Abstract
Dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (NAcc) mediate the behavioral and motivational effects of many drugs of abuse, including nicotine. Repeated intermittent administration of these drugs, a pattern often associated with initial drug exposure, sensitizes the reactivity of dopamine (DA) neurons in this pathway, enhances the locomotor behaviors the drugs emit, and promotes their pursuit and self-administration. Here we show that activation of nicotinic acetylcholine receptors (nAChRs) in the VTA, but not the NAcc, is essential for the induction of locomotor sensitization by nicotine. Repeated intermittent nicotine exposure (4 × 0.4 mg/kg, base, i.p., administered over 7 days), a regimen leading to long-lasting locomotor sensitization, also produced upregulation of nAChRs in the VTA, but not the NAcc, in the hours following the last exposure injection. Functional nAChR upregulation was observed selectively in DA but not GABA neurons in the VTA. These effects were followed by long-term potentiation of excitatory inputs to these cells and increased nicotine-evoked DA overflow in the NAcc. Withdrawal symptoms were not observed following this exposure regimen. Thus, intermittent activation and upregulation by nicotine of nAChRs in DA neurons in the VTA may contribute to the development of behavioral sensitization and increased liability for nicotine addiction.
Keywords: dopaminergic neurons, GABAergic neurons, LTP, nAChR upregulation, nucleus accumbens, sensitization, ventral tegmental area
Introduction
Nicotine and other psychomotor stimulants produce their motivating effects by stimulating the mesoaccumbens dopamine (DA) system. Repeated intermittent injection of these drugs, a pattern often associated with initial exposure, enhances drug-induced DA neurotransmission and locomotor activity and these effects can persist for weeks to months (Vezina et al., 2007). The persistence of these effects and their extension to enhanced drug self-administration in animals has led to the proposal that drug sensitization has mechanistic overlap with the development of addiction in humans (Robinson & Berridge, 1993; Vezina, 2004). Ultimately, drug-induced changes in mesoaccumbens DA neurotransmission and associated behaviors arise from cellular and synaptic changes that we are only starting to decipher (Dani et al., 2001; Changeux, 2010). Adaptations occur at glutamatergic synapses in the VTA following exposure to psychomotor stimulants including nicotine (Mansvelder & McGehee, 2000; Ungless et al., 2001; Mansvelder et al., 2002; Saal et al., 2003; Mao et al., 2011). In addition, nicotinic acetylcholine receptors (nAChRs) undergo an upregulation in ligand binding and function following nicotine exposure (Buisson & Bertrand, 2001; Mugnaini et al., 2002; Vallejo et al., 2005; Govind et al., 2009, 2012).
nAChRs are expressed both on the cell bodies and terminals of mesoaccumbens DA neurons as well as by non-DA cells and afferent terminals in the VTA (Changeux, 2010). Activation of nAChRs in either the VTA or the NAcc can regulate DA release (Nisell et al., 1994; Zhang & Sulzer, 2004) and the motivation to self-administer nicotine (Maskos et al., 2005; Brunzell et al., 2010). Thus, alterations in nAChR function and neuroadaptations initiated in these sites by repeated nAChR activation could conceivably change the ability of nicotine to stimulate mesoaccumbens DA activity, increase locomotion and maintain self-administration behaviors.
In many studies examining the effects of nicotine exposure, rodents are exposed to intense continuous nicotine exposure regimens lasting days to weeks (Vezina et al., 2007). When discontinued, these regimens produce withdrawal symptoms with magnitude and duration corresponding to the intensity and length of exposure (Epping-Jordon et al., 1998; Skjei & Markou, 2003). These regimens can upregulate radioligand binding to nAChRs in a number of brain regions including the VTA and NAcc (Mugnaini et al., 2002). They also specifically upregulate α4-containing nAChRs in midbrain GABA neurons and increase inhibition of mesoaccumbens DA activity in the period immediately following exposure, when withdrawal symptoms are prevalent (Nashmi et al., 2007). These preclinical findings have been argued to support opponent process views of drug taking (Koob & Le Moal, 1997) and, in particular, to model the maintenance of tobacco use once it is established.
Here we explore the cellular, synaptic and behavioral consequences of exposure to a moderate, intermittent nicotine exposure regimen, a pattern often associated with initial exposure to the drug that is not associated with withdrawal symptoms but known to produce long-lasting sensitization. Our findings identify nicotine-induced neuroadaptations that may contribute to the development of increased liability for nicotine addiction.
Materials and Methods
Subjects and surgery
Male Sprague Dawley rats (180–250g on arrival from Harlan Sprague-Dawley, Madison, WI) were housed in a reverse light-dark cycle room with free access to food and water. They were allowed to acclimate for 1 week before any procedures. In some experiments, rats were anesthetized with a mix of ketamine (100 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.) and surgically implanted with chronic guide cannulae either aimed unilaterally at the NAcc core [anteroposterior (A/P), +3.4; lateral (L), ±1.5; dorsoventral (D/V), −6.5 to −8.5; angled at 10°] for microdialysis or bilaterally either at the VTA (A/P, −3.6; L, ±0.6; D/V, −8.9; 16°) or the NAcc core (AP, +3.4; L, ±1.5; DV, −7.5; 10°) for locomotion, using procedures described previously (Vezina et al., 2002). Coordinates are in mm from bregma and skull with the incisor bar positioned 5.0 mm above the interaural line. Only rats with the active portion of the microdialysis probe or both injection cannula tips positioned in the targeted nucleus were included in the data analyses. All experimental procedures were performed in accordance with an approved Institutional Animal Care and Use Committee protocol.
Design and procedure
Experiments involved two phases: drug exposure and test. During the exposure phase, rats received 4 injections of nicotine (0.4 mg/kg, i.p., 1 every other day). This moderate intermittent regimen is known to produce long-lasting sensitization (Schofelmeer et al., 2002). Control rats received saline. After the first and fourth injections, rats were placed in locomotor activity monitoring chambers for 2 hrs. The remaining injections were administered in the rats’ home cage. The test phase involved administering nicotine at various times following the last exposure injection. Nicotine [(−) nicotine tartrate; Sigma Inc., Saint Louis, MO] was dissolved in saline and its pH adjusted to 7.0 with NaOH. Nicotine doses are expressed as free base.
Locomotion
To assess the effect of nAChR blockade in the VTA and NAcc, the broad-spectrum nAChR antagonist mecamylamine (0, 10 or 30 μg/0.5μl/side; Tocris, Ellisville, MO) was administered intracranially immediately before each nicotine exposure injection. Testing for sensitization was conducted 3 weeks later. After 1 hr of habituation to the locomotor chambers, rats were injected with nicotine (0.4 mg/kg, i.p.) and locomotor activity measured for 2 hrs. No receptor antagonists were administered on this test. The locomotor activity monitoring chambers and intracranial microinjections were as described previously (Vezina et al., 2002).
As there is evidence that mecamylamine may block NMDA receptors at high concentrations (Snell & Johnson, 1989), cases in which it was found to block the induction of sensitization by nicotine (e.g., after VTA infusion) were confirmed in a separate experiment testing the effect of bilateral infusion of a combination of dihydro-β-erythroidine (DHβE; 10 μg/side; Tocris, Ellisville, MO) and methyllycaconitine (MLA; 3 μg/side; Tocris, Ellisville, MO), receptor antagonists with non-α7 and α7 nAChR profiles, respectively. Doses were selected from published reports of dose-effect functions for these ligands (Panagis et al., 2000; Bruijnzeel & Markou, 2004).
nAChR upregulation: 125I-epibatidine binding
Rats were sacrificed by rapid decapitation 2 hrs, 3 days, or 3 weeks after the last exposure injection and brains rapidly removed and flash-frozen on dry ice. Punches of VTA (1 mm) and NAcc (2 mm) were subsequently obtained bilaterally from 1mm-thick coronal slices. These were homogenized on ice in hypotonic lysis buffer containing protease inhibitors. Supernatant was processed to isolate the membrane portion of the sample by ultracentrifugation. The pellet was re-suspended in PBS and washed three times. Binding to membrane fractions was measured at room temperature by incubating triplicate samples in PBS with 0.5 nM 125I-epibatidine (PerkinElmer, Waltham, MA) for 30 min on a table top rotator. It was terminated by vacuum filtration through Whatman (Clifton, NJ) GF/B filters presoaked in 0.5% polyethyleneimine using a Brandel (Gaithersburg, MD) 24 channel cell harvester. Bound 125I-epibatidine (2200 Ci/mmol) was determined by gamma counting (PerkinElmer Wallac 1470, Gaithersburg, MD) with nonspecific binding estimated by 125I-epibatidine binding in a saturating concentration of nicotine (1mM). Total protein concentration in each brain membrane sample was measured by micro BCA (Pierce). 125I-epibatidine binding was subsequently expressed as fmol/mg protein.
nAChR upregulation: Slice electrophysiology
Electrophysiology procedures were as described previously (Fagan et al., 2007). Immediately after the last nicotine exposure injection, rats were anesthetized with isofluorane and the brain removed to ice-cold sucrose-artificial cerebrospinal fluid (sucrose-aCSF) with 10 mM ascorbic acid (in mM: 252 sucrose, 2.5 KCl, 1 MgCl2, 2.5 CaCl, 20 glucose, 25 NaHCO3; bubbled with 95%O2/5%CO2; pH=7.4). Horizontal slices (250 μm) including the VTA were made with a Vibrating Microtome (Leica). Slices were transferred to a 32°C holding chamber with normal aCSF (sucrose replaced by 125 mM NaCl) and perfused at ~20 ml/min for at least 15 min prior to recording.
Cells were visualized using infrared illumination on an upright microscope (Axioskop FS, Zeiss). Recording electrodes (5–8MΩ) were filled with potassium-gluconate internal solution (in mM: 154 K-gluconate, 1 KCl, 1 EGTA, 10 HEPES, 10 Glucose, 5 ATP, 0.1 GTP; pH adjusted to 7.4 with KOH). Series resistance for recordings was 5–30MΩ. Whole-cell voltage-clamp recordings were conducted at room temperature with constant bath perfusion at ~3 ml/min with normal aCSF containing DNQX (10 μM), bicuculine (20 μM), atropine (1 μM), TTX (1 μM), and Cd2+ (100 μM) to isolate nAChR currents. All cells were analyzed for the presence of the Ih current while holding at −50mV, and stepping to −115mV at −15mV increments. Cells with Ih≥150 pA at −115mV (large Ih currents), slow pacemaker-like firing rate (<5 Hz), long action potential duration (>2 ms), and anatomical location within the VTA were considered dopaminergic. The population of neurons with Ih<100 pA at −115mV (small Ih currents) were considered GABA-enriched. Neurons were selected within an area approximately 0.6 mm to 0.1 mm from the medial border of the medial terminal nucleus (MT), extending approximately 0.3 mm in A/P directions from the center of the MT (in mm from bregma: L, 0.75 to 1.25; A/P, −5.1 to −5.6; D/V, −7.9 to −8.4). This region has a small degree of overlap with the medial extreme of the substantia nigra compacta and a small number of DA neurons in this area may have been included in the data analyses. These methods of cell identification are supported by recent reports from our group and others (Zhang et al., 2010; Mao et al., 2011). To elicit nAChR currents, 1 mM acetylcholine (ACh) was focally applied (300 ms) using a Picospritzer II (General Valve). To limit response variability, pipettes had uniform tip diameter, pressures for drug delivery were equal, and the pipette tip was always 50–60 μm from the soma. Currents were analyzed off-line using Clampfit (Axon Instruments, Inc.). Only nAChR responses with a rise time of <50ms were included in the analyses to limit confounding effects of receptor desensitization. ACh-induced currents were converted to current density (pA/pF), pooled in time bins following the last nicotine exposure injection and averaged.
Synaptic plasticity
The ratio of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) to N-methyl-D-aspartate (NMDA) receptor (AMPAR/NMDAR) components of excitatory postsynaptic currents (EPSCs) were determined in brain slices taken from rats 17–26 hrs after the last nicotine exposure injection. This assay provides a relative assessment of AMPA receptor trafficking using previously described methods (Mao et al., 2011).
Nicotine withdrawal symptoms
The day after the last nicotine exposure injection, rats were administered the nAChR antagonist mecamylamine (1.0 mg/kg, s.c.; O’Dell et al., 2006), placed in a circular plastic observation chamber and the frequency of the following somatic signs of precipitated withdrawal recorded at three times (20–30, 60–70, and 110–120 minutes after the mecamylamine injection) and summed for each rat: writhes, cheek tremors, teeth chattering, yawns, and body shakes (Epping-Jordan et al., 1998). To provide comparative data, separate rats were exposed to an intense continuous exposure regimen lasting two weeks. These rats were surgically prepared with osmotic mini-pumps (2ML2; Alzet, Cupertino, CA) set to deliver 3.0 mg/kg/day s.c. of nicotine (Epping-Jordan et al., 1998). Control rats were fitted with a dummy pump. Pumps were removed after two weeks and precipitated withdrawal signs recorded the following day.
NAcc DA
The day after the last exposure injection, extracellular levels of DA in the NAcc core were assessed with microdialysis before and after a challenge injection of nicotine (0.4 mg/kg, i.p.). Comparative data were again provided by rats exposed to 3.0mg/kg/day of nicotine for two weeks and tested the day following pump removal. The day before testing, concentric microdialysis probes were lowered into the NAcc core and perfused with modified Ringer’s dialysate at 0.3 μl/min overnight and 1.5 μl/min during testing the following day. Microdialysis samples were collected at 20 minute intervals, 3 before and 9 after the nicotine challenge injection. No significant group differences were detected in the basal DA levels observed in the 1 hour before the nicotine challenge injection [intermittent: exposure, F1,12=0.01, NS, time, F2,24=0.9, NS, exposureXtime, F2,24=2.54, NS; continuous: exposure, F1,20=0.01, NS, time, F2,40=2.76, NS, exposureXtime, F2,40=1.79, NS; analyzed by 2-way between-within ANOVA with exposure as the between factor and time as the within factor]. The maximal DA level achieved after the nicotine challenge was recorded. All microdialysis and chromatography procedures were as described previously (Vezina et al., 2002).
Data analyses
Locomotor data were analyzed with 2-way between analyses of variance (ANOVA) with exposure and mecamylamine (or DHβE+MLA) as the two factors. The nAChR epibatidine binding data were also analyzed with 2-way between ANOVA but with exposure and test time as the two factors. Current density data were analyzed with between-within ANOVA with exposure as the between factor and test time as the within factor. All post-hoc comparisons were conducted with the Scheffé test. Remaining data (somatic signs, NAcc DA) were analyzed using independent samples t-tests.
Results
Activation of nAChRs in the VTA but not the NAcc is necessary for locomotor sensitization by nicotine
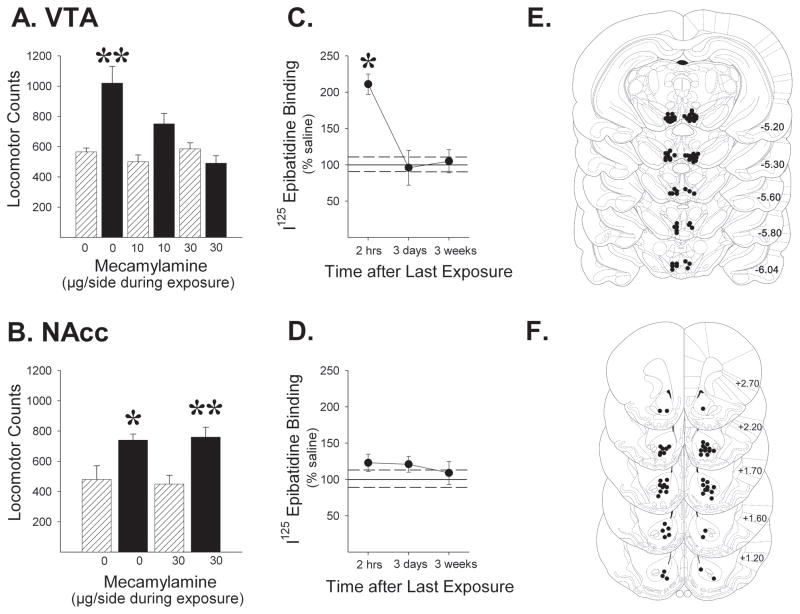
As expected, previous exposure to repeated intermittent nicotine produced robust locomotor sensitization. A subsequent nicotine challenge (0.4 mg/kg, IP) given 3 weeks after the final exposure injection produced a greater locomotor response in nicotine vs. saline exposed animals (Fig. 1; up to 2-fold increase; P<0.05–0.01). Bilateral infusion of the broad-spectrum nAChR antagonist mecamylamine into the VTA prior to each exposure injection dose-dependently blocked the induction of locomotor sensitization, as evidenced by the lack of enhanced responding on this test (Fig. 1A; exposure, F1,30=13.19, P<0.01; mecamylamine, F2,30=6.06, P<0.01; exposure x mecamylamine interaction, F2,30=7.15, P<0.01). Similarly blocking nAChRs in the NAcc during nicotine exposure had no effect on the induction of sensitization (Fig. 1B; exposure, F1,22=24.48, P<0.001; mecamylamine, F1,22=0.002, NS; exposure x mecamylamine interaction, F1,22=0.28, NS). As with VTA mecamylamine, infusing DHβE+MLA into the VTA prior to each nicotine exposure injection also blocked the induction of locomotor sensitization. These receptor antagonists exhibit non-α7 (DHβE) and α7 (MLA) nAChR profiles. No evidence for sensitization was observed in these rats. Means ± SEMs obtained were 554.0±33.17 (VTA vehicle IP saline, n=6), 1014.67±132.08 (VTA vehicle IP nicotine, n=6), 483.57±74.74 (VTA DHβE+MLA IP saline, n=7), and 642.25±110.51 (VTA DHβE+MLA IP nicotine, n=8). The ANOVA conducted on these data revealed significant effects of exposure (F1,23=9.25, P<0.01) and DHβE+MLA (F1,23=4.73, P<0.05) but a non-significant exposure x DHβE+MLA interaction (F1,23=2.20, NS). Again, VTA vehicle rats exposed to nicotine showed greater nicotine-evoked locomotor counts on the sensitization test compared to their saline exposed controls (P<0.05). This was not observed in VTA DHβE+MLA rats. These results indicate that nAChR activation in the VTA but not the NAcc is critical for the induction of locomotor sensitization by nicotine.
Figure 1.
Blockade of nAChRs in the VTA (A), but not the NAcc (B), prevents locomotor sensitization by nicotine. Rats were exposed to 4 injections of nicotine (0.4mg/kg over 7 days, i.p.; ▬) or saline (
 ), with or without an i.c. injection of the nAChR antagonist mecamylamine and tested 3 weeks later for their locomotor response to nicotine (0.4 mg/kg). Mecamylamine was not injected on this test. n/group=6–7. Data are shown as group mean (+SEM) 2-hr session total locomotor counts obtained after the challenge injection. Line drawings (Paxinos & Watson, 1997; numbers indicate mm from bregma) indicating location of injection cannula tips in the VTA (E) and NAcc (F) are shown for rats included in the data analyses. C. Exposure to the same regimen increased 125I-epibatidine binding in the VTA observed at 2-hrs but not 3 days or 3 weeks following exposure. D. Significant nAChR upregulation was not observed in the NAcc at any time tested. n/group=7–10. Data (mean±SEMs) are expressed as % of saline controls (solid and dashed lines). Values for the saline control groups ranged from 90.0±15.81–130.09±10.22 fmol/mg protein in VTA and 55.68±6.96–64.62±8.91 fmol/mg protein in NAcc. No significant differences between control groups were observed in either the VTA (F(2,24)=2.07, NS) or the NAcc (F(2,32)=0.40, NS). *, p<0.05, **, p<0.01, compared to respective saline exposure controls.
), with or without an i.c. injection of the nAChR antagonist mecamylamine and tested 3 weeks later for their locomotor response to nicotine (0.4 mg/kg). Mecamylamine was not injected on this test. n/group=6–7. Data are shown as group mean (+SEM) 2-hr session total locomotor counts obtained after the challenge injection. Line drawings (Paxinos & Watson, 1997; numbers indicate mm from bregma) indicating location of injection cannula tips in the VTA (E) and NAcc (F) are shown for rats included in the data analyses. C. Exposure to the same regimen increased 125I-epibatidine binding in the VTA observed at 2-hrs but not 3 days or 3 weeks following exposure. D. Significant nAChR upregulation was not observed in the NAcc at any time tested. n/group=7–10. Data (mean±SEMs) are expressed as % of saline controls (solid and dashed lines). Values for the saline control groups ranged from 90.0±15.81–130.09±10.22 fmol/mg protein in VTA and 55.68±6.96–64.62±8.91 fmol/mg protein in NAcc. No significant differences between control groups were observed in either the VTA (F(2,24)=2.07, NS) or the NAcc (F(2,32)=0.40, NS). *, p<0.05, **, p<0.01, compared to respective saline exposure controls.
Intermittent nicotine exposure upregulates nAChR radioligand binding in the VTA but not the NAcc
To test whether nicotine exposure alters nAChRs in either of these regions, tissue punches were obtained from the VTA and NAcc of nicotine- and saline-exposed rats for binding assays with radiolabeled epibatidine, a high affinity nAChR agonist. Repeated intermittent nicotine exposure increased epibatidine binding in the VTA at 2 hours, but not 3 days or 3 weeks following nicotine exposure (Fig. 1C; >2-fold increase relative to saline-exposure; P<0.05). The ANOVA conducted on these data (expressed as % of saline-exposed controls) revealed significant effects of exposure (F1,52=4.31, P<0.05) and time (F2,52=3.96, P<0.05) as well as a significant exposure x time interaction (F2,52=3.96, P<0.05). No significant effects of nicotine exposure on epibatidine binding were observed in the NAcc at any of the times tested (Fig. 1D; exposure, F1,67=2.56, NS; time, F2,67=0.13, NS, exposure x time interaction, F2,67=0.13, NS). Thus, transient upregulation was observed in VTA nAChRs that are critical for the induction of sensitization by nicotine.
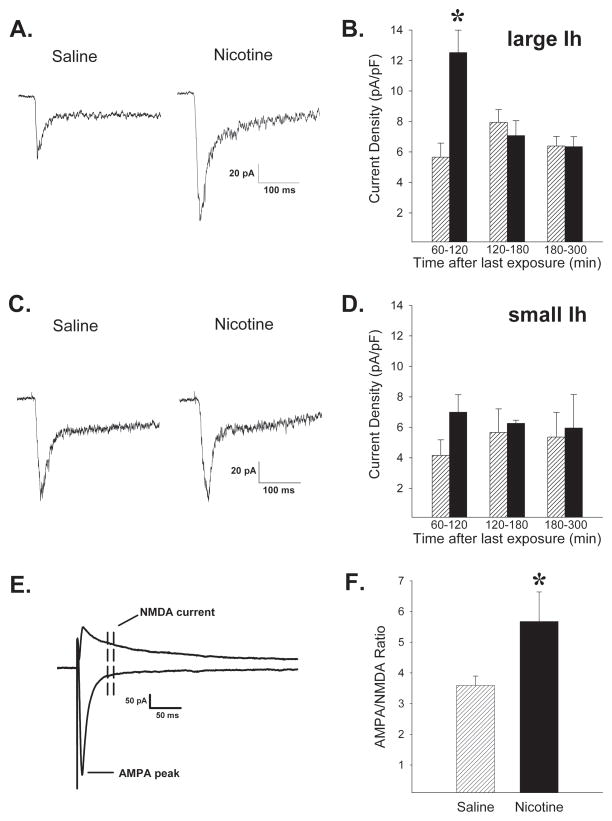
Intermittent nicotine exposure functionally upregulates nAChRs specifically in VTA DA neurons
To further assess the extent and time course of nicotine induced functional upregulation of nAChRs in the VTA, electrophysiological measures of nicotinic currents were obtained in horizontal brain slices prepared from rats previously exposed to repeated intermittent nicotine or saline. Pharmacologically isolated whole-cell nAChR inward currents were elicited by pressure application of 1mM ACh onto identified VTA neuronal somata in the presence of atropine (1μM) to block muscarinic effects. DA neurons were identified on the basis of cell size, location, action potential properties, and large Ih currents (Mao et al 2011), while cells with small Ih currents were considered GABA-enriched neurons. The ACh responses were larger in the DA (large Ih) neurons of nicotine- relative to saline-exposed rats (Fig. 2A–B; exposure, F(1,86)=6.03, P<0.05, time, F(2,86)=3.81, P<0.05, exposure-time interaction, F(2,86)=6.68, P<0.01). The effect decayed rapidly and was seen only in recordings performed ≤2 hrs after the last nicotine exposure injection (P<0.05). Studies conducted in cultured rat cortical neurons have observed similar transient nAChR upregulation that manifests rapidly, decays within 2 hrs, and corresponds to rapid changes in the conformation of the receptor (Govind et al., 2012). Thus, paralleling the epibatidine binding results in the present experiments, exposure to a sensitizing nicotine regimen produced a transient functional upregulation of nAChRs in VTA DA neurons. Notably, functional upregulation was not observed in the small Ih current expressing GABA-enriched neurons (Fig. 2C–D; exposure, F(1,42)=0.86, NS; time, F(2,42)=0.02, NS; exposure-time interaction, F(2,42)=0.33, NS). These findings are consistent with enhanced momentary excitability in VTA DA neurons that could favor longer-lasting potentiation of excitatory inputs to these cells by facilitating NMDA receptor dependent plasticity (Mao et al., 2011). Thus, the status of synaptic plasticity was assessed by determining the AMPAR/NMDAR ratios of the excitatory inputs to VTA DA (large Ih) neurons. When tested 17–26 hrs after the last exposure injection, the AMPAR/NMDAR ratios were larger in tissue from nicotine relative to saline exposed rats (Fig. 2E–F; t(25)=2.05, P<0.05). These results suggest higher synaptic levels of AMPA relative to NMDA receptors following repeated intermittent nicotine injections and are consistent with the induction of long term potentiation at these synapses (Ungless et al., 2001). No differences in rectification of AMPA receptor EPSCs were observed between nicotine and saline exposed rats, suggesting that the relative contribution of GluR1- vs GluR2-containing AMPA receptors was similar following synaptic modulation (data not shown).
Figure 2.
Exposure to sensitizing nicotine injections (4 × 0.4mg/kg over 7 days, i.p.; ▬) leads to functional upregulation of nAChRs in VTA DA neurons and potentiation of excitatory inputs to these cells compared to saline exposed controls (
 ).Traces of nAChR current responses to 300 msec focal application of 1 mM ACh onto (A) DA (large Ih) and (C) GABA-enriched (small Ih) neurons ≤120 min after the last saline or nicotine exposure injection. B. Average peak currents normalized to cell capacitance were significantly greater in DA (large Ih) neurons of nicotine exposed rats up to 2 hours following the last nicotine exposure injection. n=38,54 cells from 10,10 rats exposed to nicotine, saline, respectively. D. No significant effects of nicotine exposure were detected in GABA-enriched neurons (small Ih). n=23,25 cells from 14,15 rats. E. Traces of evoked AMPA and NMDA EPSCs. Peak AMPA current was measured at −70 mV and the NMDA current was averaged from a window 30–40 msec after the peak of the EPSC at +40 mV. F. Mean AMPAR/NMDAR ratios were significantly greater in DA (large Ih) neurons from nicotine relative to saline exposed animals. n=13,14 cells from 8,9 rats. *, p<0.05, compared to respective saline exposure controls.
).Traces of nAChR current responses to 300 msec focal application of 1 mM ACh onto (A) DA (large Ih) and (C) GABA-enriched (small Ih) neurons ≤120 min after the last saline or nicotine exposure injection. B. Average peak currents normalized to cell capacitance were significantly greater in DA (large Ih) neurons of nicotine exposed rats up to 2 hours following the last nicotine exposure injection. n=38,54 cells from 10,10 rats exposed to nicotine, saline, respectively. D. No significant effects of nicotine exposure were detected in GABA-enriched neurons (small Ih). n=23,25 cells from 14,15 rats. E. Traces of evoked AMPA and NMDA EPSCs. Peak AMPA current was measured at −70 mV and the NMDA current was averaged from a window 30–40 msec after the peak of the EPSC at +40 mV. F. Mean AMPAR/NMDAR ratios were significantly greater in DA (large Ih) neurons from nicotine relative to saline exposed animals. n=13,14 cells from 8,9 rats. *, p<0.05, compared to respective saline exposure controls.
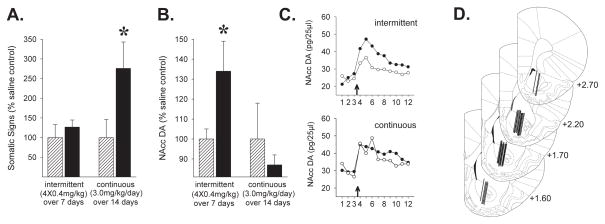
Absence of withdrawal symptoms and enhanced DA neuron reactivity following moderate intermittent nicotine exposure
Unlike more intense continuous exposure regimens often used to model the effects of smoking in humans (high nicotine concentrations delivered by osmotic minipump over 7 or more days; Epping-Jordan et al., 1998; Rahman et al., 2004; Nashmi et al., 2007), the moderate intermittent exposure regimen used in the present experiments (4 × 0.4 mg/kg, i.p., 1 injection every other day) did not produce withdrawal symptoms and increased nicotine-induced DA overflow in the period soon after exposure. One day following exposure, the nAChR antagonist mecamylamine failed to elicit somatic signs of withdrawal in nicotine relative to saline exposed rats (Fig. 3A; t(10)=0.82, NS). In contrast and as expected, rats exposed to an intense continuous exposure regimen (3.0 mg/kg/day over two weeks) exhibited a significant increase in evoked withdrawal signs at this time (Fig. 3A; t(9)=1.84, P<0.05). In addition, nicotine induced NAcc DA overflow was significantly enhanced relative to controls one day following moderate intermittent exposure (Fig. 3B; t(12)=2.27, P<0.05), a time when increased synaptic levels of AMPA receptors could have acted synergistically with nAChR stimulation to potentiate activation by nicotine of VTA DA neurons (Carlezon & Nestler, 2002; Saal et al., 2003). This effect was not observed following the intense continuous exposure regimen (Fig. 3B; t(19)=0.39, NS). To ease comparison of the different effects, data are illustrated and were analyzed as % of saline-exposed control values. These findings are consistent with the VTA DA neuron specific transient upregulation of nAChRs and the subsequent increase in the AMPAR/NMDAR ratio observed following intermittent nicotine exposure in the present experiments as well as the VTA GABA neuron specific nAChR upregulation and concurrent manifestation of withdrawal symptoms reported by others after intense continuous nicotine exposure (Epping-Jordan et al., 1998; Rahman et al., 2004; Nashmi et al., 2007).
Figure 3.
Evoked somatic signs of withdrawal (A) and maximal nicotine-evoked NAcc DA overflow (B) following an intermittent (4 × 0.4mg/kg, i.p. over 7 days) or continuous (3.0 mg/kg/day, s.c. over 14 days) nicotine exposure regimen (▬). Compared to saline exposed controls (
 ), evoked withdrawal signs were elevated 1-day following continuous but not intermittent nicotine. Nicotine-induced NAcc DA overflow was enhanced 1-day following intermittent but not continuous nicotine. n/group=5–11. Data (mean±SEMs) are expressed as % of saline-exposed controls. Values for the saline control groups were 4.5±2.1 (continuous) and 6.0±1.78 (intermittent) for number of somatic signs and 54.8±10.6 pg/25μl (continuous) and 38.5±1.7 pg/25μl (intermittent) for maximal nicotine-evoked NAcc DA overflow. No significant differences between control groups were observed for either somatic signs (t(10)=0.54, NS) or NAcc DA (t(17)=1.29, NS). *, p<0.05, compared to respective saline exposure controls. C. Time course for DA levels (pg/25μl) in the NAcc before and after the nicotine challenge injection (arrows at abscissae) for both exposure conditions. Numbers at abscissae indicate 20-min sampling intervals. ●, nicotine exposed; ○, saline exposed. D. Line drawings (Paxinos & Watson, 1997; numbers indicate mm from bregma) illustrating the location of the active portion of the microdialysis probes in the NAcc of rats included in the data analyses.
), evoked withdrawal signs were elevated 1-day following continuous but not intermittent nicotine. Nicotine-induced NAcc DA overflow was enhanced 1-day following intermittent but not continuous nicotine. n/group=5–11. Data (mean±SEMs) are expressed as % of saline-exposed controls. Values for the saline control groups were 4.5±2.1 (continuous) and 6.0±1.78 (intermittent) for number of somatic signs and 54.8±10.6 pg/25μl (continuous) and 38.5±1.7 pg/25μl (intermittent) for maximal nicotine-evoked NAcc DA overflow. No significant differences between control groups were observed for either somatic signs (t(10)=0.54, NS) or NAcc DA (t(17)=1.29, NS). *, p<0.05, compared to respective saline exposure controls. C. Time course for DA levels (pg/25μl) in the NAcc before and after the nicotine challenge injection (arrows at abscissae) for both exposure conditions. Numbers at abscissae indicate 20-min sampling intervals. ●, nicotine exposed; ○, saline exposed. D. Line drawings (Paxinos & Watson, 1997; numbers indicate mm from bregma) illustrating the location of the active portion of the microdialysis probes in the NAcc of rats included in the data analyses.
Discussion
The present results show that the induction of sensitization by intermittent nicotine requires nAChR activation in the VTA but not the NAcc. Further, VTA but not NAcc nAChRs showed nicotine-induced upregulation of radioligand binding in the hours following the last nicotine exposure injection and functional upregulation was observed selectively in DA but not GABA neurons in the VTA. These findings provide a mechanistic basis whereby repeated intermittent exposure to nicotine, a pattern often associated with initial exposure to tobacco, can ultimately lead to long-lasting predisposition to pursue the drug.
Intense continuous nicotine exposure regimens often used to model smoking in humans are associated with somatic signs of withdrawal (Epping-Jordan et al., 1998; present results). Together with upregulation of nAChRs in VTA GABA neurons (Nashmi et al., 2007) and inhibition of neighboring DA neurons (Rahman et al., 2004; present results), these could conceivably lead to a dysphoric state that contributes to maintenance of nicotine intake once it is established (Koob & Le Moal, 1997). Conversely, in the present experiments, exposure to moderate intermittent nicotine was not associated with somatic withdrawal signs but with transient upregulation of nAChRs in VTA DA neurons followed by long-term potentiation of excitatory inputs to these cells and increased nicotine-evoked DA overflow. These conditions in turn promote long-lasting sensitization of DA neuron reactivity, drug-induced locomotion and self-administration (Vezina, 2004; Vezina et al., 2007). Indeed, exposure to nicotine under these conditions has been shown to enhance the subsequent self-administration of nicotine (Neugebauer et al., 2012), amphetamine (Cortright et al., 2012), and cocaine (Horger et al., 1992).
How different nicotine exposure conditions cause such different patterns of nAChR upregulation in DA versus GABA neurons in the VTA remains an open question. Repeated intermittent nicotine exposure may favor upregulation of nAChRs in DA neurons as they, in contrast to GABA and glutamate neurons, express functional α6-containing nAChRs (Gotti et al., 2010; cf, Yang et al., 2011) which may require shorter nicotine exposure than α4β2-containing nAChRs for upregulation (Walsh et al., 2008). Different patterns of nAChR upregulation in brain are also observed following moderate intermittent and intense continuous nicotine exposure regimens. In the present experiments, nAChRs exposed to intermittent nicotine showed a transient increase in ligand binding in the VTA but not the NAcc or in the prefrontal cortex, another A10 DA neuron projection field (data not shown). On the other hand, following exposure to intense continuous nicotine regimens, nAChRs show increased ligand binding in a greater number of brain regions including the NAcc and the prefrontal cortex (Collins et al. 1988, 1990; Rowell & Li, 1997; Mugnaini et al., 2002) and it persists for a longer period of time (Collins et al. 1988, 1990; for more references and discussion, see Vezina et al., 2007). Similarly, exposing cultured rat cortical neurons to nicotine for 25-hrs leads to both transient upregulation corresponding to nAChR conformational changes and long-lasting upregulation correlating with increased nAChR numbers whereas exposure to nicotine for a shorter period (e.g., ≤4 hr) produces only transient nAChR upregulation (Govind et al., 2012). These findings reflect the differential recruitment by different patterns of nicotine exposure of multiple processes in the upregulation nAChRs (Govind et al., 2012). Determining the subunit composition of nAChRs found in different neurons and brain regions should provide additional insights into how and where nAChR upregulation occurs and help decipher the immediate and long-term consequences for behavior of upregulation in multiple brain regions. Interestingly, continuous stimulant exposure regimens are not known to produce sensitization (Robinson & Becker, 1986), suggesting that the nAChR upregulation observed in the NAcc and in VTA GABA neurons following continuous nicotine exposure may be associated with other endpoints. The present findings are consistent with others showing that intermittent exposure to stimulants initiates neuroadaptations in VTA DA neurons that lead ultimately to sensitized responding (Ferrari et al., 2002; Vezina, 2004).
It has been known for some time that nAChR upregulation does not always correlate either with tolerance to the locomotor depressant (Clarke & Kumar, 1983; Collins et al., 1988, 1990) or sensitization of the locomotor activating effects of nicotine (Ksir et al., 1987). The relatively transient time course of nAChR upregulation observed here (hours) and the subsequent increase in AMPAR-mediated excitatory transmission reported previously in the VTA (days; Ungless et al., 2001) clearly preclude these phenomena as primary mechanisms underlying the expression of sensitized locomotion. It is possible however that nAChR activation might initiate a series of sequential neuroadaptations in the VTA that lead ultimately to the expression of long-lasting behavioral sensitization known to persist for weeks to months. The upregulation of nAChRs observed selectively in VTA DA neurons in the present experiments would be expected to increase the excitability of these neurons and likely contributed to the induction of long-term potentiation of their excitatory synaptic inputs. This potentiation of excitatory inputs onto VTA DA neurons has been proposed to contribute to the induction of longer lasting sensitization as both are prevented by NMDA receptor blockade during drug exposure (Wolf et al., 1994; Vezina & Queen, 2000; Ungless et al., 2001; Saal et al., 2003). As such, transient nicotine-induced nAChR upregulation in DA neurons of the midbrain may initiate a cascade of neuroadaptations that come to support the long-lasting changes in the intensity and repertoire of motivated behaviors an organism ultimately displays. Proof of this possibility awaits development and expression in the VTA of a nAChR mutant that prevents upregulation without compromising receptor functionality.
Acknowledgments
This study was supported by National Institutes of Health Grants R01 NS043782 (WNG), R01 DA015918 (DSM), P01 DA019695 (WNG, DSM, PV) and T32 DA07255 (HC, YFV, MI, JJC).
Abbreviations
- ACh
acetylcholine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ANOVA
analysis of variance
- DA
dopamine
- DHβE
dihydro-β-erythroidine
- EPSC
excitatory postsynaptic current
- MLA
methyllycaconitine
- NAcc
nucleus accumbens
- nAChR
nicotine acetylcholine receptor
- NMDA
N-methyl-D-aspartate
- VTA
ventral tegmental area
References
- Bruijnzeel AW, Markou A. Adaptations in cholinergic transmission in the ventral tegmental area associated with the affective signs of nicotine withdrawal in rats. Neuropharmacology. 2004;47:572–579. doi: 10.1016/j.neuropharm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. α-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2010;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Bertrand D. Chronic exposure to nicotine upregulates the human α4β2 nicotinic acetylcholine receptor function. J Neurosci. 2001;21:1819–1829. doi: 10.1523/JNEUROSCI.21-06-01819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Nestler EJ. Elevated levels of GluR1 in the midbrain: A trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Changeux JP. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nature Rev Neurosci. 2010;11:389–401. doi: 10.1038/nrn2849. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Kumar R. Characterization of the locomotor stimulant action of nicotine in tolerant rats. Br J Pharmacol. 1983;80:587–594. doi: 10.1111/j.1476-5381.1983.tb10733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Nicotine tolerance: An analysis of the time course of its development and loss in the rat. Psychopharmacology. 1988;96:7–14. doi: 10.1007/BF02431526. [DOI] [PubMed] [Google Scholar]
- Collins AC, Romm E, Wehner JM. Dissociation of the apparent relationship between nicotine tolerance and up-regulation of nicotine receptors. Brain Res Bull. 1988;25:373–379. doi: 10.1016/0361-9230(90)90222-l. [DOI] [PubMed] [Google Scholar]
- Cortright JJ, Sampedro GR, Neugebauer NM, Vezina P. Previous exposure to nicotine enhances the incentive motivational effects of amphetamine via nicotine-associated contextual stimuli. Neuropsychopharmacology. 2012;37:2277–2284. doi: 10.1038/npp.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Ji D, Zhou FM. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Fagen ZM, Mitchum R, Vezina P, McGehee DS. Enhanced nicotinic receptor function and drug abuse vulnerability. J Neurosci. 2007;27:8771–8778. doi: 10.1523/JNEUROSCI.2017-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari R, Le Novere N, Picciotto MR, Changeux JP, Zoli M. Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci. 2002;15:1810–1818. doi: 10.1046/j.1460-9568.2001.02009.x. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: Primary role of ventral tegmental area α6β2* receptors in mediating systemic nicotinic effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Vezina P, Green WN. Nicotine-induced upregulation of nicotinic receptors: Underlying mechanisms and relevance to nicotine addiction. Biochem Pharmacol. 2009;78:756–765. doi: 10.1016/j.bcp.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind AP, Walsh H, Green WN. Nicotine-induced upregulation of native neuronal nicotinic receptors is caused by multiple mechanisms. J Neurosci. 2012;32:2227–2238. doi: 10.1523/JNEUROSCI.5438-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: Hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan RL, Kellar KJ. Chronic nicotine and locomotor activity: Influences of exposure dose and test dose. Psychopharmacology. 1987;92:25–29. doi: 10.1007/BF00215474. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–367. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskos U, Molles BE, Pons S, Besson M, Guiard BP, Guilloux JP, Evrard A, Cazala P, Cormier A, Mameli-Engvall M, Dufour N, Cloez-Tayarani I, Bemelmans AP, Mallet J, Gardier AM, David V, Faure P, Granon S, Changeux JP. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Tessari M, Tarter G, Pich EM, Chiamulera C, Bunnemann B. Upregulation of [3H]methyllycaconitine binding sites following continuous infusion of nicotine, without changes of α7 or α6 subunit mRNA: An autoradiography and in situ hybridization study in rat brain. Eur J Neurosci. 2002;16:1633–1646. doi: 10.1046/j.1460-9568.2002.02220.x. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: Basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Cortright JJ, Sampedro GR, Vezina P. The effect of contextual stimuli previously paired with non-contingent nicotine on the subsequent acquisition of nicotine self-administration. Soc Neurosci Abst. 2012;38 [Google Scholar]
- Nisell M, Nomikos G, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A. Diminished nicotine withdrawal in adolescent rats: Implications for vulnerability to addiction. Psychopharmacology. 2006;186:612–619. doi: 10.1007/s00213-006-0383-6. [DOI] [PubMed] [Google Scholar]
- Panagis G, Kastellakis A, Spyraki C, Nomikos G. Effects of methyllycaconitine (MLA), an α7 nicotine receptor antagonist, on nicotine- and cocaine-induced potentiation of brain stimulation reward. Psychopharmacology. 2000;149:388–396. doi: 10.1007/s002130000384. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3. Academic Press; New York, NY: 1997. [Google Scholar]
- Rahman S, Zhang J, Engleman EA, Corrigall WA. Neuroadaptive changes in the mesoaccumbens dopamine system after chronic nicotine self-administration: A microdialysis study. Neurosci. 2004;129:415–424. doi: 10.1016/j.neuroscience.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: A review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rowell PP, Li M. Dose-response relationship for nicotine-induced upregulation of rat brain nicotinic receptors. J Neurochem. 1997;68:1982–1989. doi: 10.1046/j.1471-4159.1997.68051982.x. [DOI] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, De Vries TJ, Wardeh G, van de Ven HWM, Vanderschuren LJMJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–3276. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skjei KL, Markou A. Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology. 2003;168:280–292. doi: 10.1007/s00213-003-1414-1. [DOI] [PubMed] [Google Scholar]
- Snell LD, Johnson KM. Effects of nicotinic agonists and antagonists on n-methyl-d-aspartate-induced 3H-norepinephrine release and 3H-(1-[1-(2-thienyl)cyclohexyl]-piperidine) binding in rat hippocampus. Synapse. 1989;3:129–135. doi: 10.1002/syn.890030204. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Vallejo YF, Buisson B, Bertrand D, Green WN. Chronic nicotine exposure upregulates nicotinic receptors by a novel mechanism. J Neurosci. 2005;25:5563–5572. doi: 10.1523/JNEUROSCI.5240-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, McGehee DS, Green WN. Exposure to nicotine and sensitization of nicotine-induced behaviors. Progress in Neuro-Psychopharmacol. Biol Psychiatry. 2007;31:1625–1638. doi: 10.1016/j.pnpbp.2007.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Queen AL. Induction of locomotor sensitization by amphetamine requires the activation of NMDA receptors in the rat ventral tegmental area. Psychopharmacology. 2000;151:184–191. doi: 10.1007/s002130000463. [DOI] [PubMed] [Google Scholar]
- Walsh H, Govind AP, Mastro R, Hoda JC, Bertrand D, Vallejo Y, Green WN. Up-regulation of nicotinic receptors by nicotine varies with receptor subtype. J Bio Chem. 2008;283:6022–6032. doi: 10.1074/jbc.M703432200. [DOI] [PubMed] [Google Scholar]
- Wolf ME, White FJ, Hu XT. MK-801 prevents alterations in the mesoaccumbens dopamine system associated with behavioral sensitization to amphetamine. J Neurosci. 1994;14:1735–1745. doi: 10.1523/JNEUROSCI.14-03-01735.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, Whiteaker P, Lukas RJ, Wu J. Functional nicotinic acetylcholine receptors containing α6 subunits are on GABAergic neuronal boutons adherent to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:2537–2548. doi: 10.1523/JNEUROSCI.3003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nature Neurosci. 2004;7:581–582. doi: 10.1038/nn1243. [DOI] [PubMed] [Google Scholar]
- Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010;59:431–436. doi: 10.1016/j.neuropharm.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]