Abstract
Background
The goal of this study was to examine cross-sectional and longitudinal associations between cognitive performance and beta amyloid (Aβ) load determined by florbetapir F18 positron emission tomography (PET) in non-demented oldest-old.
Methods
Thirteen non-demented (normal or cognitively impaired non-demented) participants (median age=94.2 years) from The 90+ Study underwent florbetapir-PET scanning within 3 months of baseline neuropsychological testing. Amyloid load was measured with a semiautomated quantitative analysis of average cortical to cerebellar standard uptake values (SUVr) ratio and a visual interpretation (Aβ- or Aβ+). Neuropsychological testing was repeated every 6 months.
Results
At baseline, SUVr correlated significantly with tests of global cognition and memory. During follow-up (median=1.5 years), the Aβ+ group had steeper declines on most cognitive tests, particularly global cognitive measures.
Conclusion
This preliminary study suggests that greater amyloid load is associated with poorer cognition and faster cognitive decline in non-demented oldest-old. Amyloid load may identify individuals at increased risk of developing Alzheimer's disease.
1. Introduction
The ability to image cerebral beta amyloid (Aβ) deposition during life with positron emission tomography (PET) scanning1, 2 is a major advance in neuroscience and a powerful research tool for the investigation of Alzheimer's disease (AD) and cognition in aging. Previously, studies of amyloid deposits and cognition were dependent on clinical pathological investigations, with a single amyloid measurement performed at the end of life. Recent studies have demonstrated that PET imaging with amyloid-binding ligands correlates with the presence and density of beta amyloid at autopsy.2 It has been hypothesized that amyloid deposition is an early event in the pathogenesis of AD, rising rapidly and plateauing before the appearance of clinical symptoms.3 In this scenario, normal individuals with amyloid deposition may be at higher risk of developing AD and may be experiencing subtle cognitive decline.3, 4
The oldest-old are the fastest growing segment of the population and have high rates of dementia5 and cognitive decline. A high proportion of non-demented individuals over age 90 have significant amyloid deposition on autopsy.6, 7 It is unknown if these individuals are at higher risk of developing dementia, are experiencing cognitive decline, or perhaps are even protected from the development of clinical AD. We examined the cross-sectional and longitudinal relationship between cognitive performance and amyloid load (florbetapir PET uptake) in thirteen non-demented oldest-old individuals.
2. Methods
Participants were part of The 90+ Study, a longitudinal population-based investigation of dementia and aging in the oldest-old. Individuals were invited to participate in this imaging study as part of an investigation to examine the relationship between measurements of brain amyloid using florbetapir PET imaging and levels of amyloid burden as measured by postmortem histopathological assessment.2
To meet inclusion criteria for our study, individuals had to be non-demented: normal or cognitively impaired non-demented (CIND) and agree to post-mortem examination. Participants were followed every 6 months with procedures that include the Mini-Mental State Exam (MMSE), Modified MMSE (3MS), Animal Fluency, Boston Naming Test (BNT), and the California Verbal Learning Test (CVLT) short form.8 At each visit, a trained neurological examiner determined cognitive status (normal, CIND, or dementia). Dementia was diagnosed using the Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria9. Participants with either cognitive or functional impairment due to cognition not severe enough to meet DSM–IV diagnostic criteria for dementia were classified as CIND.9 The neurological examiner had access to the 3MS and MMSE, but was blinded to the remainder of the neuropsychological testing.
Each participant underwent a 10-minute PET scan at approximately 50 minutes after injection of 370 MBq of Florbetapir F18. A semi-automated quantitative analysis was performed to calculate the standard uptake value (SUVr) ratio using the mean of 6 predefined anatomically relevant cortical regions (frontal, temporal, parietal, anterior cingulate, posterior cingulate, and precuneus), relative to entire cerebellum. These SUVr values were used as our primary amyloid load variable for our cross-sectional analyses. Florbetapir-PET images were also assessed visually by three trained nuclear medicine physicians using a semi-quantitative score ranging from 0 (no amyloid) to 4 (high levels of cortical amyloid). The median of the three visual scores was used to dichotomize participants into Aβ– (0,1) and Aβ+ (2,3, 4) groups for our longitudinal analyses. All procedures were approved by the UCI Institutional Review Board.
3. Statistical Methods
The visit closest to the PET scan was considered the baseline visit. For our cross-sectional analysis, Pearson correlation coefficients were calculated to assess the correlation between SUVr and neuropsychological scores at baseline. To analyze change in cognitive performance over time in Aβ– and Aβ+ participants, we took two different approaches. First, because of the small number of participants we took a simple approach and estimated a slope for each participant with a linear regression of neuropsychological test scores as a function of years from baseline. The average slope was then compared between Aβ– and Aβ+ participants using t-tests and Wilcoxon rank tests. As a second approach, we compared cognitive decline in Aβ– and Aβ+ people using mixed effects models, which accounts for the correlation between repeated measurements in the same individuals, but usually requires more observations. The covariates in the model were years from baseline, an indicator variable for Aβ group, and the interaction between years from baseline and Aβ group. A significant interaction indicates a difference in the rate of cognitive decline in the Aβ– and Aβ+ groups. The intercept was included as a random effect. Analyses were done separately for each neuropsychological test and were performed using SAS Version 9.2.
4. Results
Table 1 shows characteristics of the 13 participants (9 women and 4 men) in the study. The baseline visit was within 90 days of the PET scan (median=42 days). At baseline, the median age of the participants was 94.1 years, eight participants had normal cognition, and five participants had CIND.
Table 1.
Characteristics of Participants by Amyloid Load Status at Baseline: The 90+ Study
| Characteristic | All Participants (N=13) | Aβ- (N=9) | Aβ+ (N=4) |
|---|---|---|---|
|
Median (Range)
|
|||
| Age at baseline, y | 94.1 (90 - 99) | 94.1 (90 - 99) | 94.4 (93 - 96) |
| Days between baseline visit and PET scan | 42 (8 - 87) | 46 (11 - 87) | 25 (8 - 66) |
| Follow-up, y | 1.5 (0 - 1.6) | 1.5 (0 - 1.6) | 1.4 (1.1 - 1.6) |
| No. of visits | 4 (1 - 4) | 4 (1 - 4) | 3.5 (3 - 4) |
| Average cortical SUVr | 1.1 (0.9 - 2.1) | 1.0 (0.9 - 1.2) | 1.6 (1.3 - 2.1) |
| Median visual score of amyloid load | 1.0 (0 - 4) | 1.0 (0 - 1) | 3.5 (2 - 4) |
| 3MS score at baseline | 93 (80 - 99) | 94 (90 - 97) | 85 (80 - 99) |
| MMSE score at baseline | 28 (24 - 30) | 28 (25 - 30) | 26.5 (24 - 29) |
| CVLT-10 min delay score at baseline | 7 (0 - 9) | 7.5 (5 - 9) | 3.5 (0 - 8) |
| Animal fluency at baseline | 12 (9 - 19) | 12 (9 - 19) | 12 (9 - 18) |
| BNT score at baseline | 14 (13 -15) | 14 (13 -15) | 14 (13 -14) |
|
Number (%)
|
|||
| Gender | |||
| Women | 9 (69) | 7 (78) | 2 (50) |
| Men | 4 (31) | 2 (22) | 2 (50) |
| Education | |||
| ≤High school | 6 (46) | 4 (44) | 2 (50) |
| >High school | 7 (54) | 5 (56) | 2 (50) |
| Cognitive diagnosis at baseline | |||
| Normal | 8 (62) | 7 (78) | 1 (25) |
| CIND | 5 (38) | 2 (22) | 3 (75) |
| Demented during follow-up | 3 (23) | 1 (11) | 2 (50) |
Abbreviations: Aβ- = low amyloid load according to median visual score (0,1); Aβ+ = high amyloid load according to median visual score (2,3,4); SUVr = semiautomated quantitative analysis of the cortical to cerebellar signal ratio; 3MS = Modified Mini-Mental State Exam; MMSE = Mini-Mental State Exam; CVLT = California Verbal Learning Test; BNT = Boston Naming Test; CIND = Cognitive impairment no dementia
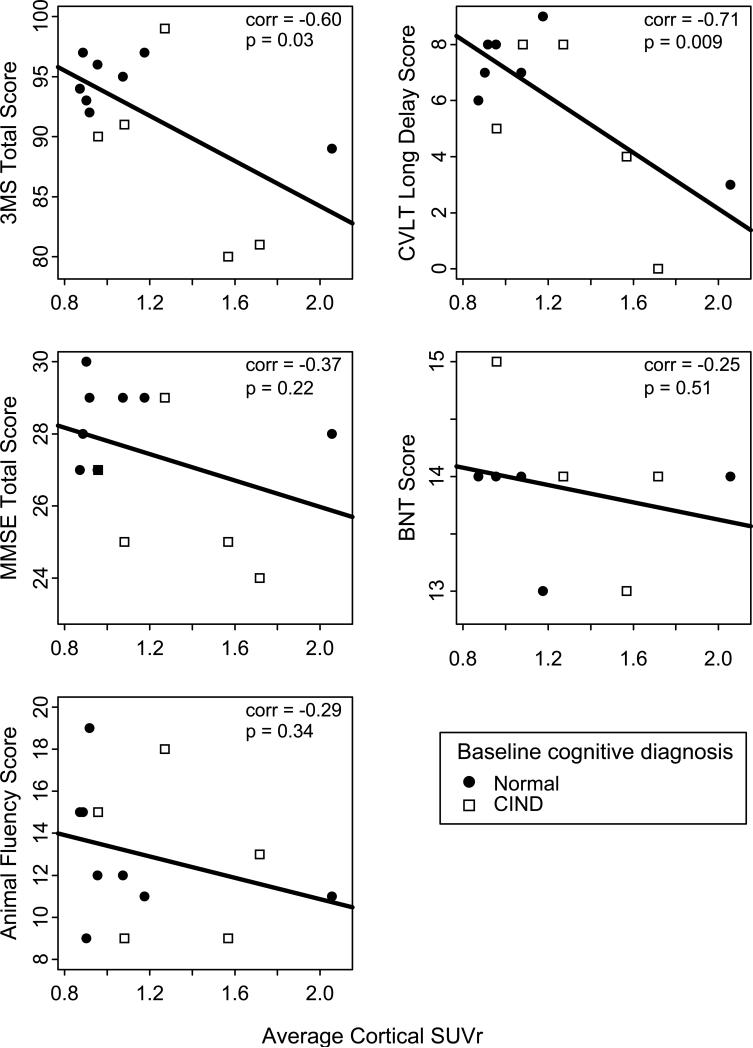
Neuropsychological test scores and SUVr at baseline are shown in Table 1. The median SUVr was 1.1 and ranged from 0.9 to 2.1. Neuropsychological scores were generally in the normal range (MMSE range=24-30) although some participants had poor memory scores consistent with their diagnosis of CIND. Figure 1 shows scatter plots of test scores at baseline versus SUVr. SUVr correlated significantly with CVLT (ρ=–0.71, p=0.009) and 3MS scores (ρ=–0.60, p=0.03) but not with MMSE (ρ=–0.37, p=0.22), animal fluency (ρ=–0.29, p=0.34), or BNT (ρ=–0.25, p=0.51) scores.
Figure 1. Scatter plot of neuropsychological test scores at baseline versus average cortical SUVr in non-demented participants from The 90+ Study.
3MS = modified Mini-Mental State Exam; MMSE = Mini-Mental State Exam; CVLT = California Verbal Learning Test; BNT = Boston Naming Test; CIND = cognitive impairment no dementia; corr = Pearson correlation; SUVr = standardized uptake value ratio Scatter plots show people with baseline cognitive diagnosis of ‘normal’ (closed circles) and ‘cognitive impairment no dementia’ (open squares).
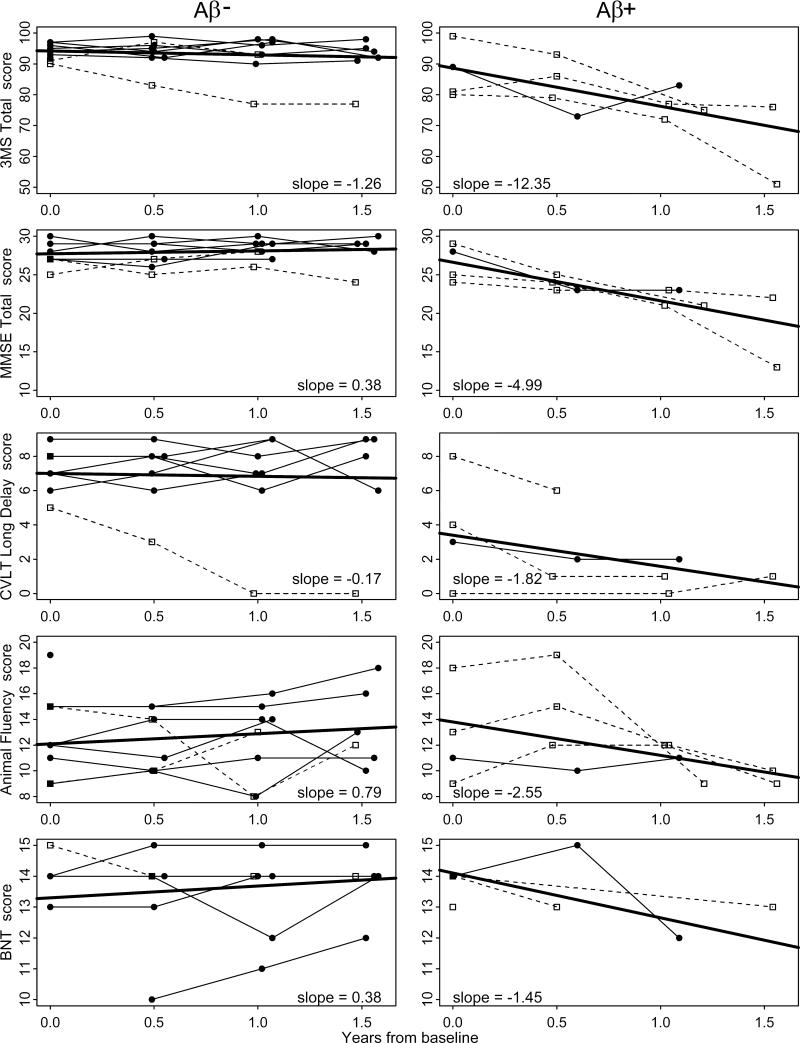
All but one participant were followed for three or four total visits: four participants had two follow-up visits and eight participants had three follow-up visits after their baseline evaluation. The remaining participant died within four months of the baseline visit and was not included in longitudinal analyses. Median follow-up time between baseline and last evaluation was 1.5 years (Table 1). Three participants developed dementia during follow-up. The three participants who developed dementia during follow-up were cognitively impaired but not demented at baseline. Figure 2 shows longitudinal trajectories for the different neuropsychological tests by amyloid load group. All but one participant in the Aβ– group appear to have stable scores across time on the 3MS, MMSE, and CVLT tests. In contrast, people in the Aβ+ group show a decline in scores, particularly in the 3MS and MMSE tests. Some of these differences were confirmed when comparing the average slopes for the two groups (Table 2). On all tests, cognitive decline was faster in the Aβ+ group compared to the Aβ– group and was significant for the 3MS, MMSE, and BNT tests. For example, people in the Aβ– group declined on average 1.26 points per year on the 3MS whereas people in the Aβ+ group declined 12.35 points per year (p=0.04). The results from the random effects models were very consistent with those of the average slope approach (Table 2).
Figure 2. Longitudinal trajectories of neuropsychological tests in Aβ- and Aβ+ non-demented participants from The 90+ Study.
Abbreviations: Aβ- = low amyloid load according to median visual score (0,1) ; Aβ+ = high amyloid load according to median visual score (2,3,4); 3MS = modified Mini-Mental State Exam; MMSE = Mini-Mental State Exam; CVLT = California Verbal Learning Test; BNT = Boston Naming Test.
Trajectories are for people with baseline cognitive diagnosis of ‘normal’ (closed circles) and ‘cognitive impairment no dementia’ (open squares). Slopes indicate the average yearly rate of change in cognitive tests (solid thick lines).
Table 2.
Average Yearly Rate of Change in Cognitive Tests by Amyloid Load Status at Baseline
| Slopes calculated from averaging individual slopes |
Slopes calculated from random effects models |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Aβ- | Aβ+ | t-test | Wilcoxon rank test | Aβ- | Aβ+ | ||||
| Cognitive Test | N | Slope (SE) | N | Slope (SE) | p-valuea | p-valuea | Slope | Slope | p-valueb |
| 3MS | 8 | -1.26 (1.20) | 4 | -12.35 (3.99) | 0.006 | 0.04 | -1.56 | -12.63 | <0.001 |
| MMSE | 8 | 0.38 (0.54) | 4 | -4.99 (1.40) | 0.001 | 0.03 | 0.24 | -4.96 | <0.001 |
| CVLT | 7 | -0.17 (0.62) | 4 | -1.82 (1.01) | 0.17 | 0.25 | -0.32 | -1.04 | 0.45 |
| Animal Fluency | 8 | 0.79 (0.77) | 4 | -2.59 (1.85) | 0.07 | 0.10 | 0.25 | -2.29 | 0.05 |
| BNT | 6 | 0.38 (0.38) | 3 | -1.45 (0.41) | 0.02 | 0.06 | 0.17 | -0.95 | 0.07 |
Abbreviations: Aβ - = low amyloid load according to median visual score (0,1) ; Aβ + = high amyloid load according to median visual score (2,3,4); 3MSE = Modified Mini-Mental State Examination; MMSE = Mini-Mental State Examination; CVLT = California Verbal learning Test 10-minute delay; BNT = Boston Naming Test
t-test and Wilcoxon rank test comparing average slopes for Aβ- and Aβ+ groups
p-value for the interaction between years from baseline and Aβ group. A significant interaction indicates a difference in the rate of cognitive decline in the Aβ– and Aβ+ groups
5. Discussion
This investigation in non-demented oldest-old individuals found that amyloid load measured with florbetapir PET scanning was related to cognitive performance at baseline and was associated with greater cognitive decline over 1.5 years. The results of this study support a model in which amyloid deposition is an early event on a path that may lead to dementia, beginning insidiously in cognitively normal individuals, and accompanied by subtle cognitive decline.10
Previous studies have suggested that the relationship between cerebral amyloid deposition and cognitive performance in the oldest-old is extremely poor.11 In large measure, these results arise because of the large percentage of non-demented oldest-old with significant amyloid deposition.
Our investigation suggests that 90 year old non-demented subjects with amyloid deposition have subtle cognitive changes compared to those without amyloid burdens, and are experiencing measurable declines over 1.5 years of follow-up. Three participants (two with high SUVr) appear to be in the early stages of dementia. We do not know if the others will develop dementia before death, nor do we know how long they have carried this amyloid load or if it is changing over time. Two-year follow-up PET studies are planned for all participants as they continue in our longitudinal study.
In this investigation of the oldest-old, scans that were interpreted visually as Aβ+ (scores = 2, 3, 4) had SUVr scores > 1.2. This value is slightly higher than the SUVr cut-point of 1.1 noted in the pivotal trial with subjects ranging in between 48 to 104 years of age.2 The pivotal trial had three Aβ+ subjects over the age of 90 and all had SUVr scores > 1.2. Of interest, despite having among the highest SUVr scores, two of these individuals exhibited only intermediate likelihood of AD on neuropathological evaluation. The appropriate cut-point for SUVr may differ for the oldest-old and requires further research.
Several limitations of this study deserve mention. First, the sample size is very small. We were somewhat surprised to find this result with our modest number of participants and have plans to increase the sample size with additional 90+ subjects to confirm our results. Second, four of the 12 participants were not followed for the full 1.5 years, thus their cognitive trajectories may have been somewhat different if they had been followed an additional six months. Third, some participants did not complete all cognitive tests at every visit; therefore we are less certain about the estimates of cognitive decline for those tests. Fourth, participants are all Caucasian, well educated, and over the age of 90, which limits our ability to generalize to other ethnic and demographic groups. Finally, oldest-old subjects have high rates of sensory loss and physical frailty, which make it challenging to administer the cognitive battery and to determine cognitive status.
This study in 90+ year olds supports a growing body of research suggesting that amyloid deposition may be an early event in the development of cognitive decline associated with AD. With additional research, we will understand better the temporal events related to amyloid deposition and the potential role of these studies in the diagnosis, treatment and hopefully, eventual prevention of AD.
Acknowledgments
Study Funding:
NIH/NIA R01AG21055
Avid Radiopharmaceuticals, Inc., Philadelphia, PA
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Claudia H. Kawas, MD - Eli Lilly & Co. - Data Monitoring Committee - Avid Radiopharmaceuticals, Inc. - Funding to obtain PET images
Dana E. Greenia, RN, MS - No Disclosures
Szofia S. Bullain, MD - No Disclosures
Christopher M. Clark, MD - Employee of Avid Radiopharmaceuticals, Inc., a wholly owned subsidiary of Eli Lilly & Co.
Michael J. Pontecorvo, PhD - Employee of Avid Radiopharmaceuticals, Inc., a wholly owned subsidiary of Eli Lilly & Co.
Abhinay Joshi - Employee of Avid Radiopharmaceuticals, Inc., a wholly owned subsidiary of Eli Lilly & Co.
María M. Corrada, ScD - No Disclosures
Florbetapir PET images were generated and read by Avid Radiopharmaceuticals, Inc. as part of the application for FDA approval of Florbetapir PET amyloid imaging tracer. Analyses of the imaging data were conducted by AVID Radiopharmaceuticals, Inc.
The 90+ Study PET investigation was designed by Drs. Kawas and Corrada independently of Avid Radiopharmaceuticals, Inc. Neurological and neuropsychological evaluations of the study participants were conducted by The 90+ Study (NIH/NIA R01AG21055 PI: C. Kawas). Data analyses were done by Dr. Corrada independent of Avid Radiopharmaceuticals, Inc.
Contributor Information
Claudia H Kawas, Department of Neurology; Department of Neurobiology and Behavior; Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, Irvine, CA 92697-4540, USA, ckawas@uci.edu.
Dana E Greenia, Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, Irvine, CA 92697-1400, USA, dgreenia@uci.edu.
Szofia S Bullain, Department of Neurology; Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, Irvine, CA 92697-1400, USA, sbullain@uci.edu.
Christopher M Clark, Avid Radiopharmaceuticals, Inc., Philadelphia, PA, 19104, USA, clark@avidrp.com.
Michael J Pontecorvo, Avid Radiopharmaceuticals, Inc., Philadelphia, PA, 19104, USA, pontecorvo@avidrp.com.
Abhinay D. Joshi, Avid Radiopharmaceuticals, Inc., Philadelphia, PA, 19104, USA, joshi@avidrp.com
María M Corrada, Department of Neurology; Institute for Memory Impairments and Neurological Disorders, University of California, Irvine, 92697-1400, USA, mcorrada@uci.edu.
References
- 1.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of neurology. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 2.Clark CM, Schneider JA, Bedell BJ, et al. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Archives of neurology. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Annals of neurology. 2010;67:114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C. Age, neuropathology, and dementia. The New England journal of medicine. 2009;360:2302–2309. doi: 10.1056/NEJMoa0806142. [DOI] [PubMed] [Google Scholar]
- 7.Corrada MM, Berlau DJ, Kawas CH. A Population-Based Clinicopathological Study in the Oldest-Old: The 90+ Study. Current Alzheimer research. 2012 doi: 10.2174/156720512801322537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whittle C, Corrada MM, Dick M, et al. Neuropsychological data in nondemented oldest-old: The 90+ Study. J Clin Exp Neuropsychol. 2007;29:290–299. doi: 10.1080/13803390600678038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSMIV. 4th ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 10.Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain : a journal of neurology. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 11.Imhof A, Kovari E, von Gunten A, et al. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm? Journal of the neurological sciences. 2007;257:72–79. doi: 10.1016/j.jns.2007.01.025. [DOI] [PubMed] [Google Scholar]