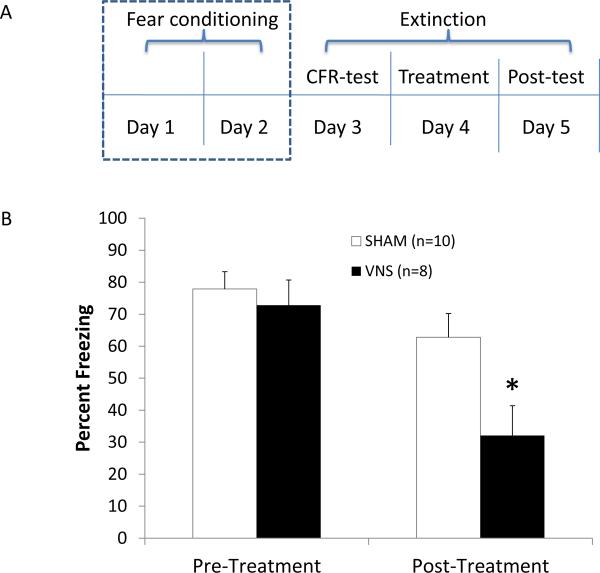
Figure 1.
VNS enhances extinction of auditory fear conditioning. (A) Auditory fear conditioning consisted of two days of training with 8 trials/day. On a Conditioned Fear Test, given 24 h later, rats were exposed to the conditioned tone 4 times in the absence of footshock and time spent freezing was assessed. On the following day (Treatment), the tone was played again 4 times and it overlapped with either sham or VNS stimulation. Freezing levels were measured again during the Post-Treatment Test trial, given 24 h later. (B) Percent of time spent freezing out of total time that rats were exposed to the tone did not differ across the two groups on the Conditioned Fear Test (left). After one day of extinction training with treatment, VNS rats spent significantly less time freezing than did sham controls (right; * p < .05).
Conditioned Fear Test
On Day 3, 1 day following the second day of fear-conditioning, 4 tones (9 khz) were presented, with an ISI of 3, 4, or 5 min (4 min average). The session was recorded by a digital camera and simultaneously viewed on a computer based outside of the behavior room and saved for later analysis. Freezing (percent time spent freezing of total duration of exposure to the tone) was used as a measure of the conditioned fear response (CFR). Freezing was defined as a period of complete immobility. Freezing posture consists of lowered head, spread paws, and rapid respiration. Time spent freezing was assessed by two independent observers who were blind to treatment conditions.
Extinction Treatment Paired with Vagus Nerve Stimulation
On Day 4, rats were again presented 4 tones in the absence of footshock (9 kHz, ISI = 3, 4, or 5 min). During this “treatment” trial, either sham stimulation (n =10), or VNS (n=8) overlapped with the conditioned 30-second tone. The 30 second-long vagus nerve (or sham) stimulation was delivered at an intensity of 0.4 mA, 500 μs pulse width at 30 Hz, and started 150 ms before the onset of the tone. These VNS parameters were selected because they were previously optimized for enhancing memory consolidation in rats and humans (10, 21).
Post-treatment Test
On Day 5, freezing was assessed as in the Conditioned Fear Test; VNS and sham stimulation were not administered and the level of freezing to the tone, in the absence of footshock, was measured. Post-treatment freezing was analyzed as percent of each individual rat's CFR.
Taking into account the two test days, where the conditioned cues were presented without footshock, all animals were essentially given the opportunity to extinguish conditioned fear over a total of 3 days. This design allowed for assessment of conditioned freezing without the potential confound of a performance effect of stimulation on freezing behavior.
Extinction of Auditory Fear Conditioning with Multiple Treatments
In a clinical setting, exposure therapy is given repeatedly until the conditioned fear is extinguished. To examine whether VNS accelerates the time to extinction of conditioned fear, VNS (n=9) and sham control rats (n=13) were conditioned to fear a 9 kHz tone followed by extinction training as described above. However alternating treatment and post-treatment tests were administered repeatedly for a total of 5 sham- or VNS-paired extinction treatments. Remission of the CFR was judged to be achieved when time spent freezing was < 10% of the CFR (no more than 12 sec).
Paired vs. Unpaired VNS
To test whether VNS alone could enhance extinction, an “unpaired VNS” group of rats (n=7) was given VNS after extinction training instead of overlapping VNS with exposure to the conditioned tone. On the treatment day, each rat in this group was removed from the conditioning boxes immediately after extinction training and VNS was administered 4 times in the home cage with an ISI of 3, 4, or 5 min.
Spontaneous Recovery of Conditioned Fear
To determine whether conditioned fear would recover spontaneously over time, 9 of the VNS rats and 4 of the sham rats were left alone and then given a follow-up retention test 2 weeks after completion of 5 treatment trials. Percent of CFR on the last retention test after extinction training was compared to the percent of CFR measured on the retention test 2 weeks later. A significant increase in this measure within a group would be taken as an indication of spontaneous recovery of fear.
Extinction of Remote Auditory Fear Memory with Multiple Treatments
Posttraumatic stress disorder and other anxiety disorders are generally not treated until long after the learning event that produces maladaptive responses. Extinction of remote memories may involve different neural pathways (22, 23) suggesting that the efficacy of a given treatment may depend upon the age of the conditioned fear (24). To test the effect of repeated extinction trials on more remote conditioned fear, rats were conditioned to fear a 9 kHz tone, as previously described. Following fear conditioning, rats were then left alone and housed individually for 2 weeks. After 2 weeks, rats were given a Conditioned Fear Test, followed by extinction treatment paired with either sham stimulation (n=10) or VNS (n = 4), and a Post-treatment Test.
Data Analysis
Single trial behavioral data were analyzed with an ANOVA subject (Treatment Group) and pairwise post-hoc comparisons determined with the Fisher's PLSD post hoc test. Multiple trial experiments were analyzed with a partially repeated ANOVA subject (Treatment Group) × Trial, and overall group differences where determined using Fisher's PLSD post hoc test.
Results
Vagus Nerve Stimulation Enhances Extinction Memory
Vagus nerve stimulation during a single paired-extinction treatment session significantly reduced the percent of CFR on the following day (Figure 1b). A statistically significant main effect was seen across auditory fear conditioning and extinction trials [F = 6.881, p < 0.05], and a post-hoc analysis revealed no significant group differences in CFR on the day before VNS or sham treatment [Fisher's PLSD, p > 0.05]. A significant reduction of percent of CFR was seen one day after extinction paired with VNS treatment [Fisher's PLSD, p < 0.05 vs. sham controls]. A comparison of percent of CFR in VNS- vs. sham-treated animals across trials on the day after treatment (Post-treatment Test) revealed a main effect of VNS [F = 6.881, p < 0.05] and a significant extinction effect of tone presentation on the freezing response during the test trial [F = 3.145, p < 0.05], however there were no significant interactions of the tone presentations and the groups [F = 1.304, p > 0.05], suggesting that the rate of extinction on the test day was the same in both groups and the observed group differences were due to treatment on the preceding day, when VNS or sham stimulation was administered. The main finding that rats given a single session of VNS paired with exposure to the conditioned cues exhibited half as much freezing as sham-treated animals indicates that VNS enhanced extinction of conditioned fear.
Vagus Nerve Stimulation Accelerates Extinction over Multiple Treatment Trials
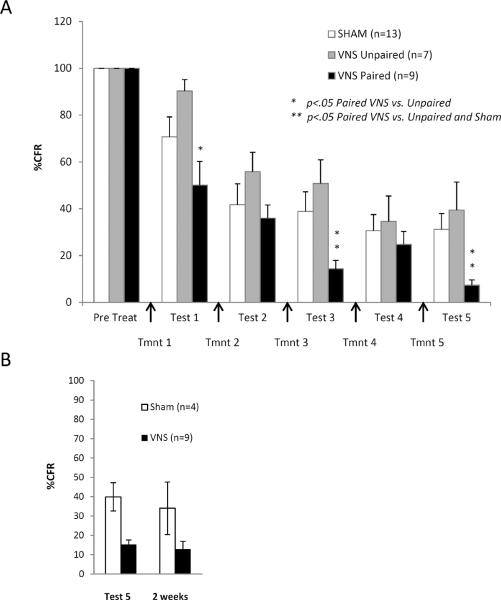
Repeated extinction and testing trials were used to determine whether VNS would remain effective over a treatment regime and if remission of the CFR could be achieved with repeated VNS-extinction pairing (Figure 2a). A significant main effect was seen between groups [F = 3.959, p < 0.05], as well as a significant extinction effect over all groups and trials [F = 76.215, p < 0.001] and a significant interaction between group and percent of CFR over trials [F = 1.98, p < 0.05]. According to the post-hoc analysis, percent of CFR was significantly lower for VNS-paired rats than either sham controls [Fisher's PLSD, p < 0.05] or animals given VNS in their home cage immediately after extinction treatment [Fisher's PLSD, p < 0.005]. Percent of CFR was significantly reduced in Paired vs. Unpaired VNS rats at Test 1 [Fisher's PLSD, p < 0.01], suggesting that pairing VNS with exposure is essential for the extinction-enhancing effect. Percent of CFR in Paired VNS rats was significantly lower than that in both unpaired and sham treated rats on Tests 3 and 5 [Fisher's PLSD, p < 0.05]. Only paired VNS animals reached the criterion for remission of the CFR at the end of 10 consecutive days of extinction (5 sham or VNS treatment trials) [Sham mean+SEM = 31.2% +− 6.9, Unpaired VNS mean+SEM = 39.4% +− 12, Paired VNS mean + SEM = 7.3% +−2.3], indicating that VNS accelerates the rate of extinction.
Figure 2.
Paired VNS accelerates extinction of conditioned fear. (A) Percent of conditioned freezing in rats given repeated extinction trials. Tests measuring freezing, and VNS or sham treatment were given on alternate days. Paired VNS overlapped with exposure to conditioned cues. Unpaired VNS was given in the home cage, immediately after extinction training. Because testing days involved exposure to the conditioned tone without footshock, extinction learning could develop over all 11 days. Paired VNS rats reached a point of remission of fear expression (< 10 % of CFR) whereas unpaired- and sham-treated rats did not (* p<.05 Paired vs. Unpaired; ** p<.05 Paired vs. Unpaired and Sham). (B) Spontaneous recovery of fear was examined in 4 Sham and 9 VNS rats 2 weeks after Test 5 (Day 11). After the passage of 2 weeks, freezing levels remained unchanged in both sham and VNS rats indicating that spontaneous recovery of fear did not occur in either group.
Nine paired VNS and 4 sham-stimulated rats were given another test 2 weeks after completion of 5 extinction and test trials (Figure 2b). A t-test comparing percent of CFR on Test Day 5 to that on the test 2 weeks later revealed no significant recovery of fear in either group [Sham = t(6) = 0.648, p > .05; VNS = t(16) = 1.365, p > .05]. VNS reduced freezing by 75% both immediately and 2 weeks after therapy, indicating that the VNS effect was long-lasting.
Vagus Nerve Stimulation Enhances Extinction of a Remote Auditory Fear Memory
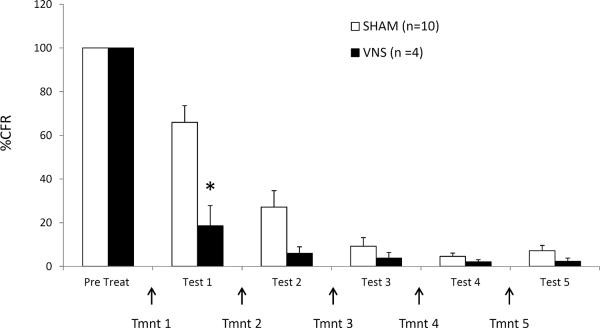
The effect of the paired-VNS treatment regime on the extinction of a relatively remote (2-week-old) fear memory was also investigated (Figure 3). Similar to the previous findings, a repeated measures ANOVA revealed a significant main effect [F=5.532, p < 0.05], a significant overall extinction effect of trials [F=26.857, p<0.0001] and a significant interaction between group and percent of CFR over trials [F=9.073, p <0.0001]. VNS significantly enhanced the extinction of a 2-week-old fear memory after a single treatment session [Fisher's PLSD, p < 0.05], indicating that the benefits of VNS persist even when the fear memory is remote.
Figure 3.
VNS enhances extinction of remote conditioned fear. Percent of conditioned freezing in sham and VNS-treated rats given delayed extinction training. Extinction trials began 2 weeks after fear conditioning. VNS or sham stimulation overlapped with the conditioned tone on treatment trials. Test trials were given on alternate days. Although both groups demonstrated remission of fear responding, VNS-treated rats reached the remission point more rapidly than sham-treated rats, indicating that remote memory is not resistant to VNS enhancement of extinction (p<.05).
Discussion
The present findings demonstrate that VNS administered during exposure to conditioned cues both enhances and accelerates extinction of conditioned fear. The significant reduction in freezing is not likely to be the result of a performance effect of VNS that is carried over to testing 24 hours later because the group given unpaired VNS and extinction showed no enhancement of extinction of conditioned fear. Neither group demonstrated spontaneous increases in fear responding 2 weeks after cessation of fear conditioning, possibly because spaced extinction training contributed to the inhibition of spontaneous return of fear (25). It remains unknown whether VNS-enhanced extinction would be susceptible to the return of fear that is frequently observed in control animals.
Although extinction of conditioned fear is typically examined within 24 hours of fear conditioning in animals (14, 26, 27), anxiety disorders stem from experiences that occur months or years before treatment is sought. It is difficult to compare the passage of 2 weeks in a rat to that of months or years in humans, especially when considering lifespan differences, but studies in rats demonstrate that 2 week-old memories and recent memories of aversive events are supported by different brain regions, and the older memories can be less sensitive to disruption (22–24, 28). The present findings demonstrate that VNS paired with the conditioned cue enhances the extinction of a relatively remote memory. When extinction training was delayed for 2 weeks after initial fear conditioning, paired VNS rats demonstrated significantly less conditioned fear than did sham controls after a single day of extinction training. Sham rats eventually demonstrated extinction of remote conditioned fear, but paired VNS rats demonstrated remission of the conditioned fear response earlier. These results indicate that VNS is no less effective when the conditioned fear is based on a remote memory.
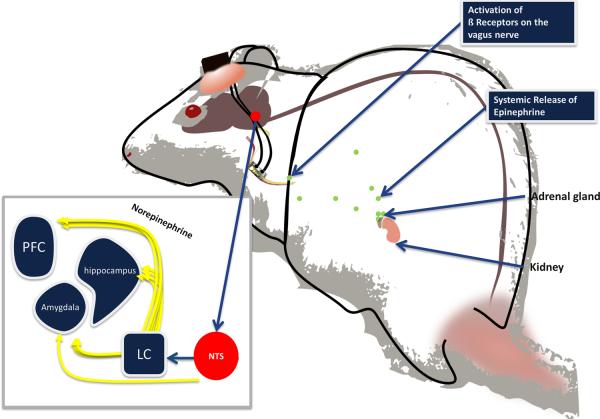
The position of the vagus nerve in the arousal pathway and the temporal specificity of electrical stimulation allow VNS to facilitate memory consolidation and plasticity (5–8). Extinction of conditioned fear requires the consolidation of newly learned associations. The role of afferent fibers of the vagus nerve in stimulating the central nervous system was proposed by Izquierdo and colleagues over 50 years ago (29). Since then, mounting evidence indicates that emotional arousal enhances memory consolidation through a pathway involving vagal afferents (5, 30, 31) which project to brain stem nuclei that regulate the release of norepinephrine throughout the forebrain (7, 32–35). Extensive evidence indicates that arousal-induced memory enhancement is mediated by noradrenergic activation of the amygdala. Infusions of the β-adrenoceptor antagonist propranolol into the amygdala block the memory-enhancing effect of posttraining, systemic administration of epinephrine in rats (36). Elevation of norepinephrine levels are seen in the amygdala following exposure to an aversive stimulus, administration of epinephrine, or VNS (7, 37–39). This evidence suggests that memory consolidation can be modulated by adrenergic actions on the vagus nerve which, through projections to the nucleus of the solitary tract and locus coeruleus, stimulates noradrenergic activation of β-adrenoceptors in the amygdala (7, 32–35) (Figure 4). Artificial stimulation of the vagus nerve takes advantage of this arousal-related pathway to enhance the consolidation of extinction memory.
Figure 4.
Model of emotional arousal effects on peripheral and central nervous systems in the rat. Under stressful conditions, epinephrine (green dots) is released into the bloodstream by the adrenal medulla. It does not cross the blood-brain barrier but binds to β-adrenoceptors located on the vagus nerve (pictured here with stimulating electrode cuff connected to a head implant). Stimulation of the vagus nerve promotes release of norepinephrine in the cortex, hippocampus, and amygdala either by direct projections to the nucleus of the solitary tract (NTS) or indirect connections with the locus coerulueus (LC). Artificial stimulation of the vagus nerve is administered through a connector that is surgically attached to the skull.
The temporal specificity of VNS was demonstrated in an earlier study in which VNS paired with a tone resulted in tone-specific plasticity in primary auditory cortex (11, 12). VNS-induced plasticity is a likely substrate of VNS enhancement of memory consolidation. This plasticity could occur in a circuit involving the infralimbic (IL) cortex and amygdala. Electrical stimulation of the IL results in a suppression of activity in the central nucleus of the amygdala, and decreased activation of the central amygdala reduces fear responding (40). Like stimulation of the vagus nerve, electrical stimulation of the IL facilitates consolidation of extinction when precisely paired with presentation of a conditioned tone; unpaired stimulation of the IL has no effect on extinction (41). VNS increases norepinephrine in the prefrontal cortex and amygdala in rats (7, 8) and posttraining administration of norepinephrine into the basolateral complex of the amygdala enhances extinction of conditioned fear of a context (42). Furthermore, activation of the prefrontal cortex during exposure therapy is correlated with positive patient outcomes (4, 43, 44). These findings suggest that VNS may enhance memory through noradrenergic modulation of plasticity in the extinction-related pathway involving the IL and amygdala.
An interesting alternative or additional factor in the observed extinction enhancement is the potential for VNS to reduce anxiety. In addition to modulating brain plasticity and memory through actions of vagal afferents, activation of efferent fibers of the vagus nerve inhibits sympathetic nervous system actions on heart rate and hypothalamic-pituitary-adrenal (HPA) activation (45, 46). Sometimes called the “vagal brake”, the vagus nerve drives the parasympathetic nervous system response that returns the body to a “rest and digest” state following exposure to a stressor. It is conceivable that this parasympathetic brake on the sympathetic response would reduce anxiety. Both human and rat studies demonstrate reduced anxiety following chronic VNS (19, 20). Although an acute effect of VNS on anxiety has not been demonstrated to our knowledge, learning to associate fear-conditioned cues with safety could have come more easily if rats were less anxious during exposure to those cues. It is possible that the benefit of reduced anxiety is sufficient to enhance extinction independently of direct effects of VNS on synaptic consolidation.
The present results may assist in the identification of an adjunct treatment for anxiety disorders as well as an avenue for investigation of the mechanisms of such disorders. Vagal tone is a term used to describe the inhibitory influence of the vagus nerve on heart rate. It is generally estimated by measurement of heart rate variability. A respiratory sinus arrhythmia (RSA) occurs naturally between respiratory phases, and is considered an indicator of vagal tone. Although heart rate variability is not necessarily an indicator of vagal activity at effectors other than the heart (47), it is interesting to note that vagal tone is consistently correlated with capacity to regulate stress responses and reduced vagal tone is associated with anxiety disorders, including PTSD (48–52). Taken together with evidence that posttraining VNS enhances consolidation of long-term memories in rats and humans (9, 10, 53), these correlative findings suggest that an adaptive response to conditioned fear, experienced in a safe environment, may involve a vagus nerve influence on the consolidation of extinction memories. Although the initial fear association is stored as a strong memory, subsequent vagal tone might identify those who are at risk of developing a trauma-related memory disorder.
VNS is approved by the federal Food and Drug Administration for the prevention of seizures and treatment of depression, making it a readily available adjunct to exposure therapy. Such paired therapy would target the cause, rather than the symptoms of anxiety disorders. Because exposure therapy is typically carried out across multiple sessions, the present findings provide promise for an adjunct treatment that may enhance the efficacy of exposure therapy and reduce the number of exposure therapy sessions required to treat anxiety disorders.
Acknowledgements
The authors thank Dr. Aage Møller for helpful feedback on a previous draft of this manuscript. We also thank Dr. Michael Kilgard, Dr. Reema Casavant, and Geoffrey White for their intellectual contributions to the research. Sherien George and Mohamed Mahmoud are acknowledged for their excellent technical support. This research was supported by the National Institute of Mental Health MH 086960-01A1 (NDE and CKM), and by the University-affiliated biotechnology company, Microtransponder Inc.
Financial Disclosures The authors received research and salary support from Microtransponder Inc. to collect the preliminary data for this project. Dr. Engineer and Dr. McIntyre are authors of a provisional patent entitled “Enhancing fear extinction using vagus nerve stimulation”.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biol Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- 2.Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: a state-of-the-science review. J Psychiatr Res. 2006;40:1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyashita T, Williams CL. Epinephrine administration increases neural impulses propagated along the vagus nerve: Role of peripheral beta-adrenergic receptors. Neurobiol Learn Mem. 2006;85:116–124. doi: 10.1016/j.nlm.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Miyashita T, Williams CL. Peripheral arousal-related hormones modulate norepinephrine release in the hippocampus via influences on brainstem nuclei. Behav Brain Res. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Hassert DL, Miyashita T, Williams CL. The effects of peripheral vagal nerve stimulation at a memory-modulating intensity on norepinephrine output in the basolateral amygdala. Behav Neurosci. 2004;118:79–88. doi: 10.1037/0735-7044.118.1.79. [DOI] [PubMed] [Google Scholar]
- 8.Follesa P, Biggio F, Gorini G, Caria S, Talani G, Dazzi L, et al. Vagus nerve stimulation increases norepinephrine concentration and the gene expression of BDNF and bFGF in the rat brain. Brain Res. 2007;1179:28–34. doi: 10.1016/j.brainres.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 9.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following vagus nerve stimulation in human subjects. Nat Neurosci. 1999;2:94–98. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 10.Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Posttraining electrical stimulation of vagal afferents with concomitant vagal efferent inactivation enhances memory storage processes in the rat. Neurobiol Learn Mem. 1998;70:364–373. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- 11.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly Pairing Vagus Nerve Stimulation with a Movement Reorganizes Primary Motor Cortex. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 13.Powers MB, Halpern JM, Ferenschak MP, Gillihan SJ, Foa EB. A meta-analytic review of prolonged exposure for posttraumatic stress disorder. Clin Psychol Rev. 2010;30:635–641. doi: 10.1016/j.cpr.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis M, Barad M, Otto M, Southwick S. Combining pharmacotherapy with cognitive behavioral therapy: traditional and new approaches. J Trauma Stress. 2006;19:571–581. doi: 10.1002/jts.20149. [DOI] [PubMed] [Google Scholar]
- 16.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 17.Frumin MJ, Herekar VR, Jarvik ME. Amnesic actions of diazepam and scopolamine in man. Anesthesiology. 1976;45:406–412. doi: 10.1097/00000542-197610000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Angus WR, Romney DM. The effect of diazepam on patients' memory. J Clin Psychopharmacol. 1984;4:203–206. [PubMed] [Google Scholar]
- 19.George MS, Ward HE, Ninan PT, Pollack M, Nahas Z, Anderson B, et al. A pilot study of vagus nerve stimulation (VNS) for treatment-resistant anxiety disorders. Brain Stimul. 2008;1:112–121. doi: 10.1016/j.brs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Furmaga H, Shah A, Frazer A. Serotonergic and Noradrenergic Pathways Are Required for the Anxiolytic-like and Antidepressant-like Behavioral Effects of Repeated Vagal Nerve Stimulation in Rats. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 21.Clark KB, Krahl SE, Smith DC, Jensen RA. Post-training unilateral vagal stimulation enhances retention performance in the rat. Neurobiol Learn Mem. 1995;63:213–216. doi: 10.1006/nlme.1995.1024. [DOI] [PubMed] [Google Scholar]
- 22.Izquierdo I, Quillfeldt JA, Zanatta MS, Quevedo J, Schaeffer E, Schmitz PK, et al. Sequential role of hippocampus and amygdala, entorhinal cortex and parietal cortex in formation and retrieval of memory for inhibitory avoidance in rats. Eur J Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 23.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 24.Milekic MH, Alberini CM. Temporally graded requirement for protein synthesis following memory reactivation. Neuron. 2002;36:521–525. doi: 10.1016/s0896-6273(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 25.Urcelay GP, Wheeler DS, Miller RR. Spacing extinction trials alleviates renewal and spontaneous recovery. Learn Behav. 2009;37:60–73. doi: 10.3758/LB.37.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learn Mem. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 29.IZQUIERDO JA, INSUA JA, BISCARDI AM, IZQUIERDO IA. Some observations on the responses to the afferent stimulation of the vagus. Med Exp Int J Exp Med. 1959;1:325–332. doi: 10.1159/000134813. [DOI] [PubMed] [Google Scholar]
- 30.Schreurs J, Seelig T, Schulman H. Beta 2-adrenergic receptors on peripheral nerves. J Neurochem. 1986;46:294–296. doi: 10.1111/j.1471-4159.1986.tb12961.x. [DOI] [PubMed] [Google Scholar]
- 31.Lawrence AJ, Watkins D, Jarrott B. Visualization of beta-adrenoceptor binding sites on human inferior vagal ganglia and their axonal transport along the rat vagus nerve. J Hypertens. 1995;13:631–635. doi: 10.1097/00004872-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Williams CL, McGaugh JL. Reversible inactivation of the nucleus of the solitary tract impairs retention performance in an inhibitory avoidance task. Behav Neural Biol. 1992;58:204–210. doi: 10.1016/0163-1047(92)90482-j. [DOI] [PubMed] [Google Scholar]
- 33.Kalia M, Sullivan JM. Brainstem projections of sensory and motor components of the vagus nerve in the rat. J Comp Neurol. 1982;211:248–265. doi: 10.1002/cne.902110304. [DOI] [PubMed] [Google Scholar]
- 34.Sumal KK, Blessing WW, Joh TH, Reis DJ, Pickel VM. Synaptic interaction of vagal afferents and catecholaminergic neurons in the rat nucleus tractus solitarius. Brain Res. 1983;277:31–40. doi: 10.1016/0006-8993(83)90904-6. [DOI] [PubMed] [Google Scholar]
- 35.Van Bockstaele EJ, Peoples J, Telegan P. Efferent projections of the nucleus of the solitary tract to peri-locus coeruleus dendrites in rat brain: evidence for a monosynaptic pathway. J Comp Neurol. 1999;412:410–428. doi: 10.1002/(sici)1096-9861(19990927)412:3<410::aid-cne3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 36.Liang KC, Juler RG, McGaugh JL. Modulating effects of posttraining epinephrine on memory: involvement of the amygdala noradrenergic system. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- 37.McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 38.Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- 39.Williams CL, Men D, Clayton EC, Gold PE. Norepinephrine release in the amygdala after systemic injection of epinephrine or escapable footshock: contribution of the nucleus of the solitary tract. Behav Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- 40.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 42.Berlau DJ, McGaugh JL. Enhancement of extinction memory consolidation: the role of the noradrenergic and GABAergic systems within the basolateral amygdala. Neurobiol Learn Mem. 2006;86:123–132. doi: 10.1016/j.nlm.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–410. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. doi: 10.1093/cercor/bhq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Keane V, Dinan TG, Scott L, Corcoran C. Changes in hypothalamic-pituitary-adrenal axis measures after vagus nerve stimulation therapy in chronic depression. Biol Psychiatry. 2005;58:963–968. doi: 10.1016/j.biopsych.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 46.Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleve Clin J Med. 2009;76(Suppl 2):S86–90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jennings JR, McKnight JD. Inferring vagal tone from heart rate variability. Psychosom Med. 1994;56:194–196. doi: 10.1097/00006842-199405000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Sahar T, Shalev AY, Porges SW. Vagal modulation of responses to mental challenge in posttraumatic stress disorder. Biol Psychiatry. 2001;49:637–643. doi: 10.1016/s0006-3223(00)01045-3. [DOI] [PubMed] [Google Scholar]
- 49.Sack M, Hopper JW, Lamprecht F. Low respiratory sinus arrhythmia and prolonged psychophysiological arousal in posttraumatic stress disorder: heart rate dynamics and individual differences in arousal regulation. Biol Psychiatry. 2004;55:284–290. doi: 10.1016/s0006-3223(03)00677-2. [DOI] [PubMed] [Google Scholar]
- 50.Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Waters AM, Henry J, Neumann DL. Aversive Pavlovian conditioning in childhood anxiety disorders: impaired response inhibition and resistance to extinction. J Abnorm Psychol. 2009;118:311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]
- 52.Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. Monogr Soc Res Child Dev. 1994;59:167–186. [PubMed] [Google Scholar]
- 53.Chen CC, Williams CL. Interactions between epinephrine, ascending vagal fibers, and central noradrenergic systems in modulating memory for emotionally arousing events. Front Behav Neurosci. 2012;6:35. doi: 10.3389/fnbeh.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]