Summary
Puberty is a period characterized by brain reorganization that contributes to the development of neural and behavioral responses to gonadal steroids. Previously, we have shown that a single injection of the bacterial endotoxin, lipopolysaccharide (LPS; 1.5mg/kg IP), during the pubertal period (around 6 weeks old) in mice decreases sexual receptivity in response to estradiol and progesterone in adulthood. These findings suggest that pubertal immune challenge has an enduring effect of decreasing the behavioral responsiveness to gonadal steroid hormones. Since estradiol improves cognitive function in certain tasks in mice, we investigated the effect of pubertal immune challenge on the ability of estradiol to enhance cognitive function. We hypothesized that estradiol would be less effective at enhancing performance on particular cognitive tasks in female mice treated with LPS during puberty. Six-week old (pubertal) and ten-week old (adult) female CD1 mice were injected with either saline or LPS. Five weeks later, they were ovariectomized and implanted subcutaneously with either an estradiol- or oil-filled Silastic© capsule followed one week later with testing for cognitive function. The duration of juvenile investigation during social discrimination and recognition tests was used as a measure of social memory, and the duration of object investigation during object recognition and placement tests was used as a measure of object memory. Chronic estradiol treatment enhanced social and object memory in saline-treated females and in females treated with LPS in adulthood. In contrast, in females treated with LPS at 6 weeks old, estradiol failed to improve social and object memories. These results support the hypothesis that exposure to an immune challenge during puberty reduces at least some of the cognitive effects of estradiol. Moreover, these results support the idea that pubertal immune challenge compromises a wide variety of behavioral influences of ovarian hormones.
Keywords: Estradiol, hippocampus-dependent tasks, immune challenge, puberty
Introduction
Puberty is an important developmental stage during which sexual maturity is attained (Schulz and Sisk, 2006). This period is accompanied by numerous physiological (e.g., increases in hormone levels, development of secondary sexual characteristics), emotional, behavioral and social changes that then have an impact on behavior later in life (Marshall and Tanner, 1969;Forbes and Dahl, 2010). Puberty is also a period of significant brain reorganization (Levitt, 2003), as the brain undergoes further remodeling by sex steroid hormones (Schulz et al., 2009).
Exposure to stressors during the pubertal period can have lasting behavioral effects in adulthood. For example, exposure to heat, immobilization, or ether stress, around the time of puberty has long-term negative effects on the reproductive capacity of female mice (Paris et al., 1973). Moreover, exposing mice of both inbred and outbred strains to shipping stress or to the immune challenge, the bacterial endotoxin, lipopolysaccharide (LPS), during the pubertal period, decreases sexual receptivity in adulthood following treatment with estradiol and progesterone contrasted with mice stressed earlier or later in development (Laroche et al., 2009a;2009b;Ismail et al., 2011). This is not a response to all stressors; exposure to other stressors like restraint stress, food deprivation or a multiple stressor regimen during this period fails to reduce behavioral responsiveness to estradiol and progesterone in adulthood (Laroche et al., 2009a).
Besides playing an important role in sexual behavior in many species, estradiol also plays a prominent role in many non-reproductive behaviors. Among other functions, estrogens have direct effects on the brain areas controlling mood and cognition (Spencer et al., 2008). Hormonal fluctuations across the menstrual cycle affect mood and cognitive function in women (Schmidt et al., 1998), and estradiol treatment in mice has similar effects (Ter Horst et al., 2012). In ovariectomized mice, estradiol treatment decreases anxiety-like and depression-like behavior; however, this effect of ovarian hormones is not seen in mice treated with LPS during pubertal development (Ismail et al., 2011;Olesen et al., 2011). In fact, pubertal immune challenge reverses the effects of estradiol treatment on two tests for depression-like behavior (Ismail et al., in press). These findings demonstrate that pubertal immune challenge alters the behavioral responsiveness of non-reproductive behaviors to estradiol.
The effects of estradiol on cognition depend on the cognitive task and the brain region(s) recruited during the particular task. For example, in female rats, estradiol impairs performance on striatum-dependent tasks (Korol, 2004;Davis et al., 2005), but it enhances performance on hippocampus-dependent tasks (Daniel et al., 1997;Luine et al., 1998;Sandstrom and Williams, 2004). Similarly, estradiol treatment to ovariectomized mice enhances performance on hippocampus-dependent tasks in mice (Li et al., 2004;Xu and Zhang, 2006).
The objective of this study was to determine if immune challenge during the pubertal period decreases behavioral responsiveness to estradiol on cognitive tasks in ovariectomized mice. We hypothesized that, as is the case with other ovarian hormone-influenced behaviors, pubertal LPS treatment will decrease the ability of estradiol to enhance performance on hippocampus-dependent tasks.
Materials and Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts Amherst. Sixty-four CD1 female mice (n = 8 per group) were purchased from Charles River Laboratories (Kingston, NY), shipped at 3 weeks of age and housed in an all-female colony room under controlled temperature (24 ± 2°C) and reversed light: dark cycle (14:10; lights off at 1000h). Mice were housed in groups of four in Polycarbonate cages with ad libitum access to food (Teklad 2014, Harlan Laboratories, Madison, WI, phytoestrogen-reduced diet) and water supplied in glass water bottles. Cages were lined with a combination of pine wood shavings and CareFRESH (International Absorbents, Inc., Ferndale, WA) bedding, and a fresh Nestlet (Ancare Corp., Baltimore, NY) was placed in each cage when they were changed. Testing and handling took place during the dark phase of the illumination cycle under dim red light.
Bacterial endotoxin, lipopolysaccharide (LPS)
Mice habituated to their housing environment until they were treated with saline or LPS. All mice were injected intraperitoneally (IP) at 6 (pubertal experimental group) or 10 (adult negative control group) weeks of age at the end of the light cycle with either sterile saline or 1.5mg LPS/kg body weight (obtained from Escherichia coli serotype O26:B6; no. L3755; Sigma Chemical Co., St. Louis, MO) dissolved at a concentration of 0.1 mg/ml in sterile saline. Mice were returned to their home cage immediately after injection. Sickness behavior was scored as described below, and animals were weighed 24 and 48 hours following treatment to assess the effect of the LPS treatment on body weight. The dose of LPS was chosen based on previous work showing long-term effects on hormonal response (Laroche et al., 2009a), as well as previous work in which it caused only mild sickness lasting less than 48 hours under our housing conditions (Ismail et al., 2011).
Sickness behavior
Sickness behavior was scored 30 min, four, 24 and 48 hours following treatment by two observers, who were blind to the treatment conditions. All mice were evaluated with respect to the number of symptoms displayed (one symptom = score of 1; two symptoms = score of 2, three symptoms = score of 3) (Gibb et al., 2008). The symptoms examined were lethargy (diminished locomotion), huddling (curled body posture), and ptosis (drooping eyelids). This rating procedure correlates with other methods of sickness scoring, such as scoring the severity of each symptom independently on a four-point scale (Gandhi et al., 2007).
Ovariectomy and capsule implantation
Five weeks after saline or LPS treatment, when all the mice were adult (either 11 or 15 weeks old), they were anesthetized with isoflurane (3% at one l/min, inhaled). Incisions were made to the ventral skin and the muscle layer. The uterine horns were tied with absorbable suture, and the ovaries were removed. The muscle layer was sutured, and the skin incision was closed with wound clips. Then, an incision was made to the dorsal skin layer of the neck, a 2.5cm long Silastic© capsule (1.57mm I.D. × 3.18mm O.D) filled with 50 μg of 17β-estradiol dissolved in 25 μl of sesame oil or a vehicle implant filled only with oil was implanted. This estradiol implant yields plasma estradiol concentrations that approximate 85–120 pg/ml (Kudwa et al., 2009). The incision was closed with wound clips, and the mice were placed on a heating pad to recover until they were fully awake. They were then returned to their home cage with an additional water bottle containing a 3 % solution of Children’s Tylenol (160 mg of acetaminophen/5 ml dissolved in tap water) for 48 hours. Behavioral testing began one week later.
Behavioral testing
Object Recognition
All mice were tested during the dark phase of their light: dark cycle. Mice were placed in a square open arena (60 × 60 × 32cm) for five minutes to habituate to the environment. Using lines mapped out in the bottom of the open-field, the number of line crossings was recorded. Following habituation, two different objects were placed at opposite corners of the arena for three minutes. The time spent exploring each of the objects was recorded. Thirty minutes later, mice were again placed in the square arena for a 5-minute habituation period. Then, they were presented with the original object from the previous trial and a novel object at opposite corners of the arena for three minutes. The time spent exploring each of the objects was recorded. The objects were chosen because they were heavy enough that mice could not displace them, and results of a pilot experiment in a separate cohort of mice revealed that mice had no preference for either object or location in the arena, as they spent equal amounts of time exploring these objects, regardless of their position in the arena (adapted from (Summers et al., 2008)).
Object Placement Recognition
One week later, all mice were tested for object placement recognition during the dark phase of their light cycle. Mice were again placed in a square open arena (60 × 60 × 32cm) for five minutes to habituate to the environment. Using lines mapped out in the bottom of the open-field, the number of line crossings was recorded. Following habituation, two identical objects were placed at opposite corners of the arena for five minutes. The time spent exploring each of the objects was recorded. Thirty minutes later, mice were again placed in the square arena for a 5-minute habituation period. Then, they were presented with the same two objects, but the location of one of the objects was changed. The time spent exploring each of the objects during this five-minute trial, was recorded. As in the object recognition task, the objects used in this task were also selected based on the criteria that they were heavy enough that mice could not displace them and results of a pilot experiment in a separate cohort of mice revealed that mice had no preference for either object or location in the arena. This procedure was adapted from Luine et al., 2003 (Luine et al., 2003).
Social Discrimination
The social discrimination test began one week after the object placement test. All mice were tested during the dark phase of their light cycle. This procedure was adapted from (Engelmann et al., 1995). Mice were individually housed and tested in their home cage. A stimulus, juvenile, female mouse (3–4 weeks old) was placed in the cage of the test mouse for 5 minutes. Three hours later, the same and a novel juvenile mouse were placed simultaneously in the home cage of the test mouse. The duration of chemo-investigation of each animal was recorded for each trial. Chemo-investigation was operationally defined as sniffing of the juvenile’s head, torso or anogenital region. This test is based on the habituation-dishabituation paradigm and makes use of mice’s natural tendency towards olfactory investigation of novel conspecifics.
Social Recognition Test
One week following the social discrimination test, mice were tested for social recognition in their home cage. As in the previous tests, all mice were tested during the dark phase of their light cycle. Adult female mice were repeatedly exposed to the same juvenile female mouse for four, 5-minute trials, spaced 15-minute apart. Fifteen minutes after the fourth trial, the adult females were presented with a novel juvenile mouse for five minutes. The duration of chemo-investigation of each animal was recorded for each trial (Imwalle et al., 2002). Like the social discrimination test, this test is also based on the habituation-dishabituation paradigm. More specifically, this test also makes use of mice’s natural tendency towards olfactory investigation of novel conspecifics. Repeated exposure to a stimulus juvenile mouse leads to a marked decrease in the amount of time the test mouse spends investigating the stimulus juvenile mouse. On the last trial, when the test mouse is exposed to a novel juvenile mouse, the time spent investigating the juvenile mouse should increase and return to the original level if the test mouse has recognized the novel juvenile (Bielsky and Young, 2004). For this reason, the difference in the duration of investigation between the last two trials is analyzed to examine cognitive function and whether the test mouse successfully recognized the novel juvenile mouse.
Statistical Analysis
Three-way repeated measures analyses of variance (ANOVAs) were used to examine the effect of age (six or ten weeks old) and treatment (saline or LPS) on sickness response following saline or LPS treatment and on the percent change in body weight from baseline (on the day of the treatment) following treatment. Three-way ANOVAs were used to examine the effect of age (six or ten weeks old), treatment (saline or LPS) and hormone (oil or estradiol) on the percentage of time spent investigating novel object or placement (during object and object placement recognition) and chemo-investigation of novel juvenile (during social discrimination), and post hoc tests (t-tests) were used to assess pairwise contrasts when appropriate. To calculate the percentage of time spent investigating the novel object, placement or chemo-investigation of the novel conspecific, the time spent investigating novelty was divided by the total time spent investigating the novel and familiar object, placement or conspecific and multiplied by 100. Three-way repeated measures ANOVAs were also used to examine the effect of age (six or ten weeks old), treatment (saline or LPS), hormone (oil or estradiol) and testing trials (1–5) on chemo-investigation during the social recognition test. Post hoc t-tests were used to assess pairwise contrasts between trials 4 and 5. All analyses were run in SPSS Inc. statistical package 11.5 (Chicago, Illinois). Outliers were eliminated using the Boxplot method (Reimann et al., 2005). The criterion for statistical reliability was set at p < 0.05 and was corrected using the bonferroni correction to correct for an inflated alpha-level.
Results
Sickness behavior
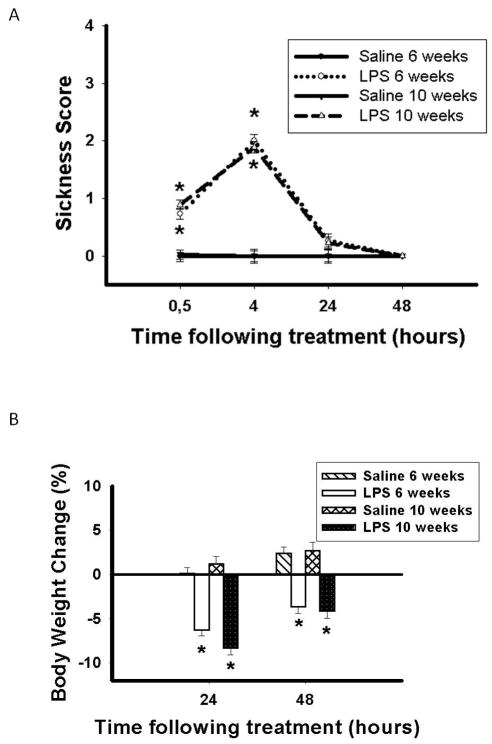
Statistical analyses revealed a main effect of time (0.5, 4, 24, 48 hours) following treatment (F(3, 183) = 120.45; p < 0.05), a main effect of treatment (saline or LPS) (F(1, 61) = 171.33; p < 0.05), and significant time x treatment interaction (F(3, 183) = 120.29; p < 0.05). Pairwise comparisons indicated that mice treated with LPS at six or ten weeks old displayed significantly more sickness symptoms than mice treated with saline at 30 min (p < 0.001 and p < 0.001, respectively) and 4 hours (p < 0.001 and p < 0.001, respectively) following treatment. Importantly, there was no difference in sickness symptoms between mice treated at 6 weeks old and those treated at 10 weeks old. Virtually no sickness symptoms were displayed 24 hours following treatment (Figure 1A).
Figure 1.
A) Sickness score (Mean ± SEM) and B) percent body weight change (Mean ± SEM) in C57Bl/6 mice treated with either saline or LPS at 6 or 10 weeks old. *Significantly greater than saline controls, p < 0.05.
Body weight
As expected, statistical analyses revealed a main effect of time (24 or 48 hours) following treatment (F(1, 61) = 54.10; p < 0.001), a main effect of treatment (saline or LPS) (F(1, 61) = 101.99; p < 0.001) and a significant time x treatment interaction (F(1, 61) = 4.75; p < 0.05). Pairwise comparisons indicated that mice treated with LPS at either age displayed significantly greater body weight loss 24 hours (p < 0.001 and p < 0.001, respectively) and 48 hours (p < 0.001 and p < 0.001, respectively) following treatment (Figure 1B).
Object Recognition
Overall statistical analyses revealed a main effect of hormone (oil or estradiol) (F(1, 46) = 20.36; p < 0.001), significant age x treatment (F(1, 46) = 10.86; p < 0.005), age x hormone (F(1, 46) = 6.01; p < 0.05), and treatment x hormone (F(1, 46) = 10.04; p < 0.005) interactions. There was also a tendency towards an age x treatment x hormone (F(1, 46) = 3.32; p = 0.075) interaction. In mice treated with saline at six weeks old, or with saline or LPS at 10 weeks, estradiol treatment in adulthood significantly increased the duration of investigation of the novel object compared to oil treatment (p < 0.01, p < 0.001, p < 0.01, respectively). In contrast, in mice treated with LPS at six weeks old, estradiol treatment failed to increase the duration of investigation of the novel object compared to oil treatment (Figure 2). The results from six mice had to be removed either because they did not examine both objects during trial 1 or because they were detected as outliers as per the boxplot method.
Figure 2.
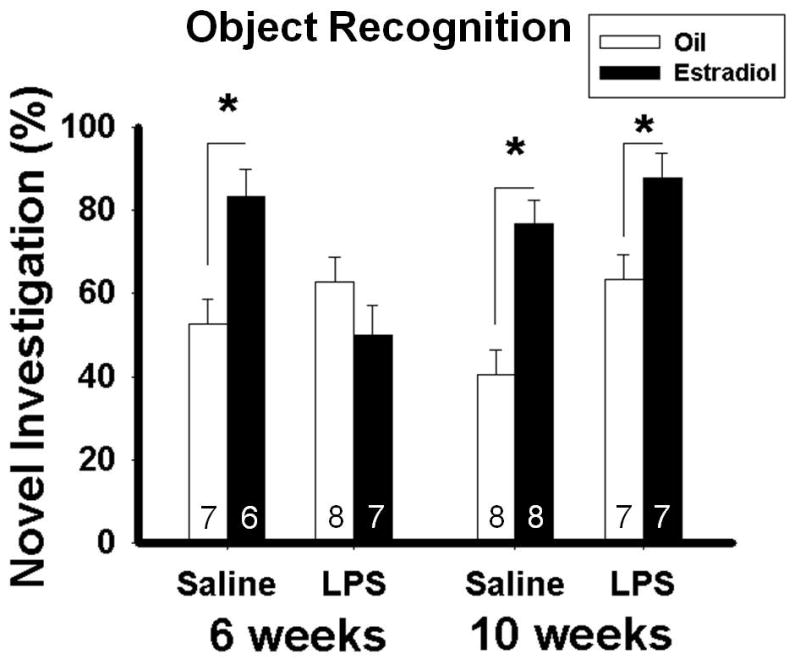
Percentage of time spent investigating the novel object (Mean ± SEM) during the object recognition test in mice treated with saline or LPS at six or ten weeks old and treated either with estradiol or oil vehicle in adulthood. *Significant difference between mice treated with estradiol and those treated with oil-vehicle, p < 0.05.
Object Placement Recognition
Overall statistical analyses revealed a main effect of hormone (oil or estradiol) (F(1, 47) = 13.50; p = 0.001). Pairwise comparisons showed that, in mice treated with saline at six weeks old, or with saline or LPS at 10 weeks old, estradiol treatment in adulthood significantly increased the duration of investigation of the object placement compared to oil treatment (p < 0.05, p < 0.05, p < 0.05, respectively). In contrast, in mice treated with LPS at six weeks old, estradiol treatment failed to increase the duration of investigation of the novel object compared to oil treatment (Figure 3). The results from five mice had to be removed either because they did not examine both object locations during trial 1 or because they were detected as outliers as per the boxplot method.
Figure 3.
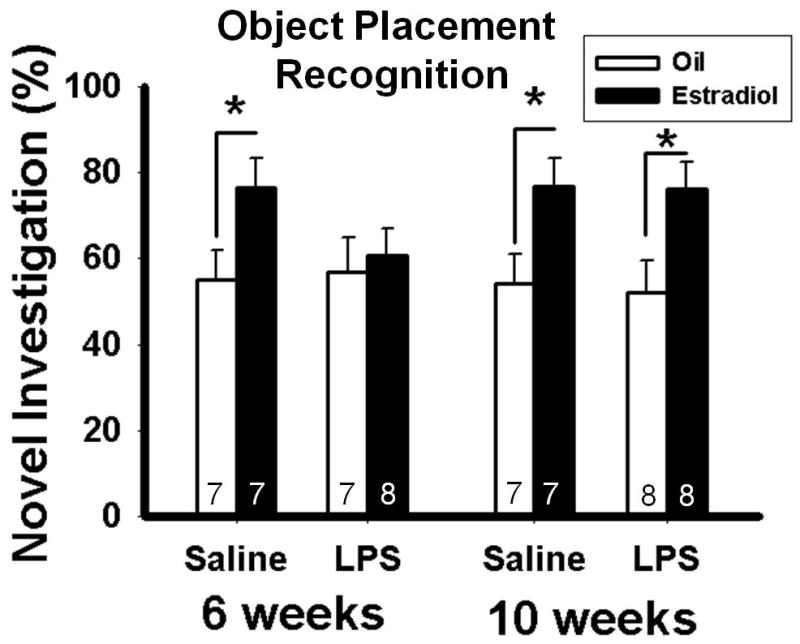
Percentage of time spent investigating the object in the novel location (Mean ± SEM) during the object placement recognition test in mice treated with saline or LPS at six or ten weeks old and treated either with estradiol or oil vehicle in adulthood. The number at the bottom of each bar represents the group size. *Significant difference between mice treated with estradiol and those treated with oil-vehicle, p < 0.05.
Social Discrimination
Similarly to the results from the object and object placement recognition, statistical analyses revealed a main effect of treatment (saline or LPS) (F(1, 51) = 15.81; p < 0.001) and of hormone (oil or estradiol) (F(1, 51) = 62.34; p < 0.001). There was a tendency towards a main effect of age (F(1, 51) = 3.42; p = 0.07). There were significant age x treatment (F(1, 51) = 10.73; p < 0.005), treatment x hormone (F(1, 51) = 10.69; p < 0.005), age x treatment x hormone (F(1, 51) = 9.80; p < 0.005) interactions. There was also a tendency towards an age x hormone (F(1, 50) = 3.05; p = 0.087) interaction. Most importantly, pairwise comparisons showed that, in mice treated with saline at six weeks old, or with saline or LPS at 10 weeks old, estradiol treatment in adulthood significantly increased the duration of chemo-investigation of the novel juvenile mouse compared to oil treatment (p < 0.001, p < 0.001, p < 0.001, respectively). However, in mice treated with LPS at six weeks old, estradiol treatment failed to increase the duration of chemo-investigation of the novel juvenile mouse compared to oil treatment (p > 0.05) (Figure 4). The results from five mice had to be removed because they were detected as outliers as per the boxplot method.
Figure 4.

Percentage of time spent investigating the novel stimulus mouse (Mean ± SEM) during the social discrimination test in mice treated with saline or LPS at six or ten weeks old and treated either with estradiol or oil vehicle in adulthood. The number at the bottom of each bar represents the group size. *Significant difference between mice treated with estradiol and those treated with oil-vehicle, p < 0.05.
Social recognition
Statistical analysis revealed main effects of trial (trials 1–5) (F(4, 220) = 13.54; p < 0.001) and of hormone (oil or estradiol) (F(1, 55) = 40.04; p < 0.001) and a significant trial x age interaction (F(4, 220) = 3.52; p < 0.01). There was also a tendency towards an age x hormone interaction (F(1, 55) = 3.29; p = 0.075). Pairwise comparisons between Trials 4 and 5 revealed no effect of LPS in mice treated at ten weeks old (data not shown). In mice treated with saline at six weeks old, estradiol treatment significantly increased the time spent investigating the novel juvenile on Trial 5 contrasted with the familiar juvenile on Trial 4 (p < 0.025). However, in mice treated with LPS at six weeks old, estradiol treatment failed to increase the time spent investigating the novel juvenile on Trial 5 contrasted with the familiar juvenile on Trial 4 (p < 0.05) (Figure 5). The results from two mice had to be removed because they were detected as outliers as per the boxplot method.
Figure 5.
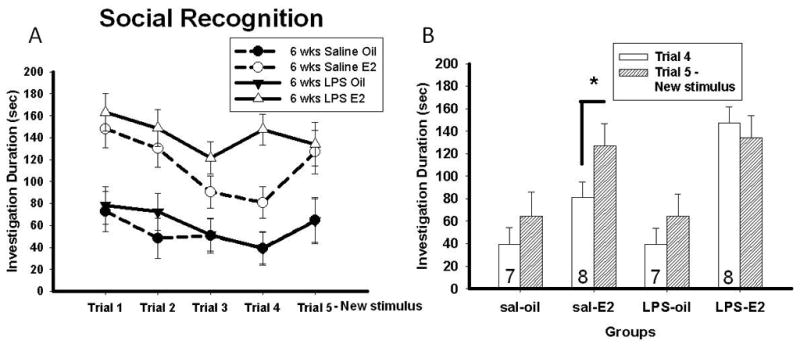
A) Duration of investigation of stimulus mouse across all five trials (Mean ± SEM) and B) duration of investigation of stimulus mouse on trials 4 and 5 (new stimulus mouse) (Mean ± SEM) in mice treated with either saline or LPS at six weeks old and treated either with estradiol or oil vehicle in adulthood. The number at the bottom of each bar represents the group size.*Significant difference in duration of investigation between trials 4 and 5 in mice treated with estradiol, p < 0.05.
Locomotion
We measured the number of lines crossed in the open field during the habituation period preceding the object and object place recognition and found no difference between our groups (data not shown).
Discussion
Exposure to stressors during the pubertal period has lasting effects on the behavioral responsiveness to estradiol and progesterone in adulthood. For example, pubertal exposure to either shipping or LPS reduces sexual receptivity in ovariectomized adult mice primed with estradiol and progesterone (Laroche et al., 2009a; 2009b;Ismail et al., 2011), and alters behavioral response to ovarian hormones on tests of depression and anxiety (Olesen et al., 2011; Ismail et al., in press). Estradiol also plays a prominent role in cognitive function. For example, estradiol treatment in ovariectomized mice also enhances performance on hippocampus-dependent tasks in mice (Li, Brake, Romeo et al. 2004; Xu & Zhang, 2006).
Because of the fact that particular pubertal stressors compromise the effects of ovarian hormones on a variety of behaviors in adulthood, and the fact that estradiol has profound influences on performance in particular cognitive tasks, we hypothesized that, in female mice treated pubertally with LPS, the cognitive enhancing effects of estradiol in adulthood would be depressed. Consistent with our predictions, estradiol failed to improve performance on hippocampus-dependent tasks in mice that were immune-challenged at six weeks old compared to mice treated with saline or to mice immune-challenged at ten weeks old. These findings extend to cognitive function, those endpoints of estradiol action which are compromised by pubertal immune challenge.
There is a controversy in the literature regarding the brain regions recruited during the object recognition task. While some researchers support the theory that it is hippocampus-dependent (Rolls et al., 1993;Clark et al., 2000;Clark et al., 2001), others argue that this task is dependent on the perirhinal cortex (Warburton et al., 2003). Recent findings suggest that both cortical regions are important for this task but on differential levels. The hippocampus seems to be important for object recognition when short-term memory (90 minutes) is tested, while the perirhinal cortex seems to be involved when long-term memory is examined (24 hours) (Balderas et al., 2012). Based on this, since in our study we examine object memory following a 3-hour delay period, we are likely still examining short-term memory and recruiting predominantly the hippocampus.
It is unlikely that the difference in the ability of estradiol to improve cognitive function between mice treated with LPS during the pubertal period or in adulthood is due to differences in LPS-induced sickness response. There was no difference in sickness symptoms following LPS treatment in mice treated with LPS at six or ten weeks old. There was only a slight difference in body weight loss as mice treated with LPS at six weeks old displayed a greater percentage of body weight loss than those treated at ten weeks old at 24 hours following treatment. This difference was not maintained at a later time point. Moreover, both groups recovered by 48 hours.
The hippocampus contains both types of classical estrogen receptors (ER), ERα and ERβ, suggesting that estrogens could affect directly the hippocampus, a major mediator of learning and memory (Barha and Galea, 2010). Our findings are consistent with previous work demonstrating that estradiol treatment enhances performance on hippocampus-dependent tasks in female rats (Korol and Kolo, 2002;Sandstrom and Williams, 2004;Daniel et al., 1997), mice (Xu and Zhang, 2006; Li, et al., 2004) and rhesus monkeys (Rapp et al., 2003;Lacreuse et al., 2002). This enhancement may be due in part to an improvement in memory consolidation (Spencer et al., 2008), because estradiol treatment after training on memory tasks still enhances performance on subsequent trials in ovariectomized rats (Luine et al., 2003;Packard and Teather, 1997).
Estradiol enhances performance on hippocampus dependent tasks and also alters cell morphology, synapse formation, membrane excitability, cell signaling pathways, neurotrophin systems, endogenous opioid systems, and neurogenesis (Spencer et al., 2008; Luine and Frankfurt, in press). For example, ovariectomy decreases the density of dendritic spines in the CA1 region of the hippocampus, and estradiol replacement increases synaptic protein expression throughout the hippocampal formation (Li et al., 2004). Moreover, natural fluctuations in estradiol levels across the estrous cycle mediate hippocampal synaptic density (Woolley and McEwen, 1992). Estradiol’s effect on the hippocampus is dependent on estrogen receptors; studies using estrogen receptor knockout mice indicate that estradiol’s ability to improve cognitive function is mediated primarily by estrogen receptor-α (Fugger et al., 2000). In future studies, it would be interesting to examine changes in estrogen receptor-α expression following pubertal LPS treatment.
In order to maintain a constant time interval across groups between the administration of the treatment and ovariectomy, the animals differed in age at the time of testing. However, because the animals were ovariectomized in adulthood, it is unlikely that the age at testing was responsible for the behavioral differences observed. In fact, in earlier work, C57BL/6 mice shipped at six weeks old and ovariectomized either one or seven weeks later displayed reduced behavioral responsiveness to estradiol and progesterone (Laroche et al., 2009b) relative to mice shipped in adulthood, suggesting that age at ovariectomy or testing is not responsible for the behavioral differences among groups.
Taken together, these findings demonstrate that immune challenge during the pubertal period, but not later, blocks estradiol’s ability to enhance cognitive function in outbred CD1 mice. These results are consistent with our recent reports that estradiol fails to decrease anxiety-like and depression-like behaviors (Olesen et al., 2011; Ismail et al., in press) in mice treated pubertally with LPS. Collectively, they suggest that exposure to particular stressors (in this case, immune challenge) during the pubertal period, but not later, remodels the brain resulting in alteration in response to ovarian hormone on a wide variety of behaviors.
Acknowledgments
We would like to thank Alex Zaltsman, Nassim Bouchentouf, Kaylyn Utley, Emmett Fitzpatrick, Bethany Rappleyea and Stacey Hronowski for technical assistance. This work was supported by NIH grant MH 093854, by the National Science Foundation under grant number 1050179, and an Isis grant from the Society for Women’s Health Research.
Footnotes
Nafissa Ismail designed and conducted the studies. She wrote this manuscript with Jeffrey Blaustein. All the research was conducted in Jeffrey Blaustein’s laboratory.
We have nothing to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Balderas I, Morin JP, Rodriguez-Ortiz CJ, Bermudez-Rattoni F. Muscarinic receptors activity in the perirhinal cortex and hippocampus has differential involvement in the formation of recognition memory. Neurobiol Learn Mem. 2012;97:418–424. doi: 10.1016/j.nlm.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta. 2010;1800:1056–1067. doi: 10.1016/j.bbagen.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Clark RE, West AN, Zola SM, Squire LR. Rats with lesions of the hippocampus are impaired on the delayed nonmatching-to-sample task. Hippocampus. 2001;11:176–186. doi: 10.1002/hipo.1035. [DOI] [PubMed] [Google Scholar]
- Clark RE, Zola SM, Squire LR. Impaired recognition memory in rats after damage to the hippocampus. J Neurosci. 2000;20:8853–8860. doi: 10.1523/JNEUROSCI.20-23-08853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Davis DM, Jacobson TK, Aliakbari S, Mizumori SJ. Differential effects of estrogen on hippocampal- and striatal-dependent learning. Neurobiol Learn Mem. 2005;84:132–137. doi: 10.1016/j.nlm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: an alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: hormonal activation of social and motivational tendencies. Brain Cogn. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883:258–264. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- Gandhi R, Hayley S, Gibb J, Merali Z, Anisman H. Influence of poly I:C on sickness behaviors, plasma cytokines, corticosterone and central monoamine activity: moderation by social stressors. Brain Behav Immun. 2007;21:477–489. doi: 10.1016/j.bbi.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Gibb J, Hayley S, Gandhi R, Poulter MO, Anisman H. Synergistic and additive actions of a psychosocial stressor and endotoxin challenge: Circulating and brain cytokines, plasma corticosterone and behavioral changes in mice. Brain Behav Immun. 2008;22:573–589. doi: 10.1016/j.bbi.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Imwalle DB, Scordalakes EM, Rissman EF. Estrogen receptor alpha influences socially motivated behaviors. Horm Behav. 2002;42:484–491. doi: 10.1006/hbeh.2002.1837. [DOI] [PubMed] [Google Scholar]
- Ismail N, Garas P, Blaustein JD. Long-term effects of pubertal stressors on female sexual receptivity and estrogen receptor-alpha expression in CD-1 female mice. Horm Behav. 2011;59:565–571. doi: 10.1016/j.yhbeh.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Kumlin AM, Blaustein JD. A pubertal immune challenge alters the antidepressant-like effects of chronic estradiol treatment in inbred and outbred adult female mice. Neuroscience. doi: 10.1016/j.neuroscience.2012.09.047. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korol DL. Role of estrogen in balancing contributions from multiple memory systems. Neurobiol Learn Mem. 2004;82:309–323. doi: 10.1016/j.nlm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Korol DL, Kolo LL. Estrogen-induced changes in place and response learning in young adult female rats. Behav Neurosci. 2002;116:411–420. doi: 10.1037//0735-7044.116.3.411. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Harada N, Honda SI, Rissman EF. Regulation of progestin receptors in medial amygdala: estradiol, phytoestrogens and sex. Physiol Behav. 2009;97:146–150. doi: 10.1016/j.physbeh.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacreuse A, Wilson ME, Herndon JG. Estradiol, but not raloxifene, improves aspects of spatial working memory in aged ovariectomized rhesus monkeys. Neurobiol Aging. 2002;23:589–600. doi: 10.1016/s0197-4580(02)00002-7. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Enduring influences of peripubertal/adolescent stressors on behavioral response to estradiol and progesterone in adult female mice. Endocrinology. 2009a;150:3717–3725. doi: 10.1210/en.2009-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009b;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–S45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Li C, Brake WG, Romeo RD, Dunlop JC, Gordon M, Buzescu R, Magarinos AM, Allen PB, Greengard P, Luine V, McEwen BS. Estrogen alters hippocampal dendritic spine shape and enhances synaptic protein immunoreactivity and spatial memory in female mice. Proc Natl Acad Sci U S A. 2004;101:2185–2190. doi: 10.1073/pnas.0307313101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. doi: 10.1016/j.yfrne.2012.07.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Luine VN, Richards ST, Wu VY, Beck KD. Estradiol enhances learning and memory in a spatial memory task and effects levels of monoaminergic neurotransmitters. Horm Behav. 1998;34:149–162. doi: 10.1006/hbeh.1998.1473. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen KM, Ismail N, Merchasin ED, Blaustein JD. Long-term alteration of anxiolytic effects of ovarian hormones in female mice by a peripubertal immune challenge. Horm Behav. 2011;60:318–326. doi: 10.1016/j.yhbeh.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Teather LA. Posttraining estradiol injections enhance memory in ovariectomized rats: cholinergic blockade and synergism. Neurobiol Learn Mem. 1997;68:172–188. doi: 10.1006/nlme.1997.3785. [DOI] [PubMed] [Google Scholar]
- Paris A, Kelly P, Ramaley JA. Effects on short-term stress upon fertility. II After puberty. Fertil Steril. 1973;24:546–552. doi: 10.1016/s0015-0282(16)39796-5. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann C, Filzmoser P, Garrett RG. Background and threshold: critical comparison of methods of determination. Sci Total Environ. 2005;346:1–16. doi: 10.1016/j.scitotenv.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Cahusac PM, Feigenbaum JD, Miyashita Y. Responses of single neurons in the hippocampus of the macaque related to recognition memory. Exp Brain Res. 1993;93:299–306. doi: 10.1007/BF00228398. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. doi: 10.1016/j.yhbeh.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–216. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- Schulz KM, Molenda-Figueira HA, Sisk CL. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm Behav. 2009;55:597–604. doi: 10.1016/j.yhbeh.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz KM, Sisk CL. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol Cell Endocrinol. 2006;254–255:120–126. doi: 10.1016/j.mce.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Spencer JL, Waters EM, Romeo RD, Wood GE, Milner TA, McEwen BS. Uncovering the mechanisms of estrogen effects on hippocampal function. Front Neuroendocrinol. 2008;29:219–237. doi: 10.1016/j.yfrne.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BL, Henry CM, Rofe AM, Coyle P. Dietary zinc supplementation during pregnancy prevents spatial and object recognition memory impairments caused by early prenatal ethanol exposure. Behav Brain Res. 2008;186:230–238. doi: 10.1016/j.bbr.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Ter Horst JP, de Kloet ER, Schachinger H, Oitzl MS. Relevance of stress and female sex hormones for emotion and cognition. Cell Mol Neurobiol. 2012;32:725–735. doi: 10.1007/s10571-011-9774-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, Aggleton JP, Bashir ZI, Brown MW. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang Z. Effects of estradiol benzoate on learning-memory behavior and synaptic structure in ovariectomized mice. Life Sci. 2006;79:1553–1560. doi: 10.1016/j.lfs.2006.04.020. [DOI] [PubMed] [Google Scholar]