Abstract
The sea lamprey (Petromyzon marinus) is a genetically programmed animal model for biliary atresia as it loses its bile ducts and gallbladder during metamorphosis. However, in contrast to patients with biliary atresia or other forms of cholestasis who develop progressive disease, the post-metamorphosis lampreys grow normally to adult size. To understand how the adult lamprey thrives without the ability to secrete bile, we examined bile salt homeostasis in larval and adult lampreys. Adult livers were severely cholestatic with levels of bile salts >1 mM, but no evidence of necrosis, fibrosis, or inflammation. Interestingly, both larvae and adults had normal plasma levels (~10 μM) of bile salts. In larvae, petromyzonol sulfate (PZS) was the predominant bile salt, whereas the major bile salts in adult liver were sulfated C27 bile alcohols. Cytotoxicity assays revealed that PZS was highly toxic. Pharmacokinetic studies in free-swimming adults revealed that ~35% of intravenously injected bromosulfophthalein (BSP) was eliminated over a 72 hr period. Collection of urine and feces demonstrated that both endogenous and exogenous organic anions, including biliverdin, bile salts and BSP, were predominantly excreted via the kidney with minor amounts also detected in feces. Gene expression analysis detected marked up-regulation of orthologs of known organic anion and bile salt transporters in the kidney with lesser effects in the intestine and gills in adults compared to larvae. These findings indicate that adult lampreys tolerate cholestasis by altering hepatic bile salt composition, while maintaining normal plasma bile salt levels predominantly through renal excretion of bile products. Therefore, we conclude that strategies to accelerate renal excretion of bile salt and other toxins should be beneficial for patients with cholestasis.
Keywords: cholestasis, bile salt homeostasis, biliverdin, adaptive response, transporter expression
Introduction
Biliary atresia is a developmental disorder of unknown etiology where infants are born without bile ducts or gallbladders, and rapidly develop obstructive cholestasis. Although some of these infants may survive after a surgical by-pass (Kasai procedure) within two months of birth or a liver transplant, a truly effective therapy is currently unknown. Similar to these patients, sea lamprey (Petromyzon marinus, hereafter lamprey) larvae lose their bile ducts and gallbladder during metamorphosis/transformation to the adult form, and presumably become cholestatic. Thus, the lamprey has been considered a naturally occurring, genetically determined animal model for biliary atresia (1, 2). However, in striking contrast to patients with biliary atresia or other chronic cholestatic disorders, who develop liver fibrosis, cirrhosis, and liver failure, the post-metamorphosis lamprey not only tolerates complete biliary atresia but grows normally to a full adult (3). It is not known how the post-metamorphosis lampreys maintain their bile salt homeostasis, and successfully adapt to this process. A better understanding of this adaptation to biliary atresia might provide new strategies for treatment of human cholestatic disorders..
Lamprey are one of the most evolutionarily primitive vertebrates (4). Their life cycle can be divided into four stages, i.e. larvae, metamorphosis/transformation, juvenile, and adult/migratory. Lamprey larvae are born in upstream rivers and remain within mudbanks for a variable number of years after which they emerge and undergo transformation into juvenile forms that then move to the ocean to feed off of other fish as they grow to adult size. After ~ 2 years they migrate back to their rivers of origin, spawn and die. Lamprey larvae are similar to other vertebrates, and are born with an intact gallbladder and biliary system, and excrete bile into the intestine to establish an enterohepatic circulation. However, during metamorphosis, lamprey’s bile ducts and gallbladder vanish, a process resembling biliary atresia in humans (1, 2). Previous studies have shown that the adult lamprey liver contains elevated levels of biliverdin and bilirubin, but bile salts have not been measured (5). Also it is not known whether lamprey can eliminate biliverdin, bilirubin and other toxins from their body through other extrahepatic routes, although prior studies suggest that bile salt pheromones might be excreted via the gills(6) and that the intestine might be a route of excretion because green pigments can be seen in the distal intestine of adult lamprey (5). In support of this hypothesis, Yeh et al. have very recently reported that post-metamorphosis lamprey can excrete 3H-taurocholic acid across the intestinal wall (7). However, in bile duct ligated rodents and cholestatic patients, elevated levels of bilirubin and bile salts are detected in the urine rather than in the intestine (8-12). Therefore, it remains to be determined what role, if any, the kidney plays in the elimination of bile pigments and bile salts in the adult lamprey.
In this report, we assessed and compared bile salt homeostasis in larval and adult migrating sea lampreys, and found that despite cholestatic levels of bile salts and biliverdin in the liver, adult lampreys convert toxic C24 bile salts into C27 bile salts in the liver, while maintaining normal levels of bile salts and bile pigments in plasma. We also found that bile salts and other organic solutes are excreted primarily via the kidney in the adult lamprey, while only minor amounts are eliminated via the intestine. These functional studies were supported by ortholog gene sequencing where the expression of organic anion transporters was markedly up-regulated in adults compared to larval kidneys. These findings suggest that strategies to enhance renal excretion of bile salts and other toxins might prove to be an effective therapy for patients with biliary atresia and other forms of cholestasis.
Materials and methods
Animals
Animal experiments were performed at the Mount Desert Island Biological Laboratory (MDIBL) in Salisbury Cove, ME. Experiments were in agreement with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and approval was given beforehand by the Institutional Animal Care and Use Committee. Larval lampreys were obtained from Acme Lamprey Company (Harrison, ME). Adult lampreys were caught while migrating upstream in the Kennebunk River, Maine, in May-June 2010 and 2011. Larval and adult lampreys were kept in dark-adapted freshwater tanks at 11°C. Adult males and females were maintained in separate dark adapted tanks. All animals were anesthetized in 0.1g/L Tricaine prior to intravenous injections, blood sampling, ureter and/or intestinal cloacae cannulation, and euthanasia. To assess the pharmacokinetics of bromosulfophthalein (BSP) clearance, animals were injected intravenously via the caudal tail vein with 10 mg BSP/kg body weight, and immediately returned to their fresh water tanks. Blood was obtained at specific timed intervals over 72 hrs . In separate experiments, cannulas with attached balloons were inserted into the ureter and intestinal cloacae and urine and feces were then collected from these free swimming lampreys for a period of 24 hrs after injection with BSP.. For clearance of radioactive bile salts, 3H-Taurocholic acid (TCA, 30 μCi/kg body weight) was injected into anesthetized animals and urine was collected via ureter cannulation while the lampreys were maintained in a Perspex chamber for 4 hours. After exsanguination by cardiac puncture, the intestine was ligated in the middle to separate the proximal and distal portions, and the contents from both sections were collected by inserting a cannula and irrigating the luminal contents with 1.0 ml lamprey Ringers solution (in mM, 130 NaCl, 2.1 KCl, 1.8 MgCl2, 4 HEPES, 4 Dextrose, 1 NaHCO3, 2.6 CaCl2). Each of the renal tubules was gavaged in a similar manner via external openings in the anal pore. Liver, intestine, kidney and muscle samples were removed and homogenized in PBS, and lysed by adding 1% Triton X-100. BSP concentration was assayed as we have previously reported (13, 14). Radioactivity in tissues was determined by liquid scintillation.
Bile salt spectrum analysis
Electrospray mass spectrometry was performed on lamprey liver, urine, and serum samples using a Perkin-Elmer Sciex API-III instrument (Perkin-Elmer, Alberta, Canada) modified with a nanoelectrospray source from Protana (Odense, Denmark). Palladium-coated borosilicate glass capillary needles (Protana) were used for sample injection. The instrument was operated in the negative mode with Q1 IS voltage set to 600V. The IN voltage was set to 110V, and the ORI voltage set to 90V. A curtain gas of ultrapure nitrogen was pumped into the interface to aid in the evaporation of solvent droplets and to prevent particulate matter from entering the analyzer. Chemical identity of peaks was confirmed by the fragmentation pattern of selected ions (Q3 mode) using argon collision gas. The presence of conjugated bile salts was confirmed by selection for m/z 97 (sulfate), and m/z 124 (taurine).
Bile salt cytotoxicity assay
The cytotoxicities of petromyzonol sulfate (PZS), 3 keto-PZS (3kPZS) and several mammalian bile salts were determined by performing cell lysis experiments using human red blood cells as previously described (15).
Liver histology and immunohistochemistry
Liver, intestine, kidney and gill tissues were obtained for histology and immunohistochemistry was performed using C219 antibody that react with a conserved region of Mdr1.
RNA extraction and quantification
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA) and cleaned up with a kit (RNeasy Clean-up Kit, Qiagen, Valencia, CA). Reverse transcription was performed using 2 μg of total RNA per sample, using a kit from Roche (Indianapolis, IN). Real-time RT-PCR using TaqMan probes was performed on an ABI 7500 Sequence Detection System (Applied Biosystems, Carlsbad, CA). Primers and probes are listed in Table S1 (Supplementary Information). Expression of larval and adult mRNA was normalized to a fixed signal of lamprey β-actin.
Statistical analysis
Statistical analysis was performed with Graphpad Prism 4 (Graphpad Software) and unpaired two-tailed t-test was used to detect differences between two groups, where a P value < 0.05 was considered statistically significant.
Results
The adult lamprey is a unique cholestatic model
Previous studies revealed that plasma levels of bilirubin and biliverdin in adult lamprey are within normal range, whereas their levels in the liver are elevated (4, 5). To assess bile salt homeostasis, we analyzed bile salt concentration and composition in the plasma and liver of lamprey adults and larvae. As shown in Table 1, hepatic bile salts in adults (both males and females), but not larvae, reached to millimolar levels. Such high levels of hepatic bile salts are similar to those described in advanced cases of cholestasis in patients or in bile duct ligated rodents, indicating that the adult lamprey liver is indeed cholestatic. However, in contrast to bile duct ligated rodents and cholestatic patients where high levels of bile salts are also detected in plasma, the plasma level of bile salts in both larvae and adults is only ~ 10 μM, within the normal range observed in mammals. Furthermore, in the plasma of male lamprey, C24 and C27 bile salts made up 79% and 21% of measured bile salt species, whereas in liver, the percentage of C24 and C27 bile salts were reversed, averaging 25% and 75%, respectively. Similar ratios were also obtained in the liver of the female lamprey, although C24 bile salts were completely absent in female plasma. In striking difference from the adults, C24 bile salts were the predominant bile salt in both plasma and liver in larvae. Together, these findings suggest that the adult lamprey has developed a unique adaptive mechanism by reducing C24 bile salts and thereby increasing the C27/C24 bile salt ratio in the liver. These data also suggest that post-metamorphosis lampreys may have developed an efficient mechanism for eliminating bile salts from the blood.
Table 1.
Bile salt concentration and composition in sea lamprey
| Plasma | Liver | |||||
|---|---|---|---|---|---|---|
| Total (μM) | % of C24 | % of C27 | Total (μM) | % of C24 | % of C27 | |
| Larvae (n=4-5) | 12.8 ± 7.1 | 95.8 ± 5.1 | 4.2 ± 5.1 | 752 ± 153 | 96.7 ± 7.4 | 3.3 ± 7.4 |
| Adult males (n=3) | 12.2 ± 10.1 | 72.4 ± 9.0 | 27.6 ± 9.0 | 1761 ± 232 | 30.4 ± 5.3 | 69.6 ± 5.3 |
| Adult females (n=3) | 3.8 ± 3.0 | 0 | 100 | 1746 ± 1161 | 12.1 ± 2.3 | 87.9 ± 2.3 |
Petromyzonol sulfate (PZS), the major bile salt in lamprey larva, is cytotoxic
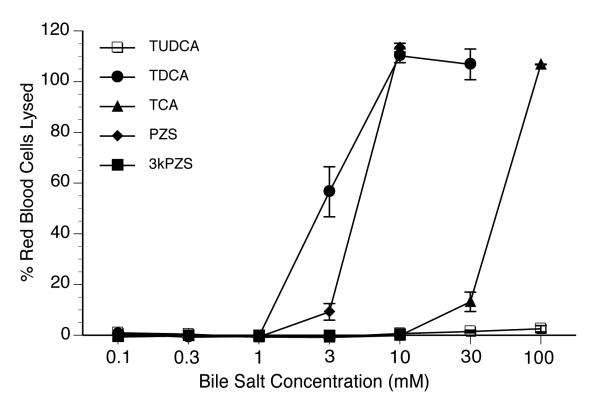
Since the adult lamprey has significantly less C24 bile salts in the liver in comparison to the larval form, we assessed the cytotoxicity of C24 PZS (the major bile salt in larva bile) as well as 3-keto PZS (3kPZS), released by the adult male as a pheromone for spawning. We compared these findings with several mammalian bile salts using a red cell cytotoxicity assay. As shown in Figure 1, 3 mM and 10 mM PZS lysed 10% and 110% red blood cells, respectively (cells lysed by distilled water were set as 100%). In contrast, 10 mM 3kPZS was without cytolytic effect. As controls, taurodeoxycholic acid (TDCA), taurocholic acid (TCA), and tauroursodeoxycholic acid (TUDCA) demonstrated different levels of cytolysis (Figure 1) as previously observed (15). These results demonstrate that 3kPZS is markedly less cytotoxic than PZS. These findings suggest that adult lamprey have developed novel mechanisms to reduce bile salt toxicity by changing their bile salt composition and by converting PZS into 3kPZS, that can then be secreted as a sex pheromone. It is likely that the C27 bile salts are also less toxic but purified samples are not available for testing.
Figure 1.
Cytotoxicity of various bile salts determined by cytolysis of human red blood cells. Cell lysis by distilled water was set as 100%. Values are expressed as means ± SD from 3 independent experiments. TDCA, taurodeoxycholic acid; TCA, taurocholic acid; TUDCA, tauroursodeoxycholic acid; PZS, petromyzonol sulfate; 3kPZS, 3 keto-petromyzonol sulfate.
Adult lampreys lose bile canaliculi without apparent hepatic injury
Macroscopically male livers were light-green in color, whereas female livers were dark-green and weighed less than the males. Homogenization of the liver generally revealed a fatty supernatant in male but not female specimens. Despite high concentrations of bile salts in the liver of the adult lamprey, and the absence of bile ducts, examination of liver histology by H&E staining revealed no necrosis, fibrosis or inflammation in either males or female livers (Figure 2A and 2B). To determine if adult hepatocytes remained polarized, we performed immunohistological staining using the monoclonal antibody C219, which interacts with a highly conserved motif in the canalicular multidrug resistance protein 1 (MDR1, ABCB1). As demonstrated in Figure 2 (lower panels) the apical bile canalicular domain was labeled only in the liver of larvae, but not in adults, further confirming the loss of an apical bile secretory mechanism in the adult lamprey liver.
Figure 2.
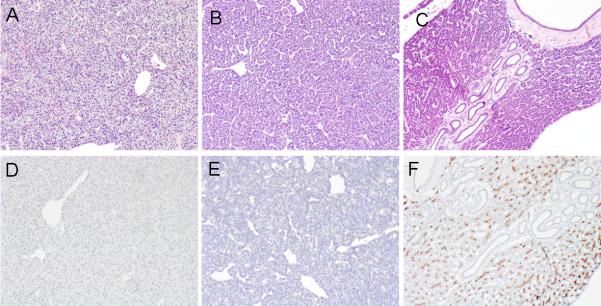
Comparative liver histology of lamprey adult females, adult males, and larvae. H&E staining demonstrates that bile duct epithelium is absent in adult male (A) and female (B) but present in larval (C) lamprey. Note the absence of inflammation, cell injury and fibrosis. Immunohistochemistry (C219 monoclonal antibody) staining Mdr1-like protein in bile canaliculi from larval (F) but not adult male (D) or female (E) livers.
BSP plasma clearance and tissue distribution in adult lamprey
To investigate the mechanisms by which the cholestatic adult lamprey maintains normal plasma levels of bile salts and bile pigments, we first assessed plasma clearance of BSP, a prototypical organic anion normally excreted into bile. As demonstrated in Figure 3, after intravenous injection, BSP was slowly removed from plasma with an initial t1/2 of ~2 hr (reflecting tissue uptake) for both males and females, and the second t1/2 of 64 hr (reflecting body elimination rate). Our previous studies in marine skates and dogfish demonstrated substantially faster BSP elimination rates (initial t1/2 was 12.5 minutes and the second t1/2 was ~ 2 hrs) albeit still considerably slower than in mammals (13, 14). The slow rate of elimination in lamprey presumably reflect the down regulation of basolateral transporters for uptake of organic anions, typically seen in cholestatic mammalian livers (16).
Figure 3.
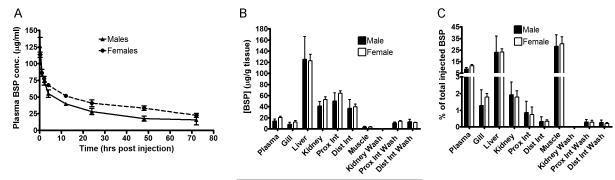
Pharmacokinetics of the organic anion BSP in adult lampreys. Animals were injected intravenously with BSP (10mg/kg body weight). Plasma samples were taken after anesthesia, and tissues were collected at 72 hrs. A, Plasma BSP concentration in adult lamprey, with an initial tissue uptake rate (t1/2) of ~2 hr while overall clearance rate (t1/2) was ~64 hr. B, BSP concentration in different tissues. C, amount of BSP in different tissues, expressed as percentage of total BSP injected. At 72 hr post injection, ~ 65% of total injected BSP could be accounted for, indicating that an estimated ~35% of the injected BSP had been excreted from the lamprey body during this time period. All values represent means (n = 4) ± SD.
Seventy-two hours after BSP injection, the highest tissue concentration of BSP was detected in the liver, followed by kidney and intestine (Figure 3B), suggesting that the adult lamprey’s cholestatic liver still maintains uptake function for organic anions. Of note, there was no BSP detected in the ureter, whereas detectable BSP was obtained when both the proximal and distal intestine were irrigated, despite the complete absence of intestinal contents in these fasting migratory lamprey. This observation initially seemed to support the concept that post-metamorphosis lamprey might use the intestine to excrete organic anions as previously suggested by the appearance of green contents in the distal intestine (5). However, we recovered only ~ 65% of injected BSP from all tissues 72 hours post injection, indicating that ~35% of the injected BSP had been excreted from the lamprey body during this time period. The amount of BSP in the intestinal washes was less than 1% of total injected BSP, suggesting that additional mechanisms/routes may contribute to the elimination of BSP.
Kidney is the major organ for the excretion of both bile salts and the organic anions
During work on this project, one lamprey was serendipitously discovered to have an obstructed ureter, presumably from a prior injury as a scar was seen on the skin. Surprisingly high concentrations of biliverdin (202 μM) and bile salts (ΔC27α(OH)-SO4, 18.3 μM; C27α(OH)4-SO4, 9.5 μM) were detected in the fluid from the dilated obstructed ureter (Supplementary information, Figure S1). This unexpected finding suggested that adult lamprey secretes endogenous metabolic substrates via the kidney. To investigate this possibility in greater detail, we collected urine from free swimming lamprey after cannulating their ureters, as previously described for bile collection in skates and dogfish (13, 14). We also assessed the rate of urine flow, and the biliverdin and bile salt content. Over a 24 hour period, urine flow in these migrating adults averaged ~ 4 ml/hr/kg body weight. Biliverdin’s green color could easily be seen by eye in the collected urine. As shown in Table 2, both biliverdin and bile salts were detected biochemically in the urine effluent. Males excreted significantly more biliverdin than females, whereas bile salt excretion rates were similar in both sexes.
Table 2.
Urine excretion of bile salt and biliverdin in adult sea lampreys
| Bile salt | Biliverdin | |||
|---|---|---|---|---|
| conc. (μM) | Rate (nmole/ hr/kg of BW) |
conc. (μM) | Rate (nmole/ hr/kg of BW) |
|
| Male (n=4) | 0.24 ± 0.11 | 1.1 ± 0.4 | 4.05 ± 1.71a | 18.7 ± 9.1 a |
| Female (n=4) | 0.29 ± 0.09 | 1.1 ± 0.5 | 1.70 ± 0.39 | 6.5 ± 3.3 |
Of note, urine bile salts are predominantly C27 at this migration stage.
p<0.05 male v/s female
To test if exogenous compounds could also be disposed through the kidney and whether this was the major route of excretion, we again injected BSP intravenously. Twelve hours post injection, we cannulated both the ureter and the intestinal orifice and collected urine and feces for a 24 hours period. In these experiments (n=7), we recovered 11.6±3.8% and 11.4 ±3.8% of the injected BSP in the urine of males and females, respectively. This 24 hr elimination rate is very similar to our estimate of ~ 35% clearance of BSP over a 72 hour period in the previous experiments. We also recovered less than 1% of the injected BSP from the intestinal cannulation in a limited volume of feces (<0.5 ml) over this same 24 hour experiment. These findings clearly indicate that the kidney, not the intestine, is the primary organ for the elimination of BSP in adult lamprey.
To verify if this was also true for the elimination of bile salts, we injected 3H-TCA intravenously into cannulated adult lamprey, and collected urine and feces for 4 hours. Again, we detected high counts of radioactive material in the urine but not in irrigations of the ureter (Figure 4), while there was no evidence for fecal excretion. Quantitatively, ~ 5% of injected 3H-TCA was excreted in the urine. Irrigation of the intestine recovered an additional 1.5% of the injected amount (Figure 4). These findings confirm that renal excretion is also the major route for excretion of bile salts. High levels of 3H-TCA were also detected in tissues of the intestine, liver and kidney, similar to the BSP experiments. We speculate that our initial failure to detect organic anions in the ureteral irrigations was the result of high urine flow and spontaneous urination during the process of handling and anesthetizing the animals.
Figure 4.
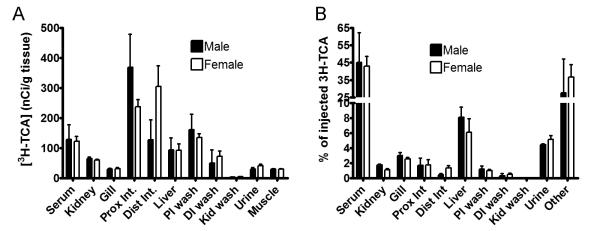
Tissue distribution of the bile salt 3H-TCA in adult lampreys. Samples were collected 4 hours after animals were administered 3H-TCA intravenously. (A) Concentration of 3H-TCA in a variety of tissues. (B) Amount of 3H-TCA in different tissues expressed as percentage of the total amount injected. All values represent means (n = 4) ± SD. PI wash, proximal intestinal wash; DI wash, distal intestinal wash; Kid wash, ureter/kidney wash.
Renal excretion of bile salts and organic anions in adult lampreys is associated with altered transporter expression
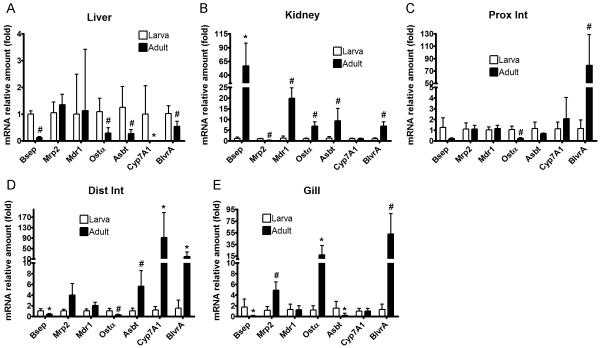
To gain insights into the molecular mechanisms that constitute the successful adaptation of adult lampreys to biliary atresia, we analyzed mRNA expression of genes involved in the metabolism and transport of bile salt, bilirubin and biliverdin. We first annotated the lamprey genome by performing ortholog searches using human genes followed by phylogenetic analysis. We were able to confidently identify the bile salt export pump (Bsep, Abcb11), multidrug resistance protein 1 (Mdr1, Abcb1), multidrug resistance-associated protein 2 (Mrp2, Abcc2), organic solute transporter α (Ostα), apical sodium-dependent bile salt transporter (Asbt, Slc10a2), cholesterol 7 alpha-hydroxylase (Cyp7A1), and biliverdin reductase A (BlvrA). We then cloned partial or full-length sequences of the coding regions, and verified them by DNA sequencing (Supplementary information). After confirming the sequence, we designed TaqMan assays and determined mRNA expression in a variety of larval and adult tissues using real-time RT-PCR. Cyp7A1 (the rate-limiting enzyme in bile salt synthesis) and Bsep (the major apical bile salt exporter) are essentially liver specific genes for lamprey larvae (Supplementary information, Figure S2) that showed greatly reduced mRNA expression levels in the livers of adults (Figure 5). In contrast, Bsep mRNA was increased more than 50-fold in adult kidney and Cyp7A1 mRNA was induced by nearly 100-fold in adult distal intestine compared to larval tissues. BlvrA (enzyme that reduces biliverdin into bilirubin) and Mrp2 (apical diconjugate bile salt and organic anion exporter) appear to be most abundant in larval kidney compared to other tissues (Figure S2). However, in adults, BlvrA mRNA was greatly increased in the intestine and gills, and further elevated in the kidney but decreased in the liver. Mrp2 was significantly reduced in the kidney but moderately increased in the gills, with no changes in the liver and intestine. Mdr1 was highly expressed in the intestine of larvae. It was significantly increased in adult kidney but no changes were observed in liver, intestine, or gills. Asbt and Ostα were most abundant in the intestine in larvae, followed by liver and kidney. In adults, Asbt was increased in kidney and distal intestine but decreased in liver and gills. In contrast, Ostα was increased in kidney and gills, but decreased in the liver and intestines. Collectively, these findings suggest that adult lampreys cope with biliary atresia by down-regulating bile salt synthesis through Cyp7A1 and canalicular export in the liver through Bsep. Renal excretion of bile salts and organic anions in the plasma appears to be facilitated by a strong kidney-specific induction of transporters Ostα, Mdr1 and Bsep.
Figure 5.
mRNA expression of genes involved in bile salt and organic anion homeostasis in larval and adult lampreys. Real-time RT-PCR was performed using cDNA from liver (A), kidney (B), the proximal half of the intestine (C), the distal half of the intestine (D), and the gills (E). Because there was no significant difference between males (n = 4) and females (n = 4), and because we were not able to differentiate the gender of lamprey larvae (n = 5), gene expression data from adult males and females were combined for analysis and presentation. Data of larvae and adults were normalized to lamprey β-actin, and presented as means ± SD by setting larval expression as 1. * p < 0.05 versus larvae; # p < 0.01 versus larvae.
Discussion
In this report, we used the sea lamprey, a genetically programmed animal model for biliary atresia, to study its adaptive response to cholestasis. We examined the larval and adult lamprey on both functional and molecular levels to gain insights in the mechanisms by which lamprey thrives after losing its bile ducts during metamorphosis. Our findings suggest that the adult lamprey is a unique cholestatic model, since they maintain a normal plasma bile salt homeostasis. Despite having a severely cholestatic liver with marked elevations in bile pigments and bile salts, adult lampreys show no evidence of liver injury on histological examination (Figure 2). This is strikingly different from what is seen in all other cholestatic models in rodents or human patients, where cholestasis is associated with elevated levels of plasma bile salts, hepatocyte necrosis/apoptosis, bile duct proliferation, inflammation, and progressive liver fibrosis. How does the adult lamprey manage to eliminate liver injury and maintain such a unique bile salt homeostasis? Based on our findings we speculate that the following adaptive mechanisms may account for this phenomenon.
First, adult lampreys down-regulate the hepatic expression of Cyp7A1, the rate-limiting enzyme in bile salt synthesis (Figure 5A). This should result in a reduction in de novo bile salt production, as occurs in mammalian cholestatic disorders (16). Second, the bile salt composition in the liver is shifted from highly toxic C24 PZS to sulfated C27 bile alcohols. As demonstrated in Figure 1, the predominant bile salt PZS in larva is as cytotoxic as TDCA, a hydrophobic bile salt that is hepatotoxic in mammals. In contrast, 3kPZS appears less toxic than PZS, since it did not lyse red blood cells even at the 10 mM concentration (Figure 1). This may explain why the male adult lamprey produces and releases 3kPZS as a sex pheromone when reaching its spawning grounds. Although the cytotoxicity of sulfated C27 bile alcohols in adult lamprey is not known, we speculate that they are also less cytotoxic than PZS. In fact, altered bile salt composition is well described in cholestatic rodents (17). Increases in sulfated bile salts are also seen in the serum and urine of patients with cholestasis (9, 18). Third, the adult lamprey liver contains very high concentrations of bilirubin and biliverdin as shown previously (5). It raises biliverdin levels perhaps by down-regulating biliverdin reductase expression in the liver as seen in Figure 5A. Both bilirubin and biliverdin have properties as antioxidants, which might provide additional hepatoprotection (19, 20). In line with this speculation, a very recent report has shown that biliverdin reduced bile salt cytotoxicity in several human hepatocyte cell lines where the best protective effects were observed in cells with lower level of biliverdin reductase activity (XXII International bile acid meeting, Vienna, 2012 –in press).
Finally, and most importantly, the adult lamprey effectively eliminates endogenous and exogenous bile salts and organic anions through renal excretion. As demonstrated in Figure 4, ~5% of injected 3H-TCA was excreted via the urine in a 4 hr period. We also demonstrated that >10% of injected BSP was excreted in urine in a 24 hr period, an excretion rate that closely approaches the plasma clearance rate of BSP, as calculated from Figure 3B. In contrast, a much smaller fraction of these compounds were detected in intestinal washes. Therefore, the kidney plays the major role in maintaining normal plasma bile salt homeostasis in these adult lampreys. Youson et al. reported that the lamprey kidney undergoes considerable structural remodeling during metamorphosis (21). This remodeling may enable the lamprey to acquire a more effective system for the disposal of toxins through renal excretion. Indeed, high concentrations of biliverdin were observed in the kidney by Makos and Youson in up-stream migrants (5). This observation may be in concordance with induced biliverdin reductase expression in the adult kidney as well as other tissues (Figure 5). In addition, we found marked increases in mRNA expression of Bsep and Mdr1 in adult kidney (Figure 5). Bsep is the major ABC export pump for bile salts in mammalian liver but not kidney (22). The mammalian ortholog of Mdr1 is also capable of transporting bile salts, albeit with lower affinity than Bsep (23). Thus it seems likely that the up-regulation of these two transporters in the kidney may be responsible for the renal excretion of bile products in adult lamprey. These findings prompt us to hypothesize that medical intervention to accelerate the renal excretion of bile products might be beneficial for patients with cholestasis. While bile products are detected in the urine of cholestatic patients, this adaptation is insufficient as high serum levels of bile salts and liver injury persists. However if renal excretory mechanisms of bile salts and other organic solutes could be further enhanced the progression of liver injury might be slowed or arrested.
During preparation of this report, Yeh et al. published evidence for intestinal synthesis and transport of bile salts based on the findings of induction of Cyp7A1 in the intestine, and serosal to mucosal transport of 3H-TCA in feeding lamprey obtained from Lake Michigan (7). We confirm the finding that Cyp7A1 is highly up-regulated in the adult lamprey intestine, particularly in the distal intestine, and that intestinal excretion of bile salts and the organic anion BSP can take place. We found that the lamprey ortholog for Mrp2 is induced in the distal intestine of adults, possibly accounting for this function. Nevertheless, our findings also show that this intestinal secretion route is quantitatively minor compared to the kidney in the migrating adult. This is in contrast to Yeh et al.’s speculation in their recent study in feeding lake dwelling juveniles, where renal excretion rates were underestimated because the ureter was not cannulated during in-vivo experiments (7). Whether renal excretion of bile salts is similarly robust in the feeding juveniles at sea will need to be determined. However, capture of feeding lampreys from the sea has proven to be difficult.
In summary, our studies indicate that the migrating adult sea lamprey is a unique cholestatic model of biliary atresia which prevents liver injury by reducing the toxicity of bile salts through decreasing their synthesis and altering their composition and by accumulating high levels of biliverdin in the liver. At the same time, they are able to maintain normal plasma levels of bile salts and bile pigments primarily by increasing their renal excretion, presumably via increased expression of renal transporters. These findings suggest that accumulating biliverdin in the liver and further inducing bile salt and organic anion transporters in the kidney may be novel approaches for treating biliary atresia and other chronic cholestatic liver diseases.
Supplementary Material
Acknowledgements
We greatly appreciate both Dr. Weiming Li and Chu-Yin Yeh (Michigan State University) for providing advice on handling of lamprey and for providing 3kPZS. We also acknowledge summer students Victoria Smith (Willamette University) and Dayne Filer (College of Idaho) for their technical assistance. These studies were supported by USPHS grants R37 DK25636 (JLB) and P30 DK34989.
Financial Support: This study was supported by National Institutes of Health Grants DK25636 (to J.L.B.) and DK34989 (Yale Liver Center).
List of Abbreviations
- Asbt
apical sodium-dependent bile salt transporter
- BlvrA
biliverdin reductase A
- Bsep
bile salt export pump
- BSP
bromosulfophthalein
- Cyp7A1
cholesterol 7 alpha-hydroxylase
- Mdr1
multidrug resistance protein 1
- Mrp2
multidrug resistance-associated protein 2
- Ostα
organic solute transporter α
- PZS
petromyzonol sulfate
- 3kPZS
3-keto PZS
- TCA
Taurocholic acid
- TDCA
taurodeoxycholic acid
- TUDCA
tauroursodeoxycholic acid
Reference List
- 1.Sidon EW, Youson JH. Morphological changes in the liver of the sea lamprey, Petromyzon marinus L., during metamorphosis. II. Canalicular degeneration and transformation of the hepatocytes. J Morphol. 1983 Dec;178(3):225–246. doi: 10.1002/jmor.1051780303. [DOI] [PubMed] [Google Scholar]
- 2.Sidon EW, Youson JH. Morphological changes in the liver of the sea lamprey, Petromyzon marinus L., during metamorphosis: I. Atresia of the bile ducts. J Morphol. 1983 Jul;177(1):109–124. doi: 10.1002/jmor.1051770109. [DOI] [PubMed] [Google Scholar]
- 3.Youson JH. Biliary atresia in lampreys. Adv Vet Sci Comp Med. 1993;37:197–255. [PubMed] [Google Scholar]
- 4.Osorio J, Retaux S. The lamprey in evolutionary studies. Dev Genes Evol. 2008 May;218(5):221–235. doi: 10.1007/s00427-008-0208-1. [DOI] [PubMed] [Google Scholar]
- 5.Makos BK, Youson JH. Tissue levels of bilirubin and biliverdin in the sea lamprey, Petromyzon marinus L., before and after biliary atresia. Comp Biochem Physiol A Comp Physiol. 1988;91(4):701–710. doi: 10.1016/0300-9629(88)90953-x. [DOI] [PubMed] [Google Scholar]
- 6.Li W, Scott AP, Siefkes MJ, Yan H, Liu Q, Yun S-S, et al. Bile Acid Secreted by Male Sea Lamprey That Acts as a Sex Pheromone. Science. 2002;296(5565):138–141. doi: 10.1126/science.1067797. [DOI] [PubMed] [Google Scholar]
- 7.Yeh CY, Chung-Davidson YW, Wang H, Li K, Li W. Intestinal synthesis and secretion of bile salts as an adaptation to developmental biliary atresia in the sea lamprey. Proc Natl Acad Sci U S A. 2012 Jul 10;109(28):11419–11424. doi: 10.1073/pnas.1203008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frohling W, Stiehl A. Bile salt glucuronides: identification and quantitative analysis in the urine of patients with cholestasis. Eur J Clin Invest. 1976 Jan 30;6(1):67–74. doi: 10.1111/j.1365-2362.1976.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 9.Makino I, Hashimoto H, Shinozaki K, Yoshino K, Nakagawa S. Sulfated and nonsulfated bile acids in urine, serum, and bile of patients with hepatobiliary diseases. Gastroenterology. 1975 Mar;68(3):545–553. [PubMed] [Google Scholar]
- 10.Takikawa H, Beppu T, Seyama Y. Urinary concentrations of bile acid glucuronides and sulfates in hepatobiliary diseases. Gastroenterol Jpn. 1984 Apr;19(2):104–109. doi: 10.1007/BF02806931. [DOI] [PubMed] [Google Scholar]
- 11.Fickert P, Zollner G, Silbert D, Fuchsbichler A, Arbeiter S, Gonzalez F, et al. Adaptive Hepatic and Renal Transporter Regulation in Cholic Acid (CA)-Fed Mice: Role of Nuclear Bile Acid Receptor (FXR). [Abstract] Hepatol. 2002;36(4):454A. doi: 10.1016/s0168-8278(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 12.Zollner G, Fickert P, Arbeiter S, Silbert D, Fuchsbichler A, Gonzalez F, et al. Ursodeoxycholic Acid (UDCA) Feeding Induces Adaptive Hepatic and Renal ABC-Transporter Expression in Mice. [Abstract] Hepatol. 2002;36(4):454A. [Google Scholar]
- 13.Boyer JL, Schwarz J, Smith N. Selective hepatic uptake and biliary excretion of 35S-sulfobromophthalein in marine elasmobranchs. Gastroenterology. 1976;70:254–256. [PubMed] [Google Scholar]
- 14.Boyer JL, Schwarz J, Smith N. Biliary secretion in elasmobranchs. II. Hepatic uptake and biliary excretion of organic anions. Am J Physiol. 1976;230:974–981. doi: 10.1152/ajplegacy.1976.230.4.974. [DOI] [PubMed] [Google Scholar]
- 15.Elferink RP, Ottenhoff R, Fricker G, Seward DJ, Ballatori N, Boyer J. Lack of biliary lipid excretion in the little skate, Raja erinacea, indicates the absence of functional Mdr2, Abcg5, and Abcg8 transporters. Am J Physiol Gastrointest Liver Physiol. 2004 May;286(5):G762–G768. doi: 10.1152/ajpgi.00424.2003. [DOI] [PubMed] [Google Scholar]
- 16.Boyer JL. Adaptive Regulation of Hepatocyte Traransporters in Cholestasis. In: Arias IM ARBJCDFNSDaWA, editor. The Liver Biology and Pathobiology. 5ifth edition John Wiley & Sons; Chichester: 2009. pp. 681–702. [Google Scholar]
- 17.Zhang Y, Hong JY, Rockwell CE, Copple BL, Jaeschke H, Klaassen CD. Effect of bile duct ligation on bile acid composition in mouse serum and liver. Liver Int. 2012 Jan;32(1):58–69. doi: 10.1111/j.1478-3231.2011.02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Berge H, Brandt KH, Eyssen H, Parmentier G. Sulphated and unsulphated bile acids in serum, bile, and urine of patients with cholestasis. Gut. 1976 Nov;17:861–869. doi: 10.1136/gut.17.11.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jansen T, Hortmann M, Oelze M, Opitz B, Steven S, Schell R, et al. Conversion of biliverdin to bilirubin by biliverdin reductase contributes to endothelial cell protection by heme oxygenase-1-evidence for direct and indirect antioxidant actions of bilirubin. J Mol Cell Cardiol. 2010 Aug;49(2):186–195. doi: 10.1016/j.yjmcc.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Sedlak TW, Saleh M, Higginson DS, Paul BD, Juluri KR, Snyder SH. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc Natl Acad Sci U S A. 2009 Mar 31;106(13):5171–5176. doi: 10.1073/pnas.0813132106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Youson JH. Differentiation of the segmented tubular nephron and excretory duct during lamprey metamorphosis. Anat Embryol (Berl) 1984;169(3):275–292. doi: 10.1007/BF00315633. [DOI] [PubMed] [Google Scholar]
- 22.Stieger B, Meier Y, Meier PJ. The bile salt export pump. Pflugers Arch. 2007 Feb;453(5):611–620. doi: 10.1007/s00424-006-0152-8. [DOI] [PubMed] [Google Scholar]
- 23.Lam P, Wang R, Ling V. Bile acid transport in sister of P-glycoprotein (ABCB11) knockout mice. Biochem. 2005 Sep 20;44(37):12598–12605. doi: 10.1021/bi050943e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.