Abstract
High-mobility group box 1 (HMGB1) was initially described as a damage-associated-molecular-pattern (DAMP) mediator that worsens acute brain injury after stroke. But recent findings suggest that HMGB1 can play a surprisingly beneficial role during stroke recovery by promoting endothelial progenitor cell (EPC) function and vascular remodeling in cortical gray matter. Here, we ask whether HMGB1 may also influence EPC responses in white matter injury. The standard lysophosphatidylcholine (LPC) injection model was used to induce focal demyelination in the corpus callosum of mice. Immunostaining showed that within the focal white matter lesions, HMGB1 was upregulated in GFAP-positive reactive astrocytes, along with the accumulation of Flk1/CD34-double positive EPCs that expressed pro-recovery mediators such as brain derived neurotrophic factor and basic fibroblast growth factor. Astrocyte-EPC signaling required the HMGB1 receptor RAGE since treatment with anti-RAGE antibodies significantly decreased EPC accumulation. Moreover, suppression of HMGB1 with siRNA in vivo significantly decreased EPC numbers in damaged white matter as well as proliferated endothelial cell numbers. Finally, in vitro cell culture systems confirmed that HMGB1 directly affected EPC function such as migration and tube formation. Taken together, our findings suggest that HMGB1 from reactive astrocytes may attract EPCs to promote recovery after white matter injury.
Keywords: endothelial progenitor cells (EPCs), high-mobility group box 1 (HMGB1), reactive astrocytes, white matter injury, white matter remodeling, neurovascular unit
Introduction
The pathophysiology of stroke and brain injury is highly complex. Both acute as well as chronic responses after injury involve multifactorial interactions between all cells in the neurovascular unit, comprising neuronal, glial, and vascular compartments (Carmichael 2006, Moskowitz et al. 2010, Zhang & Chopp 2009). More recently, it has been proposed that beyond cell-cell signaling within the brain per se, dynamic crosstalk between brain and systemic responses such as circulating blood cells may also be important (Ma et al. 2010, Meisel et al. 2005, Offner et al. 2006, Offner et al. 2009, Titova et al. 2008). In particular, endothelial progenitor cells (EPCs) that circulate in peripheral blood can be activated after stroke and these responses may significantly influence stroke outcome (Taguchi et al. 2004, Fan et al. 2010).
EPC function, migration and homing can be regulated by many different factors, including high-mobility group box 1 (HMGB1) (Chavakis et al. 2007). HMGB1 is a highly conserved non-histone nuclear DNA-binding protein, and widely expressed in most eukaryotic cells including neural cells (Yang et al. 2005). Traditionally, HMGB1 was proposed as a nuclear and cellular danger signal belonging to the damage-associated-molecular-pattern (DAMP) family of alarmins (Lotze & Tracey 2005). HMGB1 can be passively released from damaged cells and promote inflammation and cell death (Faraco et al. 2007, Goldstein et al. 2006). But while HMGB1 promotes injury in the acute phase after stroke, it may surprisingly show some beneficial effects in the chronic phase during recovery. HMGB1 signaling can promote endothelial activation and sprouting (Treutiger et al. 2003, Schlueter et al. 2005), and increase neurite outgrowth and cell survival (Huttunen et al. 2000, Huttunen et al. 2002, Passalacqua et al. 1998). Recently, our group showed that HMGB1 can be released from reactive astrocytes that augment EPC function and neurovascular remodeling in gray matter after stroke (Hayakawa et al. 2012a). In the present study, we now ask whether HMGB1 might also promote EPC responses in white matter.
Materials and Methods
Chemicals
Human recombinant HMGB1 was purchased from Sigma-Aldrich.
Antibodies
CD34 (1:100, Genway), Flk-1 (VEGFR2) (1:100, Abcam), RAGE (1:200, Abcam), BDNF (1:200, Abcam) and FGF2 (1:200, Santa cruz) were used for flow cytometory analysis. HMGB1 antibody (1:500, Abcam) and GFAP antibody (1:500, BD biosciences) were used for western blot or immunohistochemistory.
In vivo white matter injury model
This study was performed following an institutionally approved protocol in accordance with National Institutes of Health guidelines. For all experiments, group allocation, treatments and assessments were randomized and blinded. Three to 5 animals were prepared for each group in this study. Male C57BL6 mice (11–12 weeks, Charles River Laboratories) were deeply anaesthetized with isoflurane (1% to 2%) in 30%/70% oxygen/nitrous oxide. Lisophosphatidylcholine (LPC from Sigma) at a concentration of 10 µg/µl or 0.5 µl saline was injected through a 30-gauge needle over 5 minutes into the left corpus callosum. The placement coordinates were anterior: 0.5 mm from bregma, lateral: 1.0 mm from bregma, depth: 2.3 mm from the skull surface. Mouse RAGE antibody (50 mg/kg, R&D systems) or PBS (0.1 ml/10 g) was injected intraperitoneally on day 1 and day 3 after LPC injection.
siRNA Infusions in Mouse Brain
Three days after LPC injection, mice were stereotaxically injected control siRNA or HMGB1 siRNA in intra-cerebro-ventricular (i.c.v.). The placement coordinates for the left lateral ventricle were anteroposterior: 0.5 mm from bregma, lateral: 0.8 mm from bregma, depth: 2.5 mm from the skull surface. Control siRNA and HMGB1 siRNA were obtained from Santa Cruz Biotechnology, Inc. (CA, USA). The HMGB1 siRNA is a pool of 3 target-specific 19–25 nt siRNAs designed to knock down gene expression. The sequences for the mouse HMGB1 siRNAs are designed as followed; Sequence1: 5’-GGAGAGAUGUGGAACAACA-3’ Sequence2: 5’-CCAUUGUGGUAGGGUAACA-3’ Sequence3: 5’-GUACCUUCUAAUCCUUACA-3’. siRNAs for i.c.v. injection were prepared according to the in vivo siRNA transfection protocol for brain delivery from PolyPlus Transfection. Four µL of the siRNA complexes were i.c.v.-injected as 1 µL /min of flow rate of mice under anesthesia.
Immunohistochemistry
Mouse brains were taken after perfusion with PBS (pH 7.4) at day 5 after LPC injection and quickly frozen in liquid nitrogen. Coronal sections of 20-µm thickness were cut on cryostat at −20°C and collected on glass slides. Sections were fixed by cold acetone for 5 min, and rinsed three times in PBS (pH 7.4). After blocking with 3% bovine serum albumin (BSA), sections were then incubated at 4 °C overnight in a PBS solution containing the primary antibodies in PBS, 0.1% Tween 20, 0.3% BSA. Staining was performed for reactive astrocytes (GFAP) and HMGB1. The sections were washed and incubated for 1 h with secondary antibodies with fluorescence conjugations. Subsequently, the slides were covered with VECTASHIELD mounting medium with 4′, 6′-diamidino-2-phenylindole (DAPI) (H-1200 from Vector Laboratories).
Western Blotting
Tissue samples of corpus callosum were prepared using Pro-PREPTM Protein Extraction Solution (BOCA SCIENTIFIC). Samples were heated with equal volumes of SDS sample buffer (Novex) and 10 mM DTT at 95°C for 5 min, and then each sample (20 µg per lane) was loaded onto 4–20% Tris-glycine gels. After electorophoresis and transferring to polyvinylidene difluoride membranes (Novex), the membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 0.2% I-block (Tropix) for 90 min at room temperature. Membranes were then incubated overnight at 4°C with monoclonal anti-HMGB1 antibody (1:1000) followed by incubation with peroxidase-conjugated secondary antibodies and visualization by enhanced chemiluminescence (Amersham).
FACS analysis
White matter tissues were minced and then digested at 37C for 30 min with an enzyme cocktail (Collagenase typeIV, DNase I, Sigma Aldrich). Single cell suspensions were prepared by filtering through a 40-um strainer. After that, cell suspensions were pre-blocked with 3% BSA and then incubated with the following primary antibodies against CD34 (1:100, GenWay), VEGFR2/Flk1/KDR (1:100, Abcam), BDNF (1:100, Abcam), FGF2 (1:100, Santacruz), and RAGE (1:100, Abcam). Fluorescent-tagged Fab specific secondary antibodies from Jackson laboratories were incubated for 30 min at room temperature. Labeled cell populations were measured by FACS-Calibur (BD biosciences). FACS data were then analyzed by Cellquest pro software (BD biosciences). FACS analysis was performed using a variety of controls including unstained samples, isotype antibodies and single stained samples for determining appropriate gates, voltages, and compensations required in multivariate flow cytometry.
Cell cultures
The rat brain microendothelial cell line RBE.4 was maintained in EBM-2 containing EGM-2MV SingleQuots kit onto collagen-coated 25 cm2 flasks at a density of 2×105 cells/cm2 incubated in a 5% CO2 incubator at 37°C (Arai & Lo 2009a). Rat endothelial progenitor cells (EPCs) were prepared from rat spleens (Rosell et al. 2009). For each independent experiment, spleens from 11–12 weeks old Sprague-Dawley (SD) rats were kept in PBS solution. Under the hood, spleens were mechanically minced, placed at 37°C for 15 min and run through a 40-um nylon membrane to obtain cell suspension. After that, mononuclear cells (MNCs) were obtained by density gradient centrifugation with Ficoll-Paque Plus (Amersham Biosciences Corp). Isolated MNCs were shortly washed with red blood cells lysis solution and gently washed twice with complete growth media EGM-2MV (Lonza). MNCs were finally resuspended in EGM-2MV and 3 × 107 MNCs per well were seeded on collagen I-coated six-well plates (Becton Dickinson Labware) and incubated in a 5% CO2 incubator at 37°C. Under daily observation, first media change was performed 3–4 days after plating. Early EPCs (5–7 days after seeding) were used for the migration assay, and late EPCs (1–1.5 months after seeding) were for the tube formation assay.
In vitro trans-endothelial migration assay
Rat brain endothelial cells (RBE.4) (1×105 cells/well) were plated on polycarbonate membrane (3-um pore filters, Corning Costar) coated with collagen I to obtain confluent endothelial monolayer. Ac LDL-labeled EPCs (1×105 cells/well) were placed in the upper chamber on top of the RBE.4 monolayer. The chambers were placed in a 24-well culture plate containing HMGB1 (100 ng/ml). After 24 h of incubation at 37°C, labeled EPCs migrating into the lower chamber were counted in 4 random microscopic fields.
EPCs labeling
Cells were incubated with 5 ug/ml 1,19-dioctadecyl-3,3,39,39-tetramethylindocarbocyanine (DiI) labeled acetylated low density lipoprotein (ac-LDL; Molecular Probes) at 37°C for 120 min in EGM-2MV.
In vitro tube formation assay
The standard Matrigel assay was used to assess the spontaneous formation of capillary-like structures of the late EPCs. Standard 24-well plates were coated with 150 uL of cold Matrigel and allowed to solidify at 37°C for 30 min. Cells (5 ×104 cells/well) were seeded in that plates, and incubated at 37°C for 18 h.
Statistical analysis
Quantitative data were analyzed by using ANOVA followed by Tukey’s honestly significant difference tests. Data are expressed as mean ± S.E.M. A value of p < 0.05 was considered significant.
Results
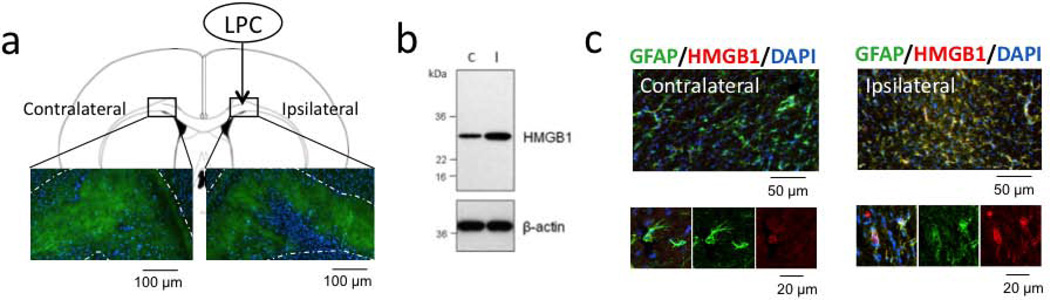
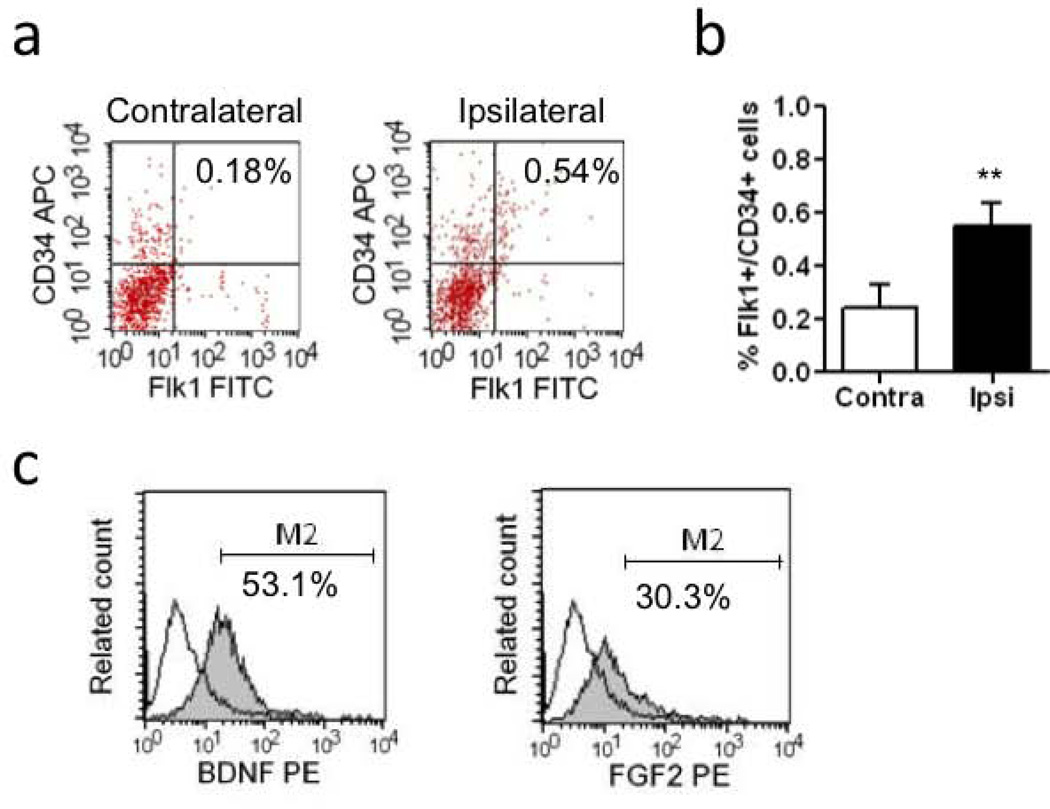
As expected, lysophosphatidylcholine (LPC) injections into the corpus callosum induced focal demyelination at 5 days (Figure 1a). HMGB1 expression was increased in the damaged white matter region (Figure 1b), with the majority of signals co-localizing with GFAP-positive reactive astrocytes (Figure 1c). Flow cytometry demonstrated an accumulation of Flk1 and CD34-double positive EPCs in these areas (Figure 2a–b). Further analysis showed that expression levels of brain derived neurotrophic factor and basic fibroblast growth factor were elevated in these EPCs within the damaged white matter regions (Figure 2c).
Figure 1. HMGB1 expression was increased in reactive astrocytes after white matter injury.
(a) Stereotaxic injection of LPC into the corpus callosum induced myelin damage in white matter tracts (green) on day 5. DAPI (blue) staining showed the cell accumulation inside the injury. N=3. (b) Western blot analysis showed the up-regulation of HMGB1 in an area of ipsilateral corpus callosum compared with contralateral side. C: contralateral side, I: ipsilateral side. N=4. (c) In ipsilateral injured area, reactive astrocytes mostly expressed HMGB1 in cell cytoplasm. N=3.
Figure 2. EPCs accumulation in ipsilateral side after white matter injury.
(a) Double positive cells for CD34 and Flk1 in ipsilateral side of white matter tract were assessed in order to detect endothelial progenitor cell (EPC) population in flow cytometory. (b) The data analysis showed significant increase of EPCs on day 5 after LPC injection. N=4. **P<0.01. (c) FACS analysis showed that trophic factors (BDNF and FGF-2) were expressed in the Flk1+/CD34+ EPC population. N=3.
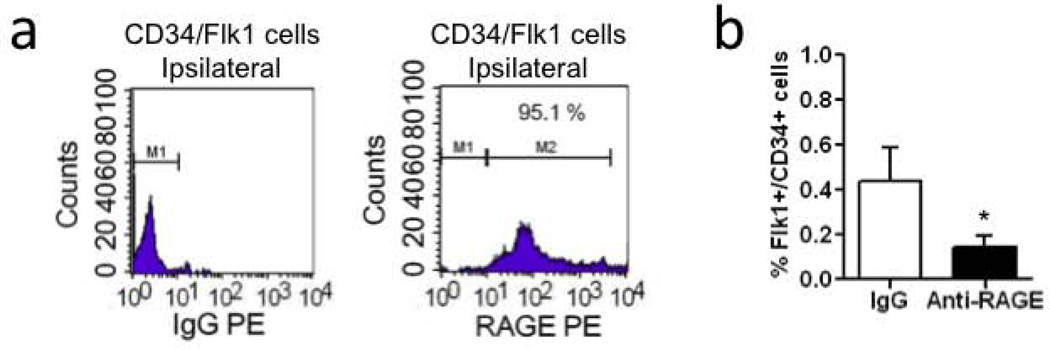
A key receptor for HMGB1 is the receptor for advanced glycation endproducts (RAGE). Flow cytometry confirmed that EPCs in damaged white matter were positive for RAGE (Figure 3a). These findings therefore suggest that HMGB1 released from reactive astrocytes can bind to RAGE receptors present on EPCs. To assess the functional significance of this cell-cell signaling, mice were treated with vehicle or neutralizing RAGE antibodies. Blockade of RAGE significantly reduced the accumulation of EPCs in LPC-damaged white matter (Figure 3b).
Figure 3. RAGE expression is required in EPCs accumulation after white matter injury.
(a) FACS analysis showed that accumulated Flk1 and CD34 double positive EPC subsets were positive for the HMGB1 receptor RAGE. N=3. (b) Peripheral treatment with neutralizing RAGE antibody significantly reduced EPCs accumulation on day 5 after LPC injection. N=5. *P<0.05.
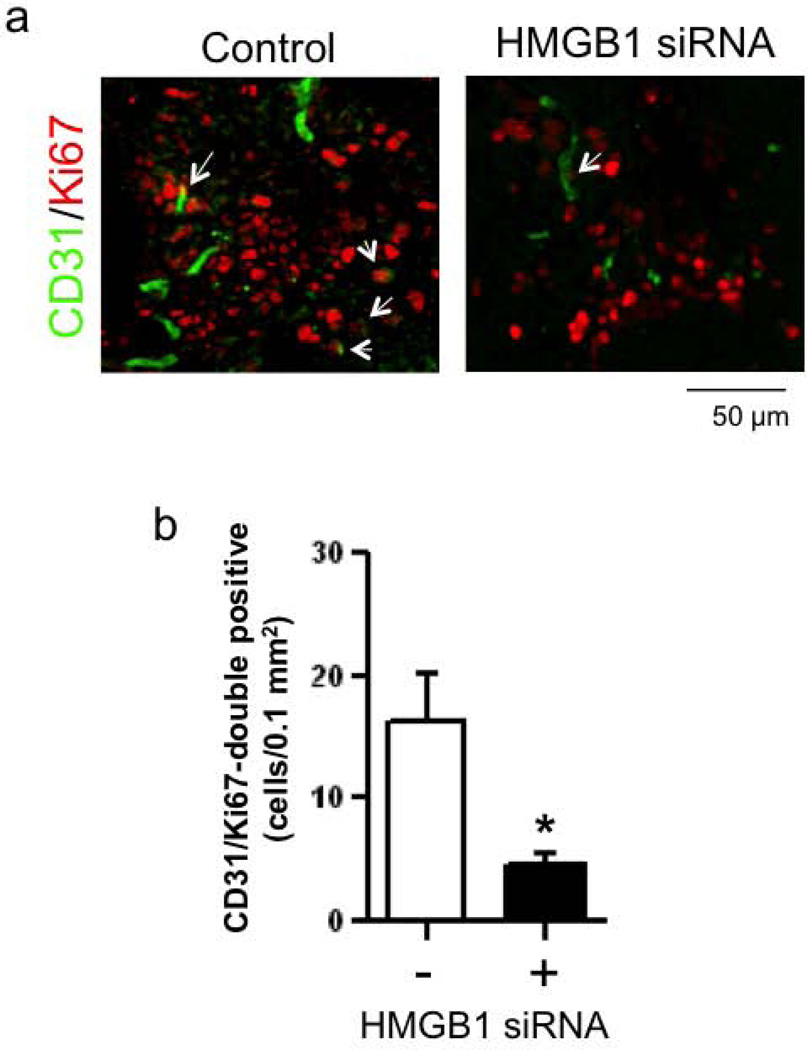
Next, we tested the ability of siRNA to interfere with HMGB1-mediated EPC responses in white matter. Western blot analysis confirmed that in vivo HMGB1 siRNA successfully downregulated HMGB1 expression in the focal LPC-induced white matter lesions (Figure 4a), without affecting the number of GFAP-positive astrocytes that were present (Figure 4b). Suppression of HMGB1 with siRNA led to a significant reduction in EPC accumulation (Figure 4c–d). Concommitantly, this reduction in EPC numbers occurred together with a decrease in microvascular response. Immunostaining with anti-CD31 (endothelial cell marker) and anti-Ki67 (proliferative cell marker) antibodies revealed that HMGB1-siRNA decreased the number of newly emerged endothelial cells within the white matter lesion area (Figure 5a–b).
Figure 4. Astrocytic HMGB1 is required in EPCs accumulation after white matter injury.
(a) HMGB1 siRNA was intracerebroventricularly injected on day 2 after LPC injection. Western blot showed HMGB1 expression levels in ipsilateral white matter tract on day 5 after LPC injection. HMGB1 siRNA successfully reduced HMGB1 protein levels in a dose-dependent manner. (b) Immunostaining analysis also showed that HMGB1 siRNA successfully decreased HMGB1 expressed by reactive astrocytes on day 5, without affecting the levels of GFAP-positive cells. (c–d) Flow cytometory showed that treatment with HMGB1 siRNA reduced the EPC accumulation in ipsilateral corpus callosum on day 5. N=5. *P<0.05.
Figure 5. HMGB1-siRNA treatment reduced proliferated endothelial number after white matter injury.
(a–b) HMGB1 siRNA was intracerebroventricularly injected on day 2 after LPC injection and brains were taken out three days later. Immunostaining showed that double positive cells with Ki67 and CD31 (i.e. newly emerged endothelial cells, arrows) were observed in the legion area, and the change was attenuated by HMGB1-siRNA treatment. N=5. *P<0.05.
Finally, cell culture experiments were conducted to directly test the effects of HMGB1 in EPCs. In a trans-well assay, HMGB1 significantly increased the migration of labeled EPCs (Figure 6a–b). In a matrigel assay, HMGB1 significantly increased the rates of tube formation and this pro-angiogenic effect was blocked with anti-RAGE antibodies (Figure 7a–b).
Figure 6. HMGB1 promoted the trans-migration of early EPCs in vitro.
(a) Schematics for our in vitro trans-migration assay. Cerebran endothelial RBE.4 cells were plated on the transwell, and once the cells were confluent, DiI-labeled early EPCs were added on the upper side. Twenty-four hours later, labeled EPCs in the lower chamber were counted. (b) HMGB1 (100 ng/mL) significantly promoted the migration of early EPCs. N=4. *P<0.05.
Figure 7. HMGB1 accelerated the tube formation of late EPCs in vitro.
(a) Representative images of tube formation in late EPC cultures. HMGB1: 1 ng/mL, anti-RAGE: 5 ug/mL. (b) HMGB1 significantly increased the number of tubes, and the HMGB1-induced tube formation was reduced by co-treatment with anti-RAGE neutralizing antibody. N=5. *P<0.05.
Discussion
The neurovascular unit describes a conceptual framework for cell-cell interactions in gray matter (del Zoppo 2009, Hawkins & Davis 2005, Iadecola 2004, Lo et al. 2004, Lo et al. 2003, Zacchigna et al. 2008, Zlokovic 2008). Correspondingly, an oligovascular niche supports signaling between oligodendrocytes and cerebral endothelium to promote oligodendrogenesis and angiogenesis in white matter (Arai & Lo 2009a, Pham et al. 2012, Hayakawa et al. 2012b). But beyond cell-cell interactions within the brain, interactions between brain and circulating blood cells are now known to be critically important as well (Ma et al. 2010, Meisel et al. 2005, Offner et al. 2006, Offner et al. 2009, Titova et al. 2008). In humans, circulating levels of EPCs tend to track the temporal profile of recovery from 7 to 14 days after stroke onset (Navarro-Sobrino et al. 2010, Navarro-Sobrino et al. 2011). In animal studies, peripheral administration of CD34+ cells enhanced endogenous neurogenesis via angiogenesis after stroke (Taguchi et al. 2004). EPCs can promote the migration of endothelial cells by releasing soluble factors such as VEGF, SDF-1 and IGF-1(Urbich et al. 2005), and EPC transplantation improved long-term stroke outcomes (Fan et al. 2010). Nevertheless, the role of EPCs has been primarily described for gray matter. The present study provides proof-of-concept that reactive astrocytes can produce HMGB1 that promotes EPCs in a mouse model of LPC-induced white matter injury. These findings may provide the basis for further investigating the crosstalk that exists between central white matter and peripheral responses after stroke, brain injury and neurodegeneration.
Our current study may have two important implications. First, although white matter damage is a key part of all neurological disorders, white matter mechanisms are relatively understudied compared to gray matter mechanisms. Our data here suggest that astrocytes may signal to EPCs after white matter injury. Indeed, low levels of circulating EPCs are associated with increased risk of age-related white matter changes in stroke and cognitive impairment (Fu et al. 2005, Jickling et al. 2009, Verdelho et al. 2007). Therefore, dissecting the mechanisms for interactions between white matter and circulating blood cells may lead us novel approaches for treating white matter dysfunction in CNS disorders. A second implication is that reactive astrocytes here show beneficial actions by promoting EPC function. The reactive astrocyte is a seminal feature of damaged or diseased brain. Traditionally, reactive astrocytic scars were thought to be detrimental because they can secrete several inhibitory substrates that retard axonal and dendritic plasticity (Silver & Miller 2004b). However, a more nuanced view of the reactive astrocyte has been recently proposed. Reactive astrocytes can also release many trophic factors (Silver & Miller 2004a, Strauss et al. 1994, Tower & Young 1973). These trophic factors may be beneficial in the chronic phase after brain injury by promoting neuronal survival and augmenting coordinated responses in synaptogenesis, neurogenesis, and angiogenesis (Mocchetti & Wrathall 1995, Tokita et al. 2001). Our findings here suggest that, along with growth factors, HMGB1 may also be positive factor by allowing reactive astrocytes to signal to pro-recovery EPCs in white matter.
Taken together, our current findings suggest that HMGB1 mediates the crosstalk between reactive astrocytes and circulating EPCs after white matter injury. Nevertheless, there are a few caveats that need to be considered for future studies. First, we only focused on the beneficial role of HMGB1. But HMGB1 has been well known as a deleterious factor after injury (Andersson et al. 2000, Dai et al. 2010, Park et al. 2003). How the recovering brains balance the deleterious versus beneficial actions of HMGB1 should be carefully dissected. Second, there are other receptors for HMGB1 such as the toll-like receptors TLR2 and TLR4 (Lotze & Tracey 2005). TLR2 and TLR4 are both expressed in brain cells (Caso et al. 2007, Hua et al. 2007, Tang et al. 2007), and they have been shown to play a critical role in infectious diseases (Tang et al. 2007, Mishra et al. 2006, Laflamme et al. 2003). Therefore, examining roles of TLR2 and TLR4 on brain remodeling would be promising direction to understand the brain pathophysiology. Third, other cells besides EPCs may also be affected by HMGB1 released from astrocytes. Further studies are warranted to examine these multi-cellular effects including those that should occur in oligodendrocyte precursor cells (Woodruff et al. 2004). Fourth, although the LPC model is commonly used in the literature, non-specific chemical toxicity is a serious caveat. Exploring these HMGB1 mechanisms in other models of white matter injury such as endothelin-1-injection (Sozmen et al. 2009) or chronic cerebral hypoperfusion (Shibata et al. 2004) will be useful. Fifth, we use siRNA and RAGE blocking antibodies for proof-of-concept. Future studies using more potent RAGE inhibitors (Deane et al. 2012, Carnevale et al. 2012) will be required for translational relevance. Finally, the precise mechanisms for how the accumulated EPCs can contribute to white matter remodeling remain to be fully assessed. Do the accumulated EPCs directly promote vascular remodeling by differentiating into mature endothelial cells? Or do they modulate the local environment for remodeling by secreting trophic factors? Our current data show that accumulated EPCs produce BDNF and FGF-2, which are well-known as pro-survival factors. But, besides those two growth factors, many other trophic factors such as VEGF should also play important roles for remodeling the neurovascular unit (Manoonkitiwongsa 2011). In white matter, trophic coupling between oligodendrocytes and cerebral endothelial cells in the oligovascular niche supports ongoing oligodendrogenesis and angiogenesis (Arai & Lo 2009b). Therefore, dissecting the roles of EPC-derived trophic factors may contribute to another aspect of the oligovascular niche in white matter remodeling after injury.
Pathophysiologic responses after brain injury are highly complex. Beyond the cell-cell interaction within the brain, factors outside the brain may affect the brain homeostasis. Our current study demonstrates that reactive astrocytes secrete HMGB1 that may attract circulating EPCs to damaged tissue in white matter injury. These findings provide proof-of-concept for a novel mechanism of dynamic crosstalk between white matter and circulating blood cells. Understanding the precise mechanisms of the communication between reactive astrocytes and EPCs will ultimately lead us new therapeutic strategies for white matter related diseases including stroke and vascular dementia.
Acknowledgements
Supported in part by the Deane Foundation, MGH ECOR Fund for Medical Discovery, American Heart Association, National Institutes of Health, Research Abroad from the Japan Society for the Promotion of Science, National Research Foundation of Korea, the World Class University Program, and the Global Research Laboratory Program.
References
- Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo E. An oligovascular niche: cerebral endothelial cells promote the survival and proliferation of oligodendrocyte precursor cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009a;29:4351–4356. doi: 10.1523/JNEUROSCI.0035-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K, Lo E. Oligovascular signaling in white matter stroke. Biological & pharmaceutical bulletin. 2009b;32:1639–1683. doi: 10.1248/bpb.32.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006;59:735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Mascio G, D'Andrea I, et al. Hypertension induces brain beta-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012;60:188–197. doi: 10.1161/HYPERTENSIONAHA.112.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- Dai S, Sodhi C, Cetin S, et al. Extracellular high mobility group box-1 (HMGB1) inhibits enterocyte migration via activation of Toll-like receptor-4 and increased cell-matrix adhesiveness. J Biol Chem. 2010;285:4995–5002. doi: 10.1074/jbc.M109.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Singh I, Sagare AP, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–1392. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67:488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco G, Fossati S, Bianchi ME, Patrone M, Pedrazzi M, Sparatore B, Moroni F, Chiarugi A. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J Neurochem. 2007;103:590–603. doi: 10.1111/j.1471-4159.2007.04788.x. [DOI] [PubMed] [Google Scholar]
- Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76:793–796. doi: 10.1136/jnnp.2003.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RS, Gallowitsch-Puerta M, Yang L, et al. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571–574. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Pham LD, Katusic ZS, Arai K, Lo EH. Astrocytic high-mobility group box 1 promotes endothelial progenitor cell-mediated neurovascular remodeling during stroke recovery. Proc Natl Acad Sci U S A. 2012a;109:7505–7510. doi: 10.1073/pnas.1121146109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K, Seo J, Pham L-D, Miyamoto N, Som A, Guo S, Kim K-W, Lo E, Arai K. Cerebral endothelial derived vascular endothelial growth factor promotes the migration but not the proliferation of oligodendrocyte precursor cells in vitro. Neuroscience letters. 2012b;513:42–48. doi: 10.1016/j.neulet.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, et al. Activation of Toll-like receptor 4 signaling contributes to hippocampal neuronal death following global cerebral ischemia/reperfusion. J Neuroimmunol. 2007;190:101–111. doi: 10.1016/j.jneuroim.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Rauvala H. Receptor for advanced glycation end products (RAGE) signaling induces CREB-dependent chromogranin expression during neuronal differentiation. J Biol Chem. 2002;277:38635–38646. doi: 10.1074/jbc.M202515200. [DOI] [PubMed] [Google Scholar]
- Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. 2000;275:40096–40105. doi: 10.1074/jbc.M006993200. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Jickling G, Salam A, Mohammad A, Hussain MS, Scozzafava J, Nasser AM, Jeerakathil T, Shuaib A, Camicioli R. Circulating endothelial progenitor cells and age-related white matter changes. Stroke. 2009;40:3191–3196. doi: 10.1161/STROKEAHA.109.554527. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Echchannaoui H, Landmann R, Rivest S. Cooperation between toll-like receptor 2 and 4 in the brain of mice challenged with cell wall components derived from gram-negative and gram-positive bacteria. Eur J Immunol. 2003;33:1127–1138. doi: 10.1002/eji.200323821. [DOI] [PubMed] [Google Scholar]
- Lo EH, Broderick JP, Moskowitz MA. tPA and proteolysis in the neurovascular unit. Stroke. 2004;35:354–356. doi: 10.1161/01.STR.0000115164.80010.8A. [DOI] [PubMed] [Google Scholar]
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoonkitiwongsa PS. Critical questions for preclinical trials on safety and efficacy of vascular endothelial growth factor-based therapeutic angiogenesis for ischemic stroke. CNS Neurol Disord Drug Targets. 2011;10:215–234. doi: 10.2174/187152711794480447. [DOI] [PubMed] [Google Scholar]
- Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- Mishra BB, Mishra PK, Teale JM. Expression and distribution of Toll-like receptors in the brain during murine neurocysticercosis. J Neuroimmunol. 2006;181:46–56. doi: 10.1016/j.jneuroim.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocchetti I, Wrathall JR. Neurotrophic factors in central nervous system trauma. J Neurotrauma. 1995;12:853–870. doi: 10.1089/neu.1995.12.853. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Boada C, Domingues-Montanari S, Ribo M, Alvarez-Sabin J, Montaner J. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis. 2011;216:205–211. doi: 10.1016/j.atherosclerosis.2011.01.030. [DOI] [PubMed] [Google Scholar]
- Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, Penalba A, Ribo M, Alvarez-Sabin J, Montaner J. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res. 2010;80:317–323. doi: 10.1016/j.mvr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Arcaroli J, Yum HK, et al. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–C879. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- Passalacqua M, Patrone M, Picotti GB, Del Rio M, Sparatore B, Melloni E, Pontremoli S. Stimulated astrocytes release high-mobility group 1 protein, an inducer of LAN-5 neuroblastoma cell differentiation. Neuroscience. 1998;82:1021–1028. doi: 10.1016/s0306-4522(97)00352-7. [DOI] [PubMed] [Google Scholar]
- Pham LD, Hayakawa K, Seo JH, et al. Crosstalk between oligodendrocytes and cerebral endothelium contributes to vascular remodeling after white matter injury. Glia. 2012 doi: 10.1002/glia.22320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell A, Arai K, Lok J, He T, Guo S, Navarro M, Montaner J, Katusic ZS, Lo EH. Interleukin-1beta augments angiogenic responses of murine endothelial progenitor cells in vitro. J Cereb Blood Flow Metab. 2009;29:933–943. doi: 10.1038/jcbfm.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter C, Weber H, Meyer B, Rogalla P, Roser K, Hauke S, Bullerdiek J. Angiogenetic signaling through hypoxia: HMGB1: an angiogenetic switch molecule. Am J Pathol. 2005;166:1259–1263. doi: 10.1016/S0002-9440(10)62344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata M, Ohtani R, Ihara M, Tomimoto H. White matter lesions and glial activation in a novel mouse model of chronic cerebral hypoperfusion. Stroke. 2004;35:2598–2603. doi: 10.1161/01.STR.0000143725.19053.60. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004a;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004b;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sozmen EG, Kolekar A, Havton LA, Carmichael ST. A white matter stroke model in the mouse: axonal damage, progenitor responses and MRI correlates. J Neurosci Methods. 2009;180:261–272. doi: 10.1016/j.jneumeth.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss S, Otten U, Joggerst B, Pluss K, Volk B. Increased levels of nerve growth factor (NGF) protein and mRNA and reactive gliosis following kainic acid injection into the rat striatum. Neurosci Lett. 1994;168:193–196. doi: 10.1016/0304-3940(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, et al. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titova E, Kevil CG, Ostrowski RP, Rojas H, Liu S, Zhang JH, Tang J. Deficiency of CD18 gene reduces brain edema in experimental intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2008;105:85–87. doi: 10.1007/978-3-211-09469-3_17. [DOI] [PubMed] [Google Scholar]
- Tokita Y, Keino H, Matsui F, Aono S, Ishiguro H, Higashiyama S, Oohira A. Regulation of neuregulin expression in the injured rat brain and cultured astrocytes. J Neurosci. 2001;21:1257–1264. doi: 10.1523/JNEUROSCI.21-04-01257.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower DB, Young OM. The activities of butyrylcholinesterase and carbonic anhydrase, the rate of anaerobic glycolysis, and the question of a constant density of glial cells in cerebral cortices of various mammalian species from mouse to whale. J Neurochem. 1973;20:269–278. doi: 10.1111/j.1471-4159.1973.tb12126.x. [DOI] [PubMed] [Google Scholar]
- Treutiger CJ, Mullins GE, Johansson AS, et al. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–385. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Verdelho A, Madureira S, Ferro JM, et al. Differential impact of cerebral white matter changes, diabetes, hypertension and stroke on cognitive performance among non-disabled elderly. The LADIS study. J Neurol Neurosurg Psychiatry. 2007;78:1325–1330. doi: 10.1136/jnnp.2006.110361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Mol Cell Neurosci. 2004;25:252–262. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol. 2005;78:1–8. doi: 10.1189/jlb.1104648. [DOI] [PubMed] [Google Scholar]
- Zacchigna S, Lambrechts D, Carmeliet P. Neurovascular signalling defects in neurodegeneration. Nat Rev Neurosci. 2008;9:169–181. doi: 10.1038/nrn2336. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]