Abstract
Dysfunction of the orexin/hypocretin neurotransmitter system causes the sleep disorder narcolepsy, characterized by intrusion of rapid-eye-movement (REM) sleep-like events into normal wakefulness. The sites where orexins act to suppress REM sleep are incompletely understood. Previous studies suggested that the lateral pontomesencephalic tegmentum (lPMT) contains an important REM sleep inhibitory area, and proposed that orexins inhibit REM sleep via orexin type 2 receptors (OxR2) in this region. However, this hypothesis has heretofore not been tested. We thus performed bilateral injection of small interfering RNAs (siRNAs) targeting Ox2R into the lPMT on two consecutive days. This led to a ~30 % increase of time spent in REM sleep in both the dark and light periods for the first two days after injection, with a return to baseline over the next two post-injection days. This increase was mainly due to more longer (>120 s) REM episodes. Cataplexy-like episodes were not observed. The percentage of time spent in wakefulness and NREM sleep, as well as the power spectral profile of NREM and REM sleep, were unaffected. Control animals injected with scrambled siRNA had no sleep changes post-injection. Quantification of the knockdown revealed that unilateral microinjection of siRNAs targeting OxR2 into the lPMT induced a ~40% reduction of OxR2 mRNA two days following the injections when compared to the contralateral side receiving control (scrambled) siRNA. Orexin type 1 receptor (OxR1) mRNA level was unaffected. Our results indicate that removal of OxR2 neurotransmission in the lPMT enhances REM sleep by increasing the duration of REM episodes.
Keywords: Hypocretin, Narcolepsy, RNA interference; Diurnal, siRNA
Introduction
Orexins (also known as hypocretins) are neuropeptides produced in the perifornical region of the hypothalamus. Two isoforms of orexins (orexin-A and-B, or hypocretin-1 and hypocretin-2) are derived from proteolytic cleavage of a precursor peptide (prepro-orexin, or prepro-hypocretin) and exert their actions through two types of G-protein-coupled receptors (OxR1 and OxR2, or Hctr1 and Hctr2) (De Lecea et al., 1998; Sakurai et al., 1998). Loss of orexin neurons, orexin peptides, or the type II orexin receptor leads to narcolepsy, a disorder characterized by loss of control of the timing of transitions between sleep-wake states and intrusion of REM-sleep events into normal wakefulness (for review, see Chen et al., 2009; Taheri et al., 2002). An understanding of the sites where orexins act to suppress REM sleep and the role of the two different orexin receptors in this process are critical for our understanding of the control of the sleep-wake cycle (reviewed in Brown et al., 2012) and for the treatment of narcolepsy.
One site where orexins may act to suppress REM sleep is the lateral pontomesencephalic tegmentum (lPMT), which includes the lateral pontine tegmentum (LPT) (Lu et al., 2006) and deep mesencephalic reticular nucleus (DpMe) (Luppi et al., 2011). Previous studies showed that inhibition of this region by application of the GABAA receptor agonist muscimol strongly enhanced the amount of REM sleep in cats (Crochet et al., 2006; Sastre et al., 1996), rats (Sapin et al., 2009), and guinea pigs (Vanini et al., 2007). Similarly, neurotoxic lesions of this area increased REM sleep in rats (Lu et al., 2006). The lPMT receives a strong orexinergic innervation (Lu et al., 2006; Luppi et al., 2011), and has relatively high levels of orexin type 2 receptors (OxR2) (Brischoux et al., 2008; Cluderay et al., 2002). Thus, this region was suggested to be important for orexinergic suppression of REM sleep (Lu et al., 2006; Luppi et al., 2011). However, direct evidence of OxR2 involvement in the lPMT to suppress REM sleep is lacking. One recent study identified OxR2 expression on putative GABA neurons in the neighboring ventrolateral periaqueductal gray (vlPAG) and showed that lesions of this region by a neurotoxin (Orexin-B-saporin) targeted to neurons expressing orexin type 2-receptors produced an increase in REM sleep (Kaur et al., 2009). However, such neuronal lesions may also destroy cells that do not express OXR2 and thus do not reveal the normal role of orexin receptors in this region. In addition, a neuronal lesion approach causes severe disruption of neuronal circuits and it is not reversible. So far, no study has selectively targeted orexin receptors in the lPMT area.
RNA mediated interference (RNAi) is a mechanism for highly selective and reversible gene suppression (Dillon et al., 2005). Previously, we successfully used short interfering RNAs (siRNA) to induce RNAi knockdown of prepro-orexin mRNA in the perifornical hypothalamus (Chen et al., 2006) and OxR1 in the locus coeruleus (Chen et al., 2010). Here, we applied the same technique to assess the role of OxR2 in the lPMT on sleep and wakefulness.
Materials & Methods
Animals
Adult Sprague Dawley male rats (300-350g; Charles River Laboratories, Wilmington, MA) were housed under a 12:12 h light-dark cycle (lights on: 0700 hr; lights off: 1900 hr) at 22±1°C. All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals and approved by the Animal Research Committee of the Veterans Administration Boston Healthcare System.
siRNA
A pool of 3 siRNAs (equal concentration) was used. All of them were designed individually to target OxR2 (OxR2-siRNA). Their sequences were: #1 sense: 5′-GCUUGCAGCACUGAGCCGAtt-3′; antisense 5′-AGGGAUAUGGCUCUAGCUCtg-3′; #2 sense 5′-CCGGACCAGUCCGUGAUGUtt-3′; antisense 5′-ACAUCACGGACUGGUCCGGtg-3′; #3 sense 5′-AUUGGAGGAUUCCCUCCCUtt-3′; antisense 5′-AGGGAGGGAAUCCUCCAAUtt-3′. A corresponding control pool of 3 siRNAs with no homology to known rat genes (Ctrl-siRNA) was used. All siRNAs were annealed and HPLC purified (Applied Biosystems, Foster City, CA).
Assessment of sleep-wake effects of siRNA injection into the lPMT
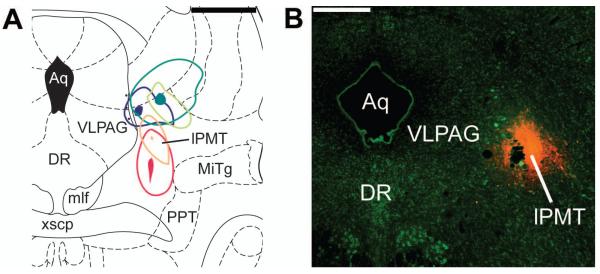
Bilateral injections of OxR2-siRNA (N=9) or Ctrl-siRNA (N=7) were made into the lPMT (Figure 1A) on two consecutive days and sleep-wakefulness monitored for 6 subsequent days.
Figure 1.
Surgery
Under sterile surgical conditions, EEG screws were implanted at 2.0 mm AP, 1.5 mm ML and −5.0 mm AP, 4 mm ML. Bilateral EMG electrodes were placed in the nuchal muscles. Bilateral guide cannulae (Plastics One, Roanoke, VA) were implanted 3 mm above lPMT (AP-7.8, DV6.3, ML1.5) for later microinjections since the injection cannula extended 3 mm beyond the tip of the guide cannula. Target coordinates were chosen based on previous literature focusing on this region (Lu et al., 2006; Sapin et al., 2009).
Microinjections
Recording cables were connected one day post-surgery. After at least a week of recovery and habituation to the recording cage, a 24-hour baseline sleep recording began at dark onset. For the next two days, 4 hr before dark onset (15:00), the rats were removed from the recording cage and gently swaddled in a towel during bilateral injection (approximately 2 min) with either OxR2-siRNA or Ctrl-siRNA (0.03 nmol in 0.3 μl water per side).
Histology
For identification of injection sites, rats were deeply anaesthetized with sodium pentobarbital and transcardially perfused with saline followed by 10% formalin (Sigma-Aldrich, St. Louis, MO). The brain was isolated and placed overnight in 10% formalin, transferred to 20% sucrose for cryoprotection, and coronal sections were cut at 30 μm on a freezing microtome. Cresyl violet staining was performed on one series of sections, encompassing the rostro-caudal extent of the lPMT, following standard procedures (Paxinos and Watson, 1998). Two rats that received OxR2-siRNA had injection tips which extended beyond the lPMT and ended in the ventrolateral tegmental nucleus (3 mm more ventral). These two cases were excluded from subsequent analysis (one rat had an increase in REM sleep during first post injection dark period; another rat had a slight increase of REM sleep during light period but decrease during dark period).
Sleep recordings and Analysis
Twenty-four hour sleep recordings were made using GAMMA software (Grass Technologies, West Warwick, RI, USA). Sleep data were recorded in 12 hour blocks (light and dark periods) and visually scored offline in 10 sec epochs of Wakefulness, NREM and REM using standard criteria (Chen et al, 2006). Scorers were blind to the specific treatment. The amount of time spent in each state was calculated along with number of episodes, as well as average duration of each behavioral state for each 12 hour block. Additionally, we calculated average REM sleep latency (time from onset of REM sleep to the preceding wakefulness) and average REM sleep to REM sleep cycle duration (R-R cycle, time between the start of two consecutive REM sleep episodes).
Assessment of the spread of injection using fluorescence conjugated siRNA
To assess the spread of our siRNA injection, we unilaterally administrated Cy3-labeled control siRNA (AM4621, Applied Biosystems, Foster City, CA) into the lPMT in 5 rats and perfused them 4 hr later. Coronal brain sections containing the injection site were cut, mounted, dried, and counterstained with a fluorescent Nissl stain (NeuroTrace; Molecular Probes).
Assessment of OxR2-knockdown: real time PCR
The extent and specificity of knockdown (KD) was assessed quantitatively by performing real time PCR to examine OxR2 and OxR1 mRNA levels.
Surgery and siRNA injections
A group of 10 rats were implanted with bilateral guide cannulae with tips 3 mm above the lPMT for later microinjections. After a one week recovery period, OxR2-siRNAs were unilaterally injected (0.03 nmol in 0.3 μl water) into one side of the lPMT. Ctrl-siRNA was injected on the contralateral side. The same procedure was repeated 24 hr later. 48 hours after treatment the animals were sacrificed by decapitation using a guillotine. The brain area containing the lPMT was punched out with a gauge 11 (2.4 mm internal diameter) micropunch, placed on dry ice, and subsequently stored at −80° C until processed for RNA extraction and real-time PCR as described previously (Chen et al., 2010).
Real time PCR
Each sample was run in duplicate in the Applied Biosystems real time PCR machine (Model 7300, Applied Biosystems, Foster City, CA). The primer/probe sets from the TaqMan Gene Expression Assays with efficiency of 100% for rat OxR2, OxR1 and endogenous control 18S RNA were used (OxR2: Rn00565155_m1; OxR1: Rn00565032_m1; Eukaryotic 18S rRNA Endogenous Control: 4333760T). The PCR reaction mixture contained 2X TaqMan® Universal PCR Master Mix (25 μl) 20X TaqMan® Gene Expression Assay (2.5μl), cDNA (4 or 8 μl) and water with a total volume of 50 μl. The amplification was run by 40 cycles of denaturation at 95°C followed by annealing/extending at 60°. We employed the comparative Ct method (ΔΔCt method) to calculate relative changes in mRNA levels (Livak and Schmittgen, 2001). The ΔCt values were determined by subtracting the reference 18S RNA values from the target gene Ct values. Our own experience and other’s work have shown that 18S rRNA is the most stable house-keeping gene among reference genes used in quantitative PCR (Port, 2007). The fold-change between the mRNA expression levels of the OxR2-siRNA treated and the Ctrl-siRNA treated samples was calculated using the formula 2-(ΔΔCt), where ΔΔCt equals the difference in ΔCt between the control and the experimental sample. The expression levels of OxR2 or OxR1 in Ctrl-siRNA treated samples were normalized to 100% and their respective levels in OxR2 treated samples were expressed as percentage of control.
Statistics
SigmaStat version 1.0 (Jandel Corporation, Sanfael, CA, USA) was used for statistical calculation. Sleep data were analyzed by one-way repeated measures analysis of variance (ANOVA) followed by multiple comparisons to the control group using Dunnett’s method. PCR data were analyzed by paired t-tests comparing OxR2 and OxR1 receptor values and differences between Ctrl-siRNA and OxR2-siRNA treated sides. Values were considered significant if p<0.05.
Results
siRNA injection successfully targeted the lPMT region
As described in methods, to assess the spread of our siRNA injection, we unilaterally administrated Cy3-labeled control siRNA into the lPMT in 5 rats and perfused them 4 hr later. Analysis of these animals revealed that cases the injection was centered in the lPMT (Figure 1). In one of the injections Cy3 fluorescence encroached on the vlPAG and in another there was a small overlap with the Cuneiform nucleus. The area that was 100~300 μm from the injection tip showed a strong Cy3 fluorescence that was not seen in areas further away from the injection tip (Figure 1), confirming that the correct site was targeted and giving an estimate for the volume of tissue affected by our injection. We found timing of perfusion to be critical, since 2 rats injected with Cy3-labeled siRNA into the lPMT and perfused 24 hr later showed a level of fluorescence that was indistinguishable from the background.
Sleep changes after siRNA-Ox2R injection into the lPMT
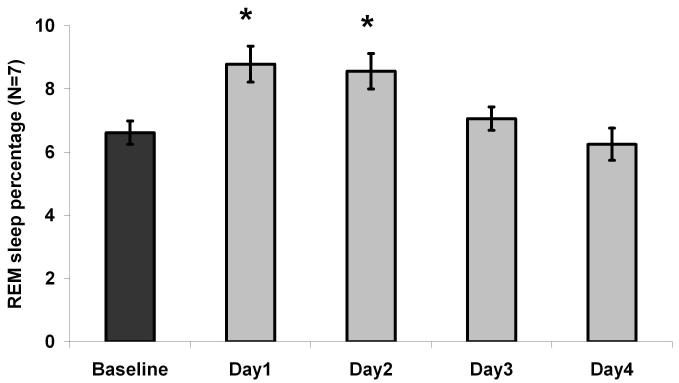
In the days following the last injection, the OxR2-siRNA treated animals (N=7) showed a significant increase (F4, 24=11.7, P=0.00002; Figure 2; Table 1) in the amount of time spent in REM sleep for the first 2 post-injection days (an increase of 32.8 and 29.4%, respectively) compared to the pre-injection day baseline. NREM sleep was not significantly changed. In contrast to OxR2-siRNA injected rats (N=7), the Ctrl-siRNA treated rats (N=7) did not have significant sleep changes after injection (Table 1).
Figure 2.
Table 1.
Sleep % in OxR2-siRNA and ctrl-siRNA treated rats.
| OxR2-siRNA (N=7) | Ctrl-siRNA (N=7) | |||||
|---|---|---|---|---|---|---|
| Period (12 hr) | Mean (± SEM) % time spent in | Mean (± SEM) % time spent in | ||||
| Wakefulness | NREMS | REMS | Wakefulness | NREMS | REMS | |
| Dark 0 | 68.7±1.4 | 27.2±1.0 | 4.0±0.5 | 62.3±2.6 | 33.2+2.2 | 4.5+0.5 |
| Dark 1 | 64.6±2.6 | 29.4±2.2 | 6.0±0.9 * | 61.7±2.6 | 32.9+2.2 | 5.0+0.5 |
| Dark 2 | 62.3±2.6 | 25.9±2.0 | 6.1±0.7 * | 62.6±3.6 | 32.2+2.9 | 4.9+0.7 |
| Dark 3 | 65.2±2.3 | 29.5±1.5 | 5.2±1.0 | |||
| Dark 4 | 66.9±3.4 | 28.8±2.7 | 4.3±0.8 | |||
| Light0 | 30.7±2.8 | 60.2±2.5 | 9.2±0.7 | 29.8±3.3 | 62.0±3.7 | 8.2±0.5 |
| Light 1 | 28.2±2.1 | 60.3±1.7 | 11.6±0.8 | 34.1±3.8 | 58.2±3.9 | 7.4±0.9 |
| Light 2 | 29.1 ±1.2 | 60.0±1.0 | 10.9±1.2 | 32.6±2.5 | 59.1±2.8 | 7.9±0.7 |
| Light 3 | 30.4±1.4 | 60.8±1.1 | 8.8±0.4 | |||
| Light 4 | 30.8±1.3 | 60.9±0.6 | 8.3±0.6 | |||
Percentage of REM sleep increases on dark periods 1 and 2 in animals treated with OxR2 siRNA. Dark= 7 PM to 7 AM; Light = 7 AM to 7 PM; Dark 0 or Light 0 = Pre-injection dark or light period. Dark 1-4 = Post-injection dark periods; Light 1-4 = post-injection light periods;
= P<0.05 versus Dark 0 (Dunnett’s test).
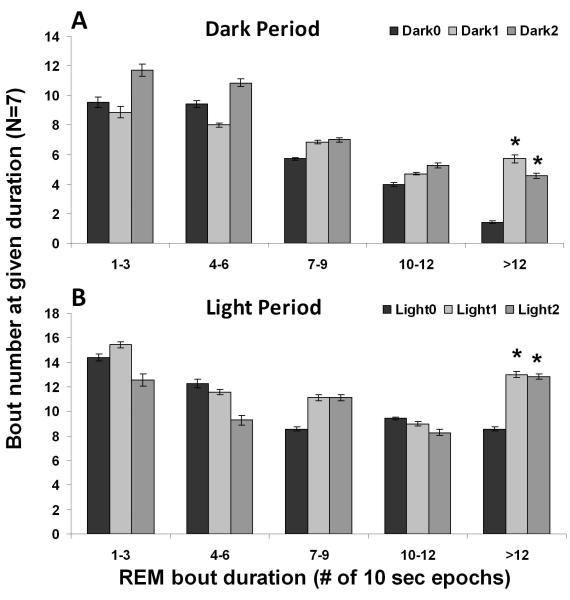
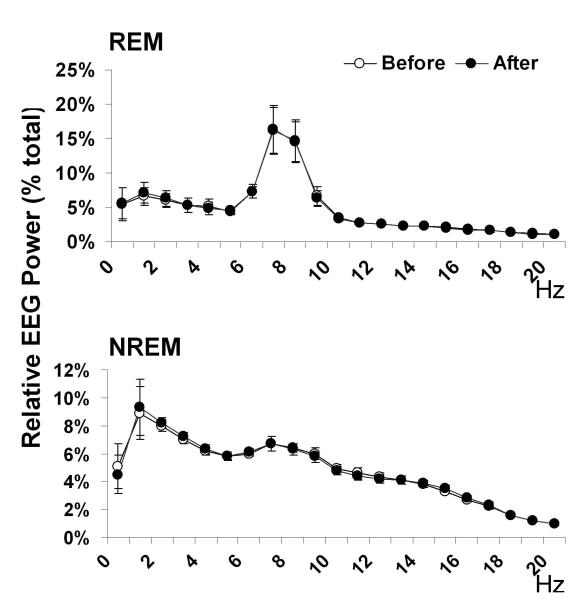
One-way ANOVA analysis showed that REM sleep amount increased both during the light period (F4, 24=4.2, P=0.01) and during the dark (active) period (F4, 24=4.3, P=0.009). Post-hoc Dunnett’s test showed that the first two post-injection dark periods had significantly more REM sleep than the baseline dark period, but no post-injection light periods showed such significant changes (Table 1). Moreover, a histogram comparison of the REM bout durations on the baseline day and days with an increase in REM sleep revealed a significant increase in the number of REM bouts longer than 2 min during both dark (F2, 12=7.1, P=0.009, Figure 3A) and light periods (F2, 12=4.5, P=0.04, Figure 3B). This increase in long bouts led to an increase in the average REM episode duration during the dark periods after OxR2 siRNA injection (F2, 12=4.8, P=0.03, Table 2). A similar trend was noted during the light periods although this did not reach significance (F2, 12=3.2, P=0.08, Table 2). During the two days following the last OxR2 siRNA injection (four 12h time blocks), the long REM bouts contributed to 89% (first light period), 88% (first dark period), 100% (second light period), and 56% (second night period) of the total REM increase, respectively. There was also a trend towards an increased frequency of REM episodes during dark periods (F2, 12=3.7, P=0.05, Table 2) but not during the light periods (F2,12=1.0, P=0.40, Table 2). REM sleep to REM sleep cycle duration (time between REM sleep episodes) was not appreciably altered. There was also no significant change in latency to REM sleep (time between previous waking to REM sleep) (Table 2). In addition, we compared the EEG power spectrum for the baseline dark period and the first 24 hour post-injection period (when the largest REM sleep change was observed) and found that the characteristics of the EEG power distribution was unaltered for both NREM and REM sleep in the range of 0-20 Hz (Figure 4). At the initial stage of our study, we conducted 24h video recordings along with sleep recordings after siRNA injections in 3 rats. However, since neither behavioral nor polysomnographic signs of cataplectic attacks or sleep onset REM episodes were identified, we discontinued this practice.
Figure 3.
Table 2.
REM sleep parameters during the dark period in OxR2-siRNA injected animals.
| REM sleep | |||||
|---|---|---|---|---|---|
| siRNA | Period | Number | Duration | Latency | R-R Cycle |
| OxR2 | Dark 0 | 23.0±2.7 | 1.2±0.1 | 6.6±0.7 | 29.2±3.0 |
| Dark 1 | 27.3±2.7 | 1.5±0.1 * | 8.5±1.2 | 24.2±2.7 | |
| Dark 2 | 30.1±3.2 | 1.4±0.1 | 6.8±0.6 | 22.0±2.0 | |
| Light0 | 39.1±3.4 | 1.7±0.1 | 5.6±0.6 | 18.3±1.5 | |
| Light1 | 45.0±3.7 | 1.8±0.1 | 5.8±0.5 | 16.1 ±1.4 | |
| Light2 | 42.6±5.3 | 1.8±0.1 | 6.3±0.7 | 18.3±2.7 | |
Compared to Dark 0, the length of REM sleep episodes increased during Dark 1. Dark= 7 PM to 7 AM; Dark 0 = Pre-injection dark period. Dark 1-2 = Post-injection dark periods; Duration and latency and REMS-REMS cycle duration (R-R cycle) are all expressed as minutes. REM sleep latency is defined as the time between the beginning of a REM sleep episode and the preceding wakefulness episode. R-R cycle is defined as from the beginning of a REM sleep episode to the beginning of the next REM sleep episode. All data are mean ± SEM.
= P<0.05 versus Dark 0 (Dunnett’s test).
Figure 4.
Verification of selective KD of Ox2R mRNA in the lPMT
Following unilateral injection of OxR2-siRNA into the lPMT area on two consecutive days, a significant reduction of OxR2 mRNA (39.1%, P=0.02) was detected 48 hrs later when compared to the contralateral side injected with Ctrl-siRNA (ΔCt value 16.2±0.3 vs.16.9±0.2 on OxR2-siRNA treated side). Thus, OxR2 mRNA was decreased at the same time point when there was a significant REM sleep increase. In contrast, there was no significant change in the non-targeted OxR1 mRNA levels between the OxR2-siRNA treated group and the Ctrl-siRNA treated group (ΔCt value 18.3±0.3 vs.18.5±0.3 on OxR2-siRNA treated side). An additional finding was that the lPMT concentration of OxR1 mRNA was about four times lower than that of OxR2 mRNA, based on the ΔCt value (2.1 cycles difference in control samples).
Discussion
Our results show that a relatively modest knockdown of OxR2 in the lPMT (39.1 % reduction of mRNA) increased REM sleep for 2 consecutive days, whereas other behavioral stages were unaffected. REM sleep was increased in both the light period and, more prominently, in the dark period. The increase in REM sleep resulted from an increased average REM sleep episode length, due to an increased number of very long REM sleep episodes. In contrast, the power spectral characteristics of NREM and REM sleep were unaffected by the siRNA injections.
Methodological issues
In our study we chose to use siRNAs against the OxR2 to inactivate the orexinergic input. In contrast to traditional gene KO techniques, the KD using this method is reversible. On the other hand, its effects last longer than pharmacological experiments, allowing study of the effect of the targeted gene on sleep wakefulness for at least one complete light-dark cycle. In this study the effect lasted for 2 days and all sleep parameters returned to baseline on the 3rd and 4th day post siRNA injection, similar to our previous studies using this technique (Chen et al., 2006; Chen et al., 2010). Most other studies, including our own, using naked siRNA microinjection in rat brain found that that the effective window of KD is around 24-48 hr after injection (Johnson et al., 2010; Liao et al., 2010; Manrique et al., 2009). Previously, we tested OxR1 mRNA levels in the locus coeruleus on post-injection day 1, 2, and 4 following OxR1 siRNA injection and found that significant KD occurred on post-injection day 1 and 2 but no reduction on post-injection day 4 (Chen et al., 2010). Although we did not test OxR2 mRNA KD on post-injection day 1 and post-injection day 4, the time course of the KD should be similar given the same approach was used to KD either OxR1 or OxR2. Injections of OxR2-siRNA on two consecutive days into the lPMT induced a KD of Ox2R mRNA of 39.1%, as measured by real-time PCR. In our previous studies (Chen et al., 2006; Chen et al., 2010), we observed a 59% reduction in prepro-orexin mRNA and 45.5% reduction of OxR1 mRNA. Our current KD level is thus comparable to previous results using the same technique. OxR1 mRNA levels in the same tissue samples collected from the lPMT were not affected, demonstrating the specificity of our approach.
Following injection of Cy3 conjugated siRNA into the lPMT area, strong Cy3 fluorescence that was distinguishable from background was restricted to an area 100~300 μm from the injection tip when assessed 4 hr following injection. siRNA uptake by cells is likely to be maximal during the first hours following injection since we failed to detect Cy3 fluorescence above background 24 hr after injection. Although it is possible that there is spread of siRNA at lower concentrations to neighbouring sleep-wake control regions, the most parsimonious explanation is that the behavioral effects observed were due to action of the siRNA on the lPMT. It is also possible to examine the spatial extent of OxR2 KD using in situ hybridization. However, the signal to noise ratio for OxR2 in brain stem detected by in situ hybridization is not very high (Greco and Shiromani, 2001; Marcus et al., 2001; Trivedi et al., 1998) and it is doubtful that this approach would be sensitive enough to detect the modest change induced by OxR2 siRNA KD. We chose not to perform OxR2 immunohistochemical staining in the lPMT or the Western Blot following OxR2-siRNA injections as the specificity of current commercially available antibodies has not been validated in OxR2 KO mice.
lPMT is a REM inhibiting area
Our experimental results are congruent with previous nonspecific lesion (Lu et al., 2006) or pharmacological inhibition (Crochet et al., 2006; Sapin et al., 2009; Sastre et al., 1996; Vanini et al., 2007) studies of vlPAG/lPMT which resulted in increased REM sleep. Orexinergic neurons project to vlPAG/lPMT (Greco and Shiromani, 2001; Lu et al., 2006; Peyron et al., 1998) and almost all studies to date have shown that orexins exert excitatory effects on their target neurons (Brown, 2003). Thus, our results that withdrawal of a (presumptively) excitatory orexinergic input leads to an increase in REM sleep is consistent with these previous studies.
Cataplexy, a sudden loss of muscle tone resembling the muscle atonia seen during REM sleep, is a prominent feature in many cases of narcolepsy associated with loss of orexins. Furthermore, OxR2 mutation causes narcolepsy with cataplexy in dogs (Lin et al., 1999). However, OxR2 knockout mice have much less frequent cataplexy-like attacks than orexin knockout mice (Willie et al., 2003). In our study cataplectic attacks were not observed. Similarly, lesion of the adjacent vlPAG with orexin conjugated saporin did not induce cataplexy (Kaur et al., 2009). However, cataplexy-like states were reported following a complete lesion of the lPMT in rats (Lu et al., 2006). Based on our results it seems that cataplexy requires one or more additional factors: (i) a more complete knockdown, in extent or amount; (ii) a more prolonged knockdown; or (iii) loss of orexin receptors in additional areas.
KD of OxR2 in the lPMT increases the duration of REM sleep episodes
Nonspecific chemical lesions of the lPMT or application of the GABA agonist muscimol increased both the number and the duration of REM bouts (Crochet et al., 2006; Lu et al., 2006; Vanini et al., 2007). Although there was a trend towards an increased number of REM episodes, our findings indicate that KD of OxR2 in the lPMT mainly enhances and stabilizes REM sleep by extending the length of REM bouts. Indeed, the bulk of REM sleep increase we found after OxR2 siRNA administration in the lPMT can be attributed to the increased number of long (>2min) REM sleep bouts. It is worth noting that orexin KO mice had increased REM sleep in the dark period (Chemelli et al., 1999). Similarly, in rats, prepro-orexin KD in the perifornical hypothalamus and OxR1 KD in the locus coeruleus increased REM sleep in dark period (Chen et al., 2006; Chen et al., 2010). Thus, it appears the primary role of orexin system is to diurnally gate REM sleep, probably via OxR1 receptors. On the other hand, our results suggest that orexinergic signaling via OxR2 in the lPMT is normally important in limiting the duration of REM episodes. Although OxR2 KO mice exhibited more modest REM sleep increases, which were restricted to the dark period, our approach may more accurately reflect the physiological role of OxR2 in the lPMT since developmental compensation may occur in KO mice (Willie et al., 2003). Alternatively, loss of OxR2 in other brain regions may limit REM sleep increases produced by the loss of OxR2 in the lPMT.
Neuronal subtypes mediating the effect of orexins in the lPMT/vlPAG
Although our study did not target the OxR2 KD to a specific cell-type in the lPMT, it is of interest to speculate as to the neurotransmitter phenotype involved in REM sleep inhibition. Previous studies hypothesized that orexins excite REM-off GABAergic neurons in the vlPAG/lPMT area (Lu et al., 2006; Luppi et al., 2011). Thus, reducing their activity by KD of OxR2 should increase REM sleep, as we found. Indeed, using c-Fos immunolabeling as a marker or neuronal activation, it was found that 72 hour REM sleep deprivation in rats activated more GABAergic neurons in the lPMT area than those in non-sleep deprived rats or in rats allowed 3 hour recovery sleep after deprivation (Sapin et al., 2009). However, contrary to the hypothesis of a REM-off profile of vlPAG/lPMT neurons, most electrophysiology studies found that lPMT/vlPAG neurons are Wake-REM-on or REM-on (Crochet et al., 2006; Datta and Maclean, 2007; Thakkar et al., 2002; Thankachan et al., 2009) with the possible caveat that small GABAergic neurons may have been missed in these electrophysiological recordings. Furthermore, with respect to the hypothesis of OxR2 receptor activation in GABA neurons, while it has been shown that GABAergic neurons in the vlPAG and other sleep related nuclei such as the laterodorsal tegmental nucleus (LDT) in the brain stem express OxR2 (Kaur et al., 2009; Mieda et al., 2011), the majority of OxR2 bearing neurons are probably non-GABAergic (Brischoux et al., 2008). Additional evidence against the lPMT GABA hypothesis was provided by a recent report indicating that disruption of GABAergic neurotransmission in the vlPAG and the lPMT by deleting vesicular GABA-glycine transporter in transgenic mice did not affect REM sleep (Krenzer et al., 2011). An alternative hypothesis is that OxR2 may be expressed on glutamatergic neurons, which target GABAergic interneurons in the pontine sublaterodorsal tegmental nucleus (SLD) or elsewhere in the brainstem (Boissard et al., 2003), which then inhibit glutamatergic REM-on neurons (Luppi et al., 2011).
Conclusions
Our experiments demonstrate that knockdown of OxR2 in the lPMT enhances REM sleep. Unlike our KD of OxR1 in the locus coeruleus (Chen et al., 2010), the increase of REM sleep produced by KD of OxR2 is not mainly due to increased REM sleep occurrence, but to enhanced REM sleep episode duration. In addition, the REM sleep increase was not limited to the dark (active) period. It is likely that orexin system regulates REM sleep via at least two routes. It relays circadian clock signals from the suprachiasmatic nucleus to the locus coeruleus via OxR1, and this mechanism may control the diurnal distribution of REM sleep (Chen et al., 2010). The orexinergic system may also control the stability and termination of REM sleep via OxR2 in the lPMT, as demonstrated here. Thus, our results suggest differential roles of orexin receptors in REM sleep regulation.
Acknowledgements
Supported by VA (Merit Award to RWM) and NIH grants R01 MH39683 & P01 HL095491 (RWM) & R21 MH094803 (REB). The authors would like to thank Courtney Birchall, Mike Leonard and Ryan Perry for their technical assistance. We also thank Dr. Robert Strecker for the support of his VA MERIT grant, and his effort in providing the staff and resources needed to perform surgery and sleep data analysis for this project.
Abbreviations
- LDT
laterodorsal tegmental nucleus
- lPMT
pontomesencephalic tegmentum
- LPT
lateral pontine tegmentum
- KD
knockdown
- KO
knockout
- NREM
non-rapid eye movement
- OxR1
orexin type 1 receptor
- OxR2
orexin type 2 receptor
- REM
rapid eye movement
- R-R cycle
REM sleep to REM sleep cycle duration
- RNAi
RNA interference
- siRNA
small interfering RNAs
- SLD
sublaterodorsal tegmental nucleus
- vlPAG
ventrolateral periaqueductal gray
Footnotes
The authors declare no conflict of interest.
References
- Boissard R, Fort P, Gervasoni D, Barbagli B, Luppi PH. Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset. Eur. J. Neurosci. 2003;18:1627–1639. doi: 10.1046/j.1460-9568.2003.02861.x. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Mainville L, Jones BE. Muscarinic-2 and orexin-2 receptors on GABAergic and other neurons in the rat mesopontine tegmentum and their potential role in sleep-wake state control. J. Comp. Neurol. 2008;510:607–630. doi: 10.1002/cne.21803. [DOI] [PubMed] [Google Scholar]
- Brown RE. Involvement of hypocretins/orexins in sleep disorders and narcolepsy. Drug News. Perspect. 2003;16:75–79. doi: 10.1358/dnp.2003.16.2.829323. [DOI] [PubMed] [Google Scholar]
- Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol. Rev. 2012;92:1087–187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Chen L, McKenna JT, Bolortuya Y, Winston S, Thakkar MM, Basheer R, Brown RE, McCarley RW. Knockdown of orexin type 1 receptor in rat locus coeruleus increases REM sleep during the dark period. Eur. J. Neurosci. 2010;32:1528–1536. doi: 10.1111/j.1460-9568.2010.07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Brown RE, McKenna JT, McCarley RW. Animal models of narcolepsy. CNS Neurol. Disord. Drug Targets. 2009;8:296–308. doi: 10.2174/187152709788921717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur. J. Neurosci. 2006;24:2039–2048. doi: 10.1111/j.1460-9568.2006.05058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul. Pept. 2002;104:131–144. doi: 10.1016/s0167-0115(01)00357-3. [DOI] [PubMed] [Google Scholar]
- Crochet S, Onoe H, Sakai K. A potent non-monoaminergic paradoxical sleep inhibitory system: A reverse microdialysis and single-unit recording study. Eur. J. Neurosci. 2006;24:1404–1412. doi: 10.1111/j.1460-9568.2006.04995.x. [DOI] [PubMed] [Google Scholar]
- Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: Reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci. Biobehav. Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van d.P., Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. U. S. A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon CP, Sandy P, Nencioni A, Kissler S, Rubinson DA, Van Parijs L. Rnai as an experimental and therapeutic tool to study and regulate physiological and disease processes. Annu. Rev. Physiol. 2005;67:147–173. doi: 10.1146/annurev.physiol.67.040403.130716. [DOI] [PubMed] [Google Scholar]
- Greco MA, Shiromani PJ. Hypocretin receptor protein and mRNA expression in the dorsolateral pons of rats. Brain Res. Mol. Brain Res. 2001;88:176–182. doi: 10.1016/s0169-328x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat. Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Thankachan S, Begum S, Liu M, Blanco-Centurion C, Shiromani PJ. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS One. 2009;4:e6346. doi: 10.1371/journal.pone.0006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenzer M, Anaclet C, Vetrivelan R, Wang N, Vong L, Lowell BB, Fuller PM, Lu J. Brainstem and spinal cord circuitry regulating REM sleep and muscle atonia. PLoS One. 2011;6:e24998. doi: 10.1371/journal.pone.0024998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao F, Taishi P, Churchill L, Urza MJ, Krueger JM. Localized suppression of cortical growth hormone-releasing hormone receptors state-specifically attenuates electroencephalographic delta waves. J. Neurosci. 2010;30:4151–4159. doi: 10.1523/JNEUROSCI.6047-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Luppi PH, Clement O, Sapin E, Gervasoni D, Peyron C, Leger L, Salvert D, Fort P. The neuronal network responsible for paradoxical sleep and its dysfunctions causing narcolepsy and rapid eye movement (REM) behavior disorder. Sleep Med Rev. 2011;15:153–163. doi: 10.1016/j.smrv.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Manrique C, Compan V, Rosselet C, Duflo SG. Specific knock-down of GAD67 in the striatum using naked small interfering RNAs. J. Biotechnol. 2009;142:185–192. doi: 10.1016/j.jbiotec.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mieda M, Hasegawa E, Kisanuki YY, Sinton CM, Yanagisawa M, Sakurai T. Differential roles of orexin receptor-1 and -2 in the regulation of non-REM and REM sleep. J. Neurosci. 2011;31:6518–6526. doi: 10.1523/JNEUROSCI.6506-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Peyron C, Tighe DK, van d.P., de LL, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sapin E, Lapray D, Berod A, Goutagny R, Leger L, Ravassard P, Clement O, Hanriot L, Fort P, Luppi PH. Localization of the brainstem GABAergic neurons controlling paradoxical (REM) sleep. PLoS One. 2009;4:e4272. doi: 10.1371/journal.pone.0004272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre JP, Buda C, Kitahama K, Jouvet M. Importance of the ventrolateral region of the periaqueductal gray and adjacent tegmentum in the control of paradoxical sleep as studied by muscimol microinjections in the cat. Neuroscience. 1996;74:415–426. doi: 10.1016/0306-4522(96)00190-x. [DOI] [PubMed] [Google Scholar]
- Taheri S, Zeitzer JM, Mignot E. The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu. Rev. Neurosci. 2002;25:283–313. doi: 10.1146/annurev.neuro.25.112701.142826. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Phasic but not tonic REM-selective discharge of periaqueductal gray neurons in freely behaving animals: Relevance to postulates of GABAergic inhibition of monoaminergic neurons. Brain Res. 2002;945:276–280. doi: 10.1016/s0006-8993(02)02914-1. [DOI] [PubMed] [Google Scholar]
- Thankachan S, Kaur S, Shiromani PJ. Activity of pontine neurons during sleep and cataplexy in hypocretin knock-out mice. J. Neurosci. 2009;29:1580–1585. doi: 10.1523/JNEUROSCI.5151-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- Vanini G, Torterolo P, McGregor R, Chase MH, Morales FR. GABAergic processes in the mesencephalic tegmentum modulate the occurrence of active (rapid eye movement) sleep in guinea pigs. Neuroscience. 2007;145:1157–1167. doi: 10.1016/j.neuroscience.2006.12.051. [DOI] [PubMed] [Google Scholar]
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, Leonard CS, Richardson JA, Hammer RE, Yanagisawa M. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: Molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]