Abstract
Background
Little is known about the specificity of the interaction of serotonin transporter 5-HTTLPR genotype x trauma exposure in relation to contemporary structural models of PTSD symptomatology, which suggest that 4- or 5-factor models provide a better representation of this phenotypic expression of this disorder.
Methods
One hundred forty-nine respondents of a representative sample of adults affected by Hurricane Ike were interviewed 2 to 5 months after this 2008 disaster.
Results
After adjustment for age, sex, and ancestral proportion scores, the interaction of 5-HTTPLR genotype x trauma exposure was significantly associated with both severity (β=.40, p<.001) and probable diagnosis (Wald=4.55, p=.033; odds ratio=3.81, 95%CI=1.11–13.03) of Ike-related PTSD. Respondents with the low-expression variant of the 5-HTTPLR polymorphism (S allele) who were highly exposed to Hurricane Ike reported significantly greater severity of PTSD symptoms and were more likely to screen positive for PTSD than respondents homozygous for the L allele who were highly exposed to Hurricane Ike. Confirmatory factor analyses revealed that a 5-factor model of intercorrelated re-experiencing, avoidance, numbing, dysphoric arousal, and anxious arousal symptoms provided the best structural representation of PTSD symptomatology. The 5-HTTPLR genotype x exposure interaction was significant only for anxious arousal (β=.44, p<.001) and re-experiencing (β=.35, p<.001) symptoms, but not avoidance, numbing, or dysphoric arousal symptoms (all β’s≤.20, all p’s>.13).
Limitations
The small sample size and employment of self-report measures may limit generalizability of these findings.
Conclusions
Results of this pilot study suggest that the low-expression variant of the 5-HTTLPR polymorphism modifies risk for PTSD, but that this effect may be specific to anxious arousal and re-experiencing symptoms.
Keywords: Serotonin transporter gene, Posttraumatic stress disorder, Trauma, Disaster
1 Introduction
Posttraumatic stress disorder (PTSD) is one of the most prevalent and disabling psychiatric disorders associated with exposure to disasters (Galea et al., 2005; Norris et al., 2002a; Norris et al., 2002b). The 5-HTTPLR variant mapped to the promoter region of the serotonin transporter (5-HTT) gene SLC6A4 has received considerable attention as a possible genetic risk factor for PTSD, with several studies demonstrating that the genotypes at this locus that are associated with lower gene expression (i.e., those containing one or more copies of the S allele) may moderate the relation between severity of trauma and stressful life event exposures and risk for PTSD (e.g., Kilpatrick et al., 2007; Koenen et al., 2009; Xie et al., 2009; Xie et al., 2012); however, some studies have found that the high expression variant of this genotype may moderate this association (e.g., Grabe et al., 2009; Thakur et al., 2009).
One notable gap in the literature on the role of the 5-HTTLPR genotype in moderating the relation between trauma exposure and risk for PTSD is that little is known about the specificity of the 5-HTTLPR x trauma exposure interaction in relation to the clinical phenomenology of PTSD. PTSD is a heterogeneous disorder characterized by symptoms of re-experiencing, avoidance/numbing, and hyperarousal symptoms. Recent confirmatory factor analytic (CFA) studies have consistently demonstrated that more refined 4- or 5-factor models provide a significantly better representation of the structure of PTSD symptoms than the DSM-IV model (Elhai et al., 2011; Elhai and Palmieri, 2011; Yufik and Simms, 2010). These models include the 4-factor dysphoria model, which is comprised of separate clusters of re-experiencing, avoidance, dysphoria, and hyperarousal symptoms; and the 4-factor emotional numbing model, which is comprised of re-experiencing, avoidance, numbing, and hyperarousal symptoms. Given that the only difference between these two 4-factor models is the assignment of three symptoms (i.e., sleep disturbance, anger/irritability, and concentration difficulties), Elhai and colleagues (2011) recently evaluated and found support for a novel, 5-factor model that separates the DSM-IV hyperarousal symptom clusters into dysphoric arousal (i.e., sleep difficulties, anger/irritability, and concentration problems) and anxious arousal (i.e., hyperviligance, exaggerated startle response). This separation of the hyperarousal cluster is based on a theoretical model proposed by Watson (2005), which describes arousal symptoms characterized by restlessness and agitation (e.g., irritability) as distinct from those characterized by fear-based, panic-like symptoms (e.g., exaggerated startle response).
A growing number of CFA studies has since demonstrated that this 5-factor model provides a significantly better representation of PTSD symptom dimensions than the DSM-IV or 4-factor models in a broad range of trauma-exposed samples (Armour et al., 2012; Elhai et al., 2011; Pietrzak et al., 2012a; Pietrzak et al., 2012b; Wang et al., 2011a; Wang et al., 2011b; Wang et al., 2011c; Wang et al., 2012a; Wang et al., 2012b). However, no study of which we are aware has evaluated whether the 5-HTTLPR x trauma exposure interaction may be differentially related to these dimensions of PTSD symptomatology. Several neuroimaging studies have found that the 5-HTTLPR S allele is associated with amygdala hyperreactivity (Hariri et al., 2002) and dysregulation of amygdala-cingulate circuitry (Pezawas et al., 2005). Thus, it is reasonable to expect that individuals with the low expression variant of the 5-HTTLPR polymorphism who are highly exposed to trauma may experience greater severity of PTSD symptoms characterized by hyperreactivity and/or emotion dysregulation, such as anxious arousal. Further, in light of neuropsychological data suggesting that the low expression variant of the 5-HTTLPR polymorphism is associated with an attentional bias toward negative stimuli (Beevers et al., 2009; Pergamin-Hight et al., 2012), it is also reasonable to expect that these individuals may experience greater severity of re-experiencing symptoms.
The purpose of this pilot study was to evaluate whether the 5-HTTLPR x trauma exposure interaction is differentially related to the expression of CFA-derived PTSD symptom dimensions in a sample of individuals who were recently exposed to a large-magnitude natural disaster. Based on prior research (Kilpatrick et al., 2007; Koenen et al., 2009; Xie et al., 2009; Xie et al., 2012), we hypothesized that low expression variant of the 5-HTTPLR genotype would moderate the association between disaster exposure and PTSD such that individuals with one or two copies of the S allele would have greater severity of and likelihood of developing PTSD than individuals homozygous for the L allele. We further expected that this interaction would be differentially associated with PTSD symptoms characterized by hyperreactivity and negative attentional bias, such as anxious arousal and re-experiencing symptoms.
2 Methods and materials
2.1 Sample
Adults aged 18 or older who had been living in Galveston County or Chambers County, Texas, for at least one month before September 13, 2008, when Hurricane Ike made landfall, participated in this study. Details regarding sampling and recruitment procedures are available elsewhere (Norris et al., 2010). Briefly, a disproportionate stratified cluster sampling was employed to acquire samples in areas that experienced more damage from Hurricane Ike and that were more likely to be exposed to hurricane-related traumas. Interviews were conducted by experienced interviewers at the University of Michigan Institute for Social Research using a computer-assisted interview system. Within one week of completing an interview, each respondent was mailed a packet that included an invitation to participate in an additional component of the study; this packet included consent documents, a brief questionnaire, and a saliva collection kit that was labeled with an anonymous ID unique to the respondent. Standard protocols were employed to obtain saliva samples (e.g., Kilpatrick et al., 2007). Of the 658 individuals who completed an interview, 163 (24.8%) returned a saliva sample. Compared to respondents who did not return a sample, respondents who did return a sample were more likely to be older, White/non-Hispanic, and more highly educated; they did not differ with respect to sex, marital status, or household income. For the current study, complete data were available for 149 respondents; missing data were due to no consent card being included with the sample (n=9); and/or leaking of or too little sample returned (n=5). This study was approved by institutional review boards of each of the participating academic institutions.
2.2 Genotyping
DNA was extracted from saliva OriGene kits (DNA Genotek). The functional polymorphism in the 5’ flanking regulatory/promoter region of the gene (SLC6A4) coding for the serotonin transporter protein was studied. This polymorphism (5-HTTLPR) has two common alleles: long (16 repeats) and short (14 repeats); other alleles have also been identified (Gelernter et al., 1997). Genotyping was performed with polymerase chain reaction followed by size fractionation (Gelernter et al., 1997) with prior Mspl restriction endonuclease digestion for triallelic classification (Stein et al., 2006), which allowed classification of long alleles into LA and LG. Thirty four additional short tandem repeat markers were genotyped to provide ancestry information (Yang et al., 2005).
Genotype frequencies for the 5-HTTLPR polymorphism, which were classified triallelically, were reclassified based on their transcriptional efficiency: LA/LA were classified as L’/L’. LA/S and LA/LG were classified as L’/S’. LG/LG, LG/S, and S/S were classified as S’/S’. Based on this classification, 30 (20.5%) of the full sample had the L’/L’ genotype, 68 (46.6%) had the L’/S’ genotype, and 48 (32.9%) had the S’/S’ genotype; three subjects had an extra long allele and were excluded from this study. L’/L’, L’/S’, and S’/S’ genotype frequencies did not differ from the Hardy-Weinberg equilibrium, χ2=.43, p=.51. Among European Americans, these frequencies were 22.1%, 45.2%, and 32.7%; among Non-European Americans, these frequencies were 20.0%, 54.3%, and 25.7%. In both groups, these frequencies did not differ from the Hardy Weinberg equilibrium, χ2=.77, p=.38 and χ2=.27, p=.60, respectively. Genotype frequencies did not differ by race in this small sample, χ2=.92, p=.63. We also genotyped an STR panel of ancestry informative markers and computed ancestry proportion scores by means of STRUCTURE (Pritchard and Rosenberg, 1999). Ancestral proportions did not differ between respondents with and without PTSD (t=1.66, p=.12).
2.3 Hurricane Ike exposure
Respondents were asked about their experiences during and after Hurricane Ike. These experiences included: (1) threat to safety of self or family/friends; (2) injury or health problem to self or household member; (3) family member or close friend injured or killed; (4) encounter with dead bodies during or after Hurricane Ike; (5) damage to three or more types of property (e.g. residence, furnishings, cars/vehicles); (6) financial loss (i.e. lost income as a result of Hurricane Ike); (7) displacement from home≥ten days; (8) lack of two or more necessities for≥one week (e.g. shelter, electricity, food/water, transportation); and (9) high level of area disruption or damage (sum of ratings of damage and disruption to area schools, churches, streets/highways in the top third for the full sample). These experiences were summed to yield a summary hurricane exposure measure. Due to the non-normal distribution of scores on this measure (Shapiro-Wilk test=.92, p<.001), a median split procedure was employed to dichotomize scores into “Low Exposure” and “High Exposure” categories.
2.4 Hurricane Ike-related PTSD
PTSD symptoms were assessed using the PTSD Checklist–Specific Stressor Version (PCL), a 17-item self-report instrument that assesses DSM-IV symptoms of PTSD in relation to a specific stressful experience (Weathers et al., 1993). Total scores on the PCL range from 17 to 85. Additional questions were also asked to assess Criteria A2, E, and F (Pietrzak et al., 2012b). A probable diagnosis of PTSD required endorsement of all DSM-IV criteria for PTSD, including: experiencing, witnessing, or being confronted with event(s) that involved actual or threatened death or serious injury, or a threat to physical integrity of self or others (Criterion A1) with response involving intense fear, helplessness, or horror (Criterion A2); endorsement of being bothered “moderately,” “quite a bit,” or “extremely” by at least one intrusion (Criterion B), three avoidance/numbing (Criterion C), and two arousal (Criterion D) symptoms (Blanchard et al., 1995); endorsement of symptom duration greater than one month (Criterion E); and endorsement of symptoms cause significant distress or impairment in social, occupational, or other important areas of functioning (Criterion F).
Table 1 shows results of confirmatory factor analyses of PCL items. Corrected scaled χ2 difference tests (Fan and Sivo, 2009) revealed that the 5-factor model provided a significantly better fit to the data than the DSM-IV (Δχ2(7, n=146)=28.53, p<.001); dysphoria (Δχ2(4, n=146)=17.61, p<.01) and numbing (Δχ2(4, n=146)=13.07, p<.05) model; there was also greater evidence of “excellent fit” for this model according to empirically defined benchmarks (Hu and Bentler, 1998; 1999). To examine the relation between 5-HTTLPR genotype, exposure, and the 5-factor model of PTSD symptoms, we computed sum scores on the PCL. Re-experiencing symptoms were computed as the sum of PCL items that correspond to Criterion B symptoms for a DSM-IV diagnosis of PTSD; avoidance symptoms were computed as the sum of PCL items that correspond to symptoms C1 and C2 of Criterion C; numbing symptoms were computed as the sum of PCL items that correspond to symptoms C3, C4, C5, C6, and C7 of Criterion C; dysphoric arousal symptoms were computed as the sum of PCL items that correspond to symptoms D1, D2, and D3 of Criterion D; and anxious arousal were computed as the sum of PCL items that correspond to symptoms D4 and D5 of Criterion D. Factor loadings for each of the PCL items on symptom clusters that comprise the 5-factor model are shown in Table 1. Internal consistency analyses suggested excellent reliability for total PCL scores (Cronbach’s α=0.96), and good-to-excellent reliability for each of the five PTSD symptom clusters that comprise the 5-factor model, with Cronbach’s α=0.88 for the re-experiencing cluster, 0.90 for the avoidance cluster, 0.87 for the numbing cluster, 0.86 for the dysphoric arousal cluster, and 0.81 for the anxious arousal cluster.
Table 1.
Fit statistics of confirmatory factor analytic models of PTSD symptom structure and factor loadings of the 5-factor model.
| Fit statistics
| ||||||
|---|---|---|---|---|---|---|
| Model | S-B χ2 | df | p | CFI | TLI | RMSEA (90%CI) |
| DSM-IV | 154.32 | 116 | .010 | .923 | .910 | .046 (.024-.065) |
| 4-factor dysphoria | 138.64 | 113 | .051 | .949 | .938 | .039 (.000-.058) |
| 4-factor numbing | 131.66 | 113 | .111 | .963 | .955 | .033 (.000-.054) |
| 5-factor | 119.05 | 109 | .240 | .980 | .975 | .025 (.000-.049) |
| Factor loadings | |||||
| DSM-IV PTSD symptom | Re-experiencing | Avoidance | Numbing | Dysphoric Arousal | Anxious Arousal |
| B1. Intrusive thoughts of trauma | .849 | ||||
| B2. Recurrent dreams of trauma | .718 | ||||
| B3. Flashbacks | .693 | ||||
| B4. Emotional reactivity to trauma cues | .803 | ||||
| B5. Physiological reactivity to trauma cues | .844 | ||||
| C1. Avoiding thoughts of trauma | .897 | ||||
| C2. Avoiding reminders of trauma | .917 | ||||
| C3. Inability to recall aspects of trauma | .674 | ||||
| C4. Loss of interest | .782 | ||||
| C5. Detachment | .824 | ||||
| C6. Restricted affect | .810 | ||||
| C7. Sense of foreshortened future | .719 | ||||
| D1. Sleep disturbance | .853 | ||||
| D2. Irritability/anger | .745 | ||||
| D3. Difficulty concentrating | .854 | ||||
| D4. Hypervigilance | .825 | ||||
| D5. Exaggerated startle response | .833 | ||||
Note: DSM-IV=Diagnostic and Statistical Manual of Mental Disorders, 4th edition; S-B χ2 =Satorra-Bentler chi-square.
statistic; df=degrees of freedom; CFI=comparative fit index; TLI=Tucker-Lewis Index; BIC=Bayesian Information Criterion.
RMSEA=root mean square error of approximation; 90%CI=90% confidence interval.
2.5 Data analysis
Due to the small sample size, a stepwise multiple linear regression analysis was conducted to examine variables associated with severity of Ike-related PTSD symptoms; and a multivariate stepwise logistic regression analysis was conducted to examine variables associated with a probable diagnosis of Ike-related PTSD. 5-HTTLPR genotype (copy of one or more S’ alleles), Hurricane Ike exposure (Low Exposure vs. High Exposure), and the interaction of 5-HTTLPR S’ allele x Hurricane Ike exposure were entered into these analyses; age and sex were additionally entered as covariates. Secondary analyses were conducted to examine associations between 5-HTTLPR genotype, exposure, and the 5-HTTLPR x exposure interaction, and PTSD symptom clusters from the 5-factor model (i.e., re-experiencing, avoidance, numbing, dysphoric arousal, and anxious arousal); α was set to.001 in these analyses to reduce the likelihood of Type I error. Following prior work (Kilpatrick et al., 2007; Xie et al., 2009; Xie et al., 2012), ancestral proportion scores were computed and also entered as a covariate in all analyses; entering these scores prevents spurious associations that can result from variation in allele frequency and prevalence of a particular trait in the population (Gelernter et al., 1997).
3 Results
The mean age of sample was 53.1 (SD=17.8; range=18-92); 86 (58.9%) were female; and 104 (71.2%) were European American, and 42 (28.8%) were non-European American (10.3% Hispanic, 9.6% Black, and 8.9% other, non-Hispanic. The mean number of Ike-related potentially traumatic exposures in the full sample was 2.6 (SD=2.0; range=0–7). In the Low Exposure group, the mean number of exposures was 0.9 (SD=0.8; range=0-2); and in the High Exposure group, the mean number of exposures was 4.3 (SD=1.2; range=3–7), t(147)=19.54, p<.001. Mean PCL scores in the full sample were 27.0 (SD=13.9; range=17-81). Thirteen (8.9%) participants met criteria for probable PTSD.
Table 2 shows results of stepwise regression analyses examining variables associated with severity and probable diagnosis of disaster-related PTSD. Results of these analyses revealed that after adjustment for age, sex, and ancestral proportion scores, the interaction of 5-HTTPLR genotype x exposure was significantly associated with both severity of and probable diagnosis of Ike-related PTSD; specifically, respondents with one or more copies of the S’ allele who were highly exposed to Hurricane Ike reported significantly greater severity of PTSD symptoms and were more likely to screen positive for probable PTSD than respondents homozygous for the L allele who were highly exposed to Hurricane Ike. Prevalence of Ike-related PTSD was 15.3% (n=9) among individuals with an S’ allele who were highly exposed to Hurricane Ike; 7.7% (n=1) among individuals homozygous for the L allele who were highly exposed; 1.7% (n=1) among individuals with an S’ allele who had low exposure; and 10.5% (n=2) among individuals homozygous for the L’ allele who had low exposure. Main effects of 5-HTTLPR genotype and Ike-related exposure severity were not significant in these analyses.
Table 2.
Results of regression analyses examining association between 5-HTTLPR genotype, and severity and probable diagnosis of disaster-related posttraumatic stress disorder.
| Severity of PTSD symptoms | |||
|---|---|---|---|
| R2=.19 | β | t | p |
| Age | .02 | .23 | .82 |
| Female sex | .20* | 2.61 | .010 |
| Ancestral proportion score | .08 | 1.05 | .29 |
| 5-HTTLPR S’ allele | .15 | 1.78 | .08 |
| High Exposure | .19 | 1.39 | .17 |
| 5-HTTLPR S’ allele x High exposure 0.5 | .40*** | 5.37 | <.001 |
| Probable diagnosis of PTSD | |||
| Nagelkerke R2=.16 | |||
| Wald | OR | 95%CI | |
| Age | .47 | 1.01 | .98–1.05 |
| Female sex | 1.91 | 2.63 | .67–10.38 |
| Ancestral proportion score | .50 | 1.65 | .41–6.68 |
| 5-HTTLPR S’ allele | 2.47 | 7.46 | .61–91.46 |
| High Exposure | .15 | 1.67 | .13–22.05 |
| 5-HTTLPR S’ allele x High exposure | 4.55* | 3.81 | 1.11–13.03 |
Note. OR=odds ratio; 95%CI=95% confidence interval.
p<.05.
p<.001.
Secondary analyses revealed that the 5-HTTLPR S’ allele x exposure interaction was significantly associated with anxious arousal (β=.44, t=5.47, p<.001) and re-experiencing (β=.35, t=4.56, p<.001) symptoms, but not avoidance (β=.08, t=.55, p=.59) numbing (β=.20, t=1.49, p=.14), or dysphoric arousal (β=.13, t=.93, p=.36) symptoms, with respondents with one or more copies of the S’ allele who were highly exposed to Hurricane Ike reporting significantly greater severity of anxious arousal and re-experiencing symptoms than respondents homozygous for the L allele who were highly exposed to this disaster. Fig. 1 illustrates the effect of the 5-HTTLPR genotype in moderating the relation between Hurricane Ike exposure and severity of total Ike-related PTSD symptoms.
Fig. 1.
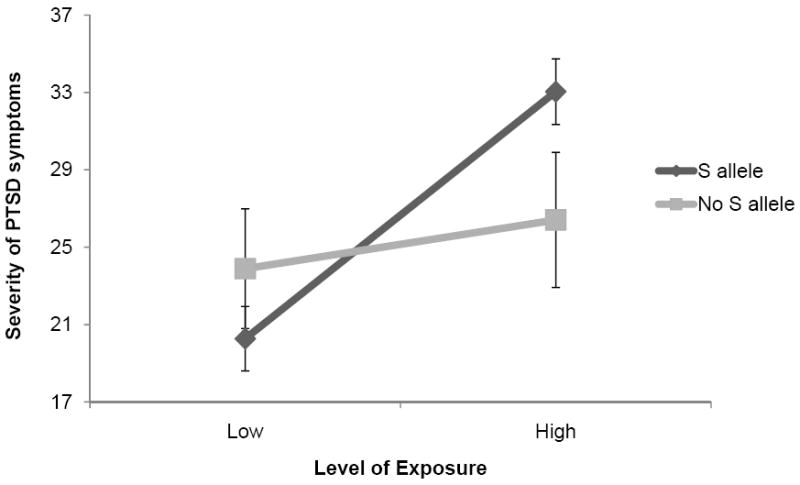
Effect of 5-HTTLPR genotype in moderating the association between level of. exposure to Hurricane Ike and severity of Ike-related PTSD symptoms Note. Error bars represent 95% confidence intervals.
Of note, when severity of DSM-IV hyperarousal symptoms was entered as the dependent variable, the relation between the 5-HTTLPR S’ allele x exposure interaction, and severity of these symptoms was only marginally significant (p=.071) and reduced in magnitude (β=.34) compared to when severity of anxious arousal symptoms was entered as the dependent variable.
4 Discussion
In this pilot study, we found that the interaction of the low expression variant of the 5-HTTLPR genotype and disaster exposure was associated only with severity of anxious arousal and re-experiencing symptoms, but not avoidance, numbing, or dysphoric arousal symptoms, thereby suggesting greater specificity of this interaction on the expression of these two clusters of PTSD symptoms among individuals recently affected by trauma.
Results of this pilot study contribute to a burgeoning body of studies demonstrating that the 5-HTTLPR genotype moderates the relation between trauma exposure and risk for PTSD (Grabe et al., 2009; Kilpatrick et al., 2007; Koenen et al., 2009; Thakur et al., 2009; Xie et al., 2009; Xie et al., 2012). The finding that the interaction of S’ allele carriage x trauma exposure on CFA-derived PTSD symptom dimensions was significant only for anxious arousal (i.e., hypervigilance, exaggerated startle) and re-experiencing (i.e., intrusive thoughts, nightmares, trauma-related physiological reactivity) symptoms aligns with results of neuroimaging studies suggesting that the 5-HTTLPR S’ allele is associated with greater amygdala hyperreactivity (Hariri et al., 2002), as well as reduced coupling of amygdala-cingulate neural circuitry implicated in emotion regulation (Pezawas et al., 2005). This finding also accords with neuropsychological studies demonstrating that the low transmission efficacy 5-HTTLPR genotype is associated with attentional vigilance toward negatively valenced stimuli (Pergamin-Hight et al., 2012), as well as greater difficulty disengaging from negative stimuli (Beevers et al., 2009). Results of the current study extend this work to suggest that, several months after a traumatic event, individuals with one or more copies of the S’ allele who are highly exposed to trauma report greater severity of PTSD-related anxious arousal and re-experiencing symptoms than individuals homozygous for the L allele who are highly exposed to trauma. To our knowledge, this study is among the first to demonstrate this specificity of association between the 5-HTTLPR x trauma exposure interaction on the phenotypic expression of PTSD symptoms.
In addition to providing empirical support for the superiority of the 5-factor model of PTSD symptomatology compared to the DSM-IV and alternative 4-factor models, these pilot findings suggest that the 5-HTTLPR genotype x trauma exposure interaction is uniquely related to hyperarousal symptom dimensions that comprise the 5-factor model, as this interaction effect was significantly associated with severity of anxious arousal, but not dysphoric arousal symptoms. This finding suggests that separation of the DSM-IV hyperarousal cluster into anxious arousal and dysphoric arousal symptoms may provide a more refined understanding of how genetic risk factors for PTSD relate to the clinical expression of PTSD symptoms. Importantly, this approach provides a theory-driven and empirically corroborated approach to characterizing heterogeneity of PTSD symptomatology that goes beyond non-specific approaches that treat PTSD as a homogeneous clinical entity.
Methodological limitations of this pilot study include the small sample size; possible misclassification of participants given that the period of assessment ranged from 2-months (i.e., acute phase of trauma) to 5-months (i.e., more chronic phase of trauma); and limited generalizability to older, White/non-Hispanic, and more highly educated trauma survivors; Nevertheless, these preliminary findings replicate prior work demonstrating the effect of the low expression variant of the 5-HTTLPR genotype in moderating the relation between trauma exposure and risk for PTSD (Kilpatrick et al., 2007; Koenen et al., 2009; Xie et al., 2009; Xie et al., 2012). Importantly, they extend this work to suggest that this effect is present in the early aftermath of a natural disaster, and that it may be specific to anxious arousal and re-experiencing symptoms. Given the somewhat mixed literature regarding whether the low or high expression variant of the 5-HTTLPR genotype moderates the relation between trauma exposure and PTSD symptoms (see Grabe et al., 2009; Thakur et al., 2009), additional research is needed to confirm our findings in larger samples of individuals affected by disasters and other traumatic events; to evaluate the relation between 5-HTTLPR, trauma exposure, and severity of PTSD symptom clusters over time; and to evaluate how the 5-HTTLPR genotype may interact with other genetic markers and environmental factors to increase risk for PTSD and related disorders, such as depression.
Acknowledgments
This study was supported by the National Center for Disaster Mental Health Research (National Institute of Mental Health Grant 5 P60 MH082598), Fran H. Norris, Center Director, Sandro Galea, Research Director. Preparation of this report was supported in part by the Clinical Neurosciences Division of the National Center for Posttraumatic Stress Disorder, a Research Career Development Award to Dr. Pietrzak from the Claude D. Pepper Older Americans Independence Center at Yale University School of Medicine (NIA Grant P30AG21342), and a private donation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Contributors
Dr. Pietrzak conducted statistical analyses and wrote the first draft of the manuscript. Drs. Galea, Southwick, and Gelernter designed the study, collected the data, and contributed to revising the manuscript.
Conflict of interest
None of the authors have any conflict of interest related to this study. Dr. Pietrzak is a scientific consultant to CogState, Ltd., for work that bears no relationship to this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric. Association Diagnostic and statistical manual of mental disorders. fourth edition. Washington, DC: American Psychiatric Press; 2000. [Google Scholar]
- Armour C, Elhai JD, Richardson D, Ractliffe K, Wang L, Elklit A. Assessing a five factor model of PTSD: Is dysphoric arousal a unique PTSD construct showing differential relationships with anxiety and depression? J Anxiety Disord. 2012;26:368–376. doi: 10.1016/j.janxdis.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Beevers CG, Wells TT, Ellis AJ, McGeary JE. Association of the serotonin transporter gene promoter region (5-HTTLPR) polymorphism with biased attention for emotional stimuli. J Abnorm Psychol. 2009;118:670–681. doi: 10.1037/a0016198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Taylor AE, Forneris CA, Loos WR, Jaccard J. Effects of varying scoring rules of the Clinician-Administered PTSD Scale (CAPS) for the diagnosis of post-traumatic stress disorder in motor vehicle accident victims. Behav Res Ther. 1995;33:471–475. doi: 10.1016/0005-7967(94)00064-q. [DOI] [PubMed] [Google Scholar]
- Elhai JD, Biehn TL, Armour C, Klopper JJ, Frueh BC, Palmieri PA. Evidence for a unique PTSD construct represented by PTSD’s D1-D3 symptoms. J Anxiety Disord. 2011;25:340–345. doi: 10.1016/j.janxdis.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Elhai JD, Palmieri PA. The factor structure of posttraumatic stress disorder: a literature update, critique of methodology, and agenda for future research. J Anxiety Disord. 2011;25:849–854. doi: 10.1016/j.janxdis.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Fan X, Sivo SA. Using goodness-of-fit indexes in assessing mean structure invariance. Struct Equ Modeling. 2009;16:54–67. [Google Scholar]
- Galea S, Nandi A, Vlahov D. The epidemiology of post-traumatic stress disorder after disasters. Epidemiol Rev. 2005;27:78–91. doi: 10.1093/epirev/mxi003. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Spitzer C, Schwahn C, Marcinek A, Frahnow A, Barnow S, Lucht M, Freyberger HJ, John U, Wallaschofski H, Volzke H, Rosskopf D. Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am J Psychiatry. 2009;166:926–933. doi: 10.1176/appi.ajp.2009.08101542. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Fit indices in covariance structural modeling: sensitivity to underparameterized model misspecification. Psychol Methods. 1998;3:424–453. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Aiello AE, Bakshis E, Amstadter AB, Ruggiero KJ, Acierno R, Kilpatrick DG, Gelernter J, Galea S. Modification of the association between serotonin transporter genotype and risk of posttraumatic stress disorder in adults by county-level social environment. Am J Epidemiol. 2009;169:704–711. doi: 10.1093/aje/kwn397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak: Part I. An empirical review of the empirical literature, 1981–2001. Psychiatry. 2002a;65:207–239. doi: 10.1521/psyc.65.3.207.20173. [DOI] [PubMed] [Google Scholar]
- Norris FH, Friedman MJ, Watson PJ. 60,000 disaster victims speak: Part II. Summary and implications of the disaster mental health research. Psychiatry. 2002b;65:240–260. doi: 10.1521/psyc.65.3.240.20169. [DOI] [PubMed] [Google Scholar]
- Norris FH, Sherrieb K, Galea S. Prevalence and consequences of disaster-related illness and injury from Hurricane Ike. Rehabil Psychol. 2010;55:221–230. doi: 10.1037/a0020195. [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Bakermans-Kranenburg MJ, van Ijzendoorn MH, Bar-Haim Y. Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biol Psychiatry. 2012;71:373–379. doi: 10.1016/j.biopsych.2011.10.030. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Tsai J, Harpaz-Rotem I, Whealin JM, Southwick SM. Support for a novel 5-factor model of posttraumatic stress symptoms in three independent samples of Veterans of the Iraq and Afghanistan wars: a confirmatory factor analytic study. J Psychiatr Res. 2012a;46:317–322. doi: 10.1016/j.jpsychires.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Van Ness PH, Fried TR, Galea S, Norris F. Diagnostic utility and factor structure of the PTSD Checklist in older adults. Int Psychogeriatr. 2012b;24:1684–1696. doi: 10.1017/S1041610212000853. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Rosenberg NA. Use of unlinked genetic markers to detect population stratification in association studies. Am J Hum Genet. 1999;65:220–228. doi: 10.1086/302449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Seedat S, Gelernter J. Serotonin transporter gene promoter polymorphism predicts SSRI response in generalized social anxiety disorder. Psychopharmacology. 2006;187:68–72. doi: 10.1007/s00213-006-0349-8. [DOI] [PubMed] [Google Scholar]
- Thakur GA, Joober R, Brunet A. Development and persistence of posttraumatic stress disorder and the 5-HTTLPR polymorphism. J Trauma Stress. 2009;22:240–243. doi: 10.1002/jts.20405. [DOI] [PubMed] [Google Scholar]
- Wang L, Cao C, Wang R, Zhang J, Li Z. The dimensionality of PTSD symptoms and their relationship to health-related quality of life in Chinese earthquake survivors. J Anxiety Disord. 2012a;26:711–718. doi: 10.1016/j.janxdis.2012.06.005. [DOI] [PubMed] [Google Scholar]
- Wang M, Elhai JD, Dai X, Yao S. Longitudinal invariance of posttraumatic stress disorder symptoms in adolescent earthquake survivors. J Anxiety Disord. 2012b;26:263–270. doi: 10.1016/j.janxdis.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Z, Shi Z, Zhang J, Zhang K, Liu Z, Elhai JD. Testing the dimensionality of posttraumatic stress responses in young Chinese adult earthquake survivors: further evidence for “dysphoric arousal” as a unique PTSD construct. Depress Anxiety. 2011a;28:1097–1104. doi: 10.1002/da.20823. [DOI] [PubMed] [Google Scholar]
- Wang L, Long D, Li Z, Armour C. Posttraumatic stress disorder symptom structure in Chinese adolescents exposed to a deadly earthquake. J Abnorm Child Psychol. 2011b:749–758. doi: 10.1007/s10802-011-9508-4. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang J, Shi Z, Zhou M, Li Z, Zhang K, Liu Z, Elhai JD. Comparing alternative factor models of PTSD symptoms across earthquake victims and violent riot witnesses in China: evidence for a five-factor model proposed by Elhai et al (2011) J Anxiety Disord. 2011c;25:771–776. doi: 10.1016/j.janxdis.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD checklist (PCL): Reliability, validity, and diagnostic utility. San Antonio, TX. Meeting of the International Society of Traumatic Stress Studies.1993. [Google Scholar]
- Xie P, Kranzler HR, Farrer L, Gelernter J. Serotonin transporter 5-HTTLPR genotype moderates the effects of childhood adversity on posttraumatic stress disorder risk: A replication study. Am J Med Genet B Neuropsychiatr Genet. 2012;159B:644–652. doi: 10.1002/ajmg.b.32068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Brady K, Weiss RD, Farrer L, Gelernter J. Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch Gen Psychiatry. 2009;66:1201–1209. doi: 10.1001/archgenpsychiatry.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Li H, Criswell LA, Gregersen PK, Alarcon-Riquelme ME, Kittles R, Shigeta R, Silva G, Patel PI, Belmont JW, Seldin MF. Examination of ancestry and ethnic affiliation using highly informative diallelic DNA markers: application to diverse and admixed populations and implications for clinical epidemiology and forensic medicine. Hum Genet. 2005;118:382–392. doi: 10.1007/s00439-005-0012-1. [DOI] [PubMed] [Google Scholar]
- Yufik T, Simms LJ. A meta-analytic investigation of the structure of posttraumatic stress disorder symptoms. J Abnorm Psychol. 2010;119:764–776. doi: 10.1037/a0020981. [DOI] [PMC free article] [PubMed] [Google Scholar]


