Abstract
β2-Adrenoceptor (β2AR) agonists are the most effective class of bronchodilators and a mainstay of asthma management. The first potent β2AR agonist discovered and widely used in reversing the airway constriction associated with asthma exacerbation was the endogenous activator of the β2AR, epinephrine. In this study, we demonstrate that activation of the β2AR by epinephrine is paradoxically required for development of the asthma phenotype. In an antigen-driven model, mice sensitized and challenged with ovalbumin showed marked elevations in three cardinal features of the asthma phenotype: inflammatory cells in their bronchoalveolar lavage fluid, mucin over production, and airway hyperresponsiveness. However, genetic depletion of epinephrine using mice lacking the enzyme to synthesize epinephrine, phenylethanolamine N-methyltransferase, or mice that had undergone pharmacological sympathectomy with reserpine to deplete epinephrine, had complete attenuation of these three cardinal features of the asthma phenotype. Furthermore, administration of the long-acting β2AR agonist, formoterol, a drug currently used in asthma treatment, to phenylethanolamine N-methyltransferase–null mice restored the asthma phenotype. We conclude that β2AR agonist–induced activation is needed for pathogenesis of the asthma phenotype. These findings also rule out constitutive signaling by the β2AR as sufficient to drive the asthma phenotype, and may help explain why chronic administration of β2AR agonists, such as formoterol, have been associated with adverse outcomes in asthma. These data further support the hypothesis that chronic asthma management may be better served by treatment with certain “β-blockers.”
Keywords: β2-adrenoceptor agonists, formoterol, epinephrine, murine model, asthma
Clinical Relevance
Epinephrine depletion by either genetic ablation of its synthesizing enzyme, or by pharmacological sympathectomy, completely abolished the asthma phenotype in a murine model. Furthermore, in mice lacking the capacity to synthesize epinephrine, the asthma phenotype can be rescued by treatment with the β2-adrenoceptor agonist, formoterol, a drug currently used in asthma management. We conclude that, in this model, β2-adrenoceptor agonists are essential in the pathogenesis of the asthma phenotype, and this may explain why some clinical trials examining chronic use of β2-adrenoceptor agonists in asthma management have shown adverse outcomes.
Asthma is a disease characterized by airway inflammation, mucin hypersecretion, and airway hyperresponsiveness (AHR). The disease affects over 300 million people, and accounts for a huge financial burden from not only its treatment, but also from the lost productivity resulting from exacerbations of the disease (1). The introduction of epinephrine, the endogenous activator of the β2-adrenoceptor (β2AR), at the beginning of the 20th century for the treatment of asthma was considered a monumental step in therapy (2). For the first time ever, a drug reversed the bronchoconstriction produced by even moderate to severe asthma attacks within minutes of administration (3). Despite intensive research, the pharmacological mainstay of treatments, β2AR agonists and glucocorticosteroids, has remained the same for decades. Indeed, β2AR agonists are a part of asthma management for all stages of disease severity (4). However, several studies have shown that constant, chronic use of β2AR agonists—in particular, long-acting β2AR agonists—have been associated with adverse outcomes, including loss of asthma control and increases in respiratory-related deaths (5–11).
We have previously shown that chronic administration of a subset of “β-blockers,” β2AR inverse agonists, such as nadolol, prevent the development of cardinal features of asthma, such as airway inflammation, AHR, and mucous metaplasia, in murine asthma models (12–14). We then tested whether deletion of the β2AR would phenocopy chronic treatment with β2AR inverse agonists. These studies showed that β2AR-null mice also exhibited attenuated asthma phenotypes, and identified β2AR signaling as required for the pathogenesis of the asthma phenotype in murine models (15). However, our previous work did not establish whether the β2AR signaling allowing the development of the asthma phenotype was due to constitutive β2AR (or spontaneous) signaling by empty receptors, or whether ligand-induced activation of the β2AR was necessary.
In this study, we investigated the role of constitutive versus ligand-induced β2AR signaling, and show that, in an antigen-driven murine model of asthma, ligand activation of the β2AR is essential for the pathogenesis of the cardinal features of the disease. The results show that genetic depletion of the endogenous β2AR agonist, epinephrine, using mice lacking the enzyme catalyzing the final step in the synthesis of epinephrine, phenylethanolamine N-phenylethanolamine N-methyltransferase (PNMT−/− mice), or pharmacologic depletion of all catecholamines using reserpine, results in complete ablation of all the asthma phenotypes that we measured (inflammatory cells in bronchoalveolar lavage (BAL) fluid, mucin volume, and AHR). Furthermore, we show that, in PNMT−/− mice, replacement of epinephrine with the β2AR agonist, formoterol, a drug currently used for asthma treatment, restores the asthma phenotype.
This requirement of β2AR agonists in development of the asthma phenotype may explain why chronic administration of β2AR agonists is associated with negative outcomes in asthma therapy (5–11), and further supports the hypothesis that chronic asthma management may be better served by treatment with certain β-blockers.
Materials and Methods
Animals
PNMT−/− mice (6- to 8-wk old; obtained from Steven Ebert, University of Central Florida) and wild-type SvJ/129 mice (Jackson Laboratory, Bar Harbor, ME) were housed under specific pathogen-free conditions. All procedures were conducted in accordance with University of Houston Institutional Animal Care and use Committee approval.
Ovalbumin Sensitization and Challenge
Mice were sensitized with three intraperitoneal injections of 2 mg/kg/d of ovalbumin (Ova) (Sigma-Aldrich, St. Louis, MO) with 2 mg of Alum (Imject Alum; Thermo Scientific, Pierce, Rockford, IL). The mice were then challenged intranasally with 1 mg/kg/d Ova, as illustrated in Figures 1A and 1B. Control mice were sensitized with Ova and challenged with saline and administered an equivalent volume of vehicle.
Figure 1.
Treatment protocols. (A) Wild type (WT) and phenylethanolamine N-methyltransferase (PNMT)–null (PNMT−/−) mice received ovalbumin (Ova) (2 mg/kg/d in 2 mg of alum) intraperitoneally (i.p.), as indicated, on Days 0, 7, and 14; followed by intranasal (i.n.) challenge with Ova (1 mg/kg/day) or vehicle on Days 24–28. In addition, a group of WT mice also received vehicle or reserpine (5 mg/kg/day; bold vertical line) on Day 23 followed by vehicle or reserpine (0.3 mg/kg/d) on subsequent days until mice were killed. (B) WT and PNMT−/− mice received Ova (2 mg/kg/d in 2 mg of alum) intraperitoneally as indicated on Days 0, 7, and 14, followed by intranasal challenge with Ova (1 mg/kg/d) or vehicle on Days 41–45. In addition, groups of PNMT−/−mice received formoterol (10 μg/kg/d) as twice-daily intraperitoneal injections of 5 μg/kg, with the different start points as indicated. Please note that we have previously established that the two different time periods between the final sensitization and the first challenge does not alter any of the asthma phenotype responses (14, 15).
Drug Administration
Reserpine (Sigma-Aldrich) was administered to wild-type mice intraperitoneally as a solution in 4% wt/vol ascorbic acid solution (pH 5.0). A loading dose of 5 mg/kg, followed by a maintenance dose of 0.3 mg/kg for the next 5 days, was used as shown in Figure 1A. Formoterol (Sigma-Aldrich) was administered to PNMT−/− mice intraperitoneally at a dose of 5 μg/kg twice daily in ∼100-μl per injection of 99.95% saline and 0.05% DMSO (Merck KGaA, Darmstadt, Germany). Formoterol was administered to different groups of Ova-sensitized and -challenged (Ova S/C) mice for 6, 9, 12, or 19 days, as shown in Figure 1B. All final concentrations resulted in injections of 100 μl/20 g mouse. Control mice received an equal volume of vehicle.
BAL
BAL fluid was obtained by perfusing 500 μl of saline into the right lung after mice were killed with 45 mg/kg pentobarbital. The BAL fluid obtained was used to determine total and differential cell counts with a Hemacytometer (Hausser Scientific, Horsham, PA) and Wright Giemsa staining (Sigma-Aldrich), respectively.
Mucin Content Analysis
The left lung was fixed with cold normal buffered formalin (Sigma-Aldrich), and 5-μm sections were stained with periodic acid fluorescent Schiff's reagent followed by quantitative mucin analysis, as described previously (16). Investigators blinded to the treatment groups performed the calculations measuring mucin volume.
AHR
Mice were anesthetized with 240 mg/kg of ketamine HCL (Ketaject; Bioniche Teoranta Inverin Co., Galway, Ireland) and 48 mg/kg of xylazine (AnaSed Akorn Inc., Lake Forest, IL). Airway responses to 0–50 mg/ml aerosolized methacholine (Sigma-Aldrich) were measured using a Flexivent (SCIREQ, Montreal, PQ, Canada). To characterize AHR, we calculated total respiratory system resistance, along with indicators of airway sensitivity represented by provocative concentration of methacholine that causes a doubling of baseline airway resistance (PC100) and airway reactivity to methacholine (K). K is calculated as the slope of the log-linear interpolation of an exponential function fitted to the methacholine dose–response curve for each animal (17) (see supplemental Materials and Methods).
High-Performance Liquid Chromatography
Tissue and plasma levels of epinephrine and norepinephrine were measured using methods described (18). Briefly, catecholamines from homogenized adrenals and plasma were processed and injected into 1,525 binary high-performance liquid chromatography pump (Waters, Milford, MA) connected to 3-μ C18 (2) LUNA column (Phenomenex, Torrance, CA). Breezev.3.30 (Waters) was used for analysis of data from Coulochem-III (ESA, Dionex, Thermo Scientific, Sunnyvale, CA) detector.
Statistical Analysis
Data are expressed as means (±SEM). Statistical significance was determined at P < 0.05 using the two-tailed unpaired Student’s t test for two groups and multiple groups were analyzed using one-way ANOVA followed by Dunnet’s multicomparison test (GraphPad Prism 4, San Diego, CA) (19).
Results
Epinephrine Depletion and Treatment Protocols
We used a standard antigen-driven murine model in which mice were sensitized to Ova, followed by intranasal challenges with Ova after the last systemic sensitization (Ova S/C), as shown in Figures 1A and 1B. In this asthma model and in control mice, the endogenous β2AR agonist, epinephrine, was depleted using both genetic and pharmacological approaches. The genetic approach consisted of using mice null for PNMT. Mice with genetic deletion of the enzyme have no detectable epinephrine in plasma, and <2% of the epinephrine in adrenal glands as compared with control mice (Table 1).
TABLE 1.
EFFECT OF GENETIC OR PHARMACOLOGICAL DEPLETION OF EPINEPHRINE ON PLASMA AND ADRENAL GLAND LEVELS OF NOREPINEPHRINE AND EPINEPHRINE
| Plasma (pg/μl) |
Adrenals (ng/mg) |
|||
| Norepinephrine | Epinephrine | Norepinephrine | Epinephrine | |
| WT | 166.94 ± 18.06 | 135.88 ± 18.87 | 233.64 ± 71.45 | 244.13 ± 50.86 |
| WT Ova S/C | 96.64 ± 25.80 | 107.93 ± 27.98 | 185.85 ± 59.33 | 217 ± 43.26 |
| PNMT−/− | 153.11 ± 7.52 | BLQ | 326.97 ± 54.06 | 12.36 + 5.28* |
| PNMT−/− Ova S/C | 86.91 ± 15.66 | BLQ | 374.12 ± 25.41 | 1.19 ± 0.37* |
| WT + reserpine | BLQ | BLQ | 8.64 ± 1.55† | 6.48 + 2.31* |
| WT + reserpine Ova S/C | BLQ | BLQ | 1.12 ± 4.85† | 5.86 ± 2.91* |
Definition of abbreviations: BLQ, below the limit of quantitation; Ova, ovalbumin; PNMT−/−, phenylethanolamine N-methyltransferase null; S/C, sensitization and challenge; WT, wild type.
Adrenal glands and plasma were collected from different groups and processed with standards as described in Materials and Methods. The samples were analyzed using high-performance liquid chromatography (HPLC) coupled to a coulometric detector. Shown are plasma and adrenal contents of epinephrine and norepinephrine in WT, PNMT−/− mice, and reserpine-treated WT mice with or without Ova S/C. Data are means (±SEM) from three to seven mice in each group. Data below 50 pg/μl are indicated as BLQ.
P < 0.05 significance as compared to epinephrine levels of respective treatment WT mice.
P < 0.05 significance as compared to norepinephrine levels of respective treatment WT mice.
As shown in Figure 1A, the pharmacologic approach consisted of administering reserpine to deplete epinephrine and other monoamines. This resulted in depletion of both epinephrine and norepinephrine below detection levels in plasma, and the epinephrine content of the adrenal glands was reduced to <3% of control mice (Table 1).
Effect of Epinephrine Depletion on Inflammatory Cells in BAL Fluid
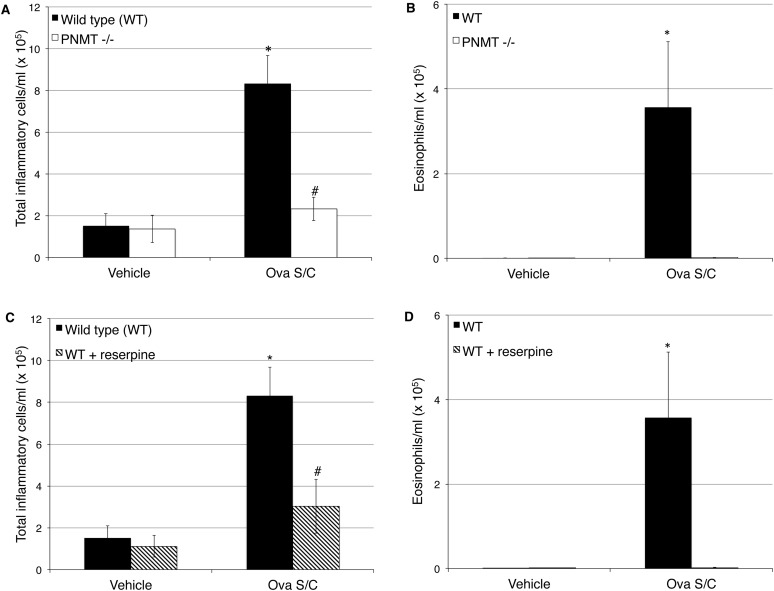
We then tested the effect of epinephrine depletion using both the genetic and pharmacologic methods on cardinal features of asthma, including airway inflammation, mucin metaplasia, and airway hyperreactivity. In wild-type mice, Ova S/C produced significant increases of roughly fivefold in total cells (Figures 2A and 2C) and even larger fold increases in eosinophils (Figures 2B and 3D) in the BAL fluid. Both methods of epinephrine depletion resulted in complete inhibition of the increase of inflammatory cells and eosinophils in the BAL fluid observed in wild-type Ova S/C mice (Figures 2A–2D).
Figure 2.
Effect of epinephrine depletion on inflammatory cells in bronchoalveolar lavage (BAL) fluid. The graphs show the total inflammatory cells and eosinophil infiltration in the BAL fluid of Ova-sensitized and -challenged (Ova S/C) mice. (A and B) Effect of genetic depletion of epinephrine. (A) Total inflammatory cell count from BAL fluid of Ova S/C WT and PNMT−/− mice in comparison to non–Ova S/C mice. (B) Eosinophil count from BAL fluid of Ova S/C WT and PNMT−/− mice in comparison to non–Ova S/C mice. (C and D) Effect of pharmacological depletion of epinephrine. (C) Total inflammatory cell count from BAL fluid of Ova S/C WT mice with and without reserpine administration in comparison to vehicle-treated control mice. (D) Eosinophil count from BAL fluid of Ova S/C WT mice with and without reserpine administration in comparison to vehicle-treated control mice. Data represent the mean (±SEM) from three to seven mice in each group. “Vehicle-treated mice” refers to Ova-sensitized and saline-challenged mice. *P < 0.05 significance as compared with respective vehicle-treated mice; #P < 0.05 significance as compared with WT Ova S/C.
Figure 3.
Effect of epinephrine depletion on mucin content. Mucin (red) content in the airway epithelia (green) was measured after periodic acid fluorescent Schiff’s reagent (PAFS) staining. (A) Mucin staining in the airway epithelia for WT mice with or without reserpine administration and PNMT−/− mice with or without Ova S/C; effect of genetic depletion of epinephrine. (B) Morphometric quantification of the mucin volume density assessed from WT and PNMT−/− mice with and without Ova S/C. Effect of pharmacological depletion of epinephrine. (C) Morphometric quantification of the mucin volume density assessed from WT and WT mice administered reserpine with and without Ova S/C. Scale bar (white), 100 μm. Data represent the mean (±SEM) from three to seven mice in each group. Vehicle-treated mice refers to Ova sensitized and saline challenged mice. *P < 0.05 significance as compared with respective vehicle-treated mice; #P < 0.05 significance as compared with WT Ova S/C.
Effect of Epinephrine Depletion on Airway Mucin
Epinephrine depletion also completely prevented increases in mucin production by the airway epithelium. The Ova S/C control mice developed an ∼40-fold increase in mucin volume density compared with vehicle-treated mice (Figures 3A–3C). However, both genetic and pharmacological depletion of epinephrine again completely prevented any increase in mucin volume density with Ova S/C (Figures 3A–3C).
Effect of Epinephrine Depletion on Airway Function
In addition to airway inflammation and mucous metaplasia, we also examined the effect of genetic and pharmacologic depletion of epinephrine on another cardinal feature of asthma, airway function. Whereas Ova S/C in wild-type mice was associated with a significant increase in methacholine-induced AHR relative to non–Ova S/C wild-type mice, Ova S/C had no effect on methacholine-induced AHR in PNMT−/− or reserpine-treated mice (Figures 4A and 4D). Airway sensitivity (as reflected by a decreased PC100) was significantly increased by OVA S/C treatment, but only in wild-type, and not in PNMT−/− or reserpine-treated mice (Figures 4B and 4E). Consistent with the observed increase in airway sensitivity, airway reactivity was significantly elevated by OVA S/C treatment, but only in wild-type, and not PNMT−/− or reserpine-treated mice (Figures 4C and 4F). Furthermore, airway sensitivity and reactivity in Ova S/C wild-type mice were significantly elevated compared with those in Ova S/C PNMT−/− mice (Figures 4B and 4C). Although Ova S/C reserpine-treated mice qualitatively also showed reduced airway sensitivity and reactivity relative to those in Ova S/C wild-type mice, statistical significance was not achieved (Figures 4E and 4F).
Figure 4.
Effect of epinephrine depletion on airway hyperresponsiveness (AHR). Total respiratory system resistance (Rrs) in response to increasing doses of nebulized methacholine (0–50 mg/ml) was measured using forced oscillation technique. Rrs was determined by averaging the two highest resistance responses produced for each mouse at each methacholine dose. A lower value for sensitivity (provocative concentration of methacholine that causes a doubling of baseline airway resistance [PC100]) and a higher value for reactivity to methacholine (K) represents increased airway responsiveness. (A–C) Effect of genetic depletion of epinephrine on Rrs, PC100, K for WT and PNMT−/− mice with or without Ova S/C. (D–F) Effect of pharmacological depletion of epinephrine on Rrs, PC100, and K for WT and reserpine-treated WT mice with or without Ova S/C. Data represent the mean (±SEM) from three to seven mice in each group. “Vehicle-treated mice” refers to Ova-sensitized and saline-challenged mice. @P < 0.05 significant effect of Ova S/C on WT mice relative to other groups; *P < 0.05 significance as compared with respective vehicle-treated mice; #P < 0.05 significance of PNMT−/− or reserpine treated mice as compared with respective WT mice.
Rescue of the Asthma Phenotypes with Formoterol in Epinephrine-Deficient Mice
We then investigated the effect on the asthma phenotypes of restoring β2AR signaling in PNMT−/− mice by administering the β2AR agonist, formoterol. We used a dose of formoterol of 10 μg/kg/d intraperitoneally (5 μg/kg intraperitoneal, administered twice daily) for the various treatment periods described in Figure 1B. For inflammatory cell infiltration in BAL fluid (Figures 5A and 5B), formoterol administration exhibited a time-dependent restoration of the inflammatory cells and eosinophils in the airways (Figures 5A and 5B). Formoterol treatment for 6 days did not recover the inflammation response, as there was no significant increase in total cells or eosinophils in BAL compared with control Ova S/C mice (Figures 5A and 5B). However, formoterol administration for 9, 12, and 19 days produced significant increases in total inflammatory cells, and for 12 and 19 days produced significant increases in eosinophils compared with Ova S/C control mice (Figures 5A and 5B). Formoterol administration for 6, 9, 12, and 19 days also resulted in a time-dependent increase in mucin production (Figures 6A and 6B). Finally, we examined the effect of formoterol administration to PNMT−/− mice on the changes in airway responsiveness. Administration of formoterol at 6 or 9 days significantly increased airway sensitivity and reactivity to methacholine in Ova S/C PNMT−/− mice (Figures 7A, 7B and 7C, respectively). This trend remained comparable with 12 and 19 days of formoterol administration. However, formoterol administration for 6 days was insufficient to completely rescue the airway responsiveness phenotype as compared with that of Ova S/C wild-type mice (Figures 7B and 7C).
Figure 5.
Effect of formoterol and duration of administration on total inflammatory cell and eosinophil count in BAL fluid. (A) Total inflammatory cell count from BAL fluid of Ova S/C WT and PNMT−/− mice with or without formoterol administration. (B) Eosinophil count from BAL fluid of Ova S/C WT and PNMT−/− mice with or without formoterol administration. The numbers indicate the days of formoterol (10 μg/kg/d) administration. Data represent the mean (±SEM) from three to seven mice in each group. *P < 0.05 significance as compared with Ova S/C WT mice; #P < 0.05 significance as compared with Ova S/C PNMT−/− mice.
Figure 6.
Effect of formoterol and duration of administration on mucin content. Mucin (red) content in the airway epithelia (green) was measured after PAFS staining for 6, 9, 12, and 19 days (6D, 9D, 12D, and 19D, respectively) of formoterol administration. (A) Mucin content in the airway epithelia for Ova S/C WT and PNMT−/− with or without formoterol administration. (B) Morphometric quantification of the mucin volume density assessed from Ova S/C WT and PNMT−/− mice with or without formoterol administration. The numbers indicate the days of formoterol (10 μg/kg/d) administration in Ova S/C PNMT−/− mice. Scale bar (white), 100 μm. Data represent the mean (±SEM) from three to seven mice in each group. *P < 0.05 significance as compared with Ova S/C WT mice; #P < 0.05 significance as compared with Ova S/C PNMT−/− mice.
Figure 7.
Effect of formoterol and duration of administration on AHR. Total Rrs in response to increasing doses of nebulized methacholine (0–50 mg/ml) was measured using forced oscillation technique. Rrs was determined by averaging the two highest resistance responses produced for each mouse by each methacholine dose. A lower value for sensitivity (PC100) and a higher value for reactivity (K) represent increased airway responsiveness. (A–C) The graphs show the Rrs, PC100, and K in Ova S/C WT mice and Ova S/C PNMT−/− mice with or without formoterol administration. The numbers indicate the days of formoterol (10 μg/kg/d) administration in Ova S/C PNMT−/− mice. Data represent the mean (±SEM) from three to five mice in each group. *P < 0.05 significance as compared with Ova S/C WT mice; #P < 0.05 significance as compared with Ova S/C PNMT−/− mice.
Discussion
Epinephrine is the endogenous ligand for the β2AR, and was the first widely accepted treatment for asthma exacerbations due to its potent bronchodilatory effect (2). However, our results show that depletion of epinephrine by either genetic deletion of the enzyme involved in the final step of its synthesis, or by “pharmacologic sympathectomy” with reserpine, prevents development of the asthma phenotype in a murine asthma model. Furthermore, replacement of β2AR signaling by administration of formoterol, a β2AR agonist currently used in asthma treatment, time-dependently restored the asthma phenotype. Thus, our results show that, although β2AR agonists are potent bronchodilators that are used at every step of asthma management, paradoxically, they are also essential for the pathogenesis of the asthma phenotype.
Our previous studies with β2AR-null mice identified β2AR signaling as required for the pathogenesis of the asthma phenotype in murine models, but did not establish whether the β2AR signaling required for the development of the asthma phenotype was due to constitutive (or spontaneous) signaling by empty receptors, or whether ligand-induced activation of the β2AR was necessary (15). Answering that question was the purpose of the present studies. This is an important question, because if ligand activation of the β2AR is necessary, it would call in to question the rationale of chronic administration of these drugs in the management of asthma. Our results indeed demonstrate that, in this model, ligand activation of the β2AR drives the asthma phenotype. Whether we performed loss of function experiments by genetically or pharmacologically depleting the endogenous β2AR ligand, epinephrine, or whether we performed gain-of-function experiments by administering the long-acting β2AR agonist, formoterol, a drug currently used in the treatment of all stages of asthma, to mice lacking endogenous epinephrine, the data indicate that β2AR agonists are essential for development of the asthma phenotype.
The genetic approach testing the effect of epinephrine depletion in the development of the asthma phenotype should selectively deplete epinephrine, because this enzyme catalyzes the final step in epinephrine synthesis. As expected, PNMT−/− mice had normal levels of norepinephrine (Table 1). The sole pharmacological difference between epinephrine and norepinephrine is that, whereas norepinephrine activates eight of the nine known adrenoceptor subtypes (α1A, α1B, α1D, α2A, α2B, α2C, β1, and β3), with near comparable potency to epinephrine, norepinephrine is much less potent than epinephrine at the β2AR (20). This difference in potency between epinephrine and norepinephrine for the β2AR was part of the evidence for the original subdivision of βARs into β1AR and β2AR subtypes by Lands and colleagues (21). These authors demonstrated that, in tissues containing the proposed β1AR, both epinephrine and norepinephrine exhibited equal potency at activating the receptor, whereas, in tissues containing the proposed β2AR, epinephrine was much more potent than norepinephrine (21).
The pharmacologic approach used reserpine administration according to the protocol illustrated in Figure 1A. Reserpine produces pharmacological sympathectomy by inhibiting the vesicular monoamine transporter (VMAT). VMAT loads monoamines into vesicles, and thus protects them from degradation by monoamine oxidase and catecholamine O-methyltransferase (22), but is not selective for epinephrine depletion, as norepinephrine and dopamine are also substrates for VMAT. The reserpine treatment protocol (Figure 1A) resulted in reductions in epinephrine very similar to those observed with the genetic approach (no detectable circulating levels of epinephrine and over 97% depletion in adrenal glands; compare in Table 1). However, unlike in PNMT mice, where norepinephrine levels remained comparable to those in wild-type mice (Table 1), reserpine treatment, as expected, depleted norepinephrine equally as effectively as it depleted epinephrine (Table 1).
One feature of the murine model that mimics asthma is the migration of inflammatory cells to the lungs. Wild-type Ova S/C mice showed significant increases in total inflammatory cells and eosinophils in BAL fluid compared with nonchallenged control mice (Figures 2A–2D). Either selective depletion of only the endogenous β2AR agonist (Figures 2A and 2B), or complete pharmacologic sympathectomy with reserpine (Figures 2C and 2D), completely suppressed the increase in inflammatory cells and eosinophilia in BAL fluid produced by Ova S/C. These results demonstrate that agonist activation of the β2AR is essential for development of inflammation in Ova S/C mice.
In murine models of asthma, it has been shown that Clara cells of the airway epithelium can differentiate into mucus-producing goblet cells (23, 24) and greatly increase mucin volume in the airways. In our model, both the genetic and pharmacological depletion methods demonstrated the necessary role of epinephrine for augmented mucin production in Ova S/C mice as compared with the control mice (Figures 3A–3C).
Ova S/C wild-type mice exhibited changes in airway function that were also consistent with the asthma phenotype, as indicated by increased methacholine-induced airway responsiveness (Figures 4A and 4D), sensitivity (as reflected by a decreased PC100; Figure 4B and 4E), and reactivity (Figures 4C and 4F). As with inflammatory cells in BAL, and mucin production, either genetic depletion of epinephrine (Figures 4A–4C) or pharmacologic sympathectomy with reserpine treatment (Figures 4D–4F) attenuated the changes in airway responsiveness, and resulted in readings similar to those in non–Ova S/C mice. The lack of a significant difference in airway sensitivity and reactivity between Ova S/C wild-type and Ova S/C reserpine-treated mice may have resulted from the differences discussed previously here regarding genetic depletion of epinephrine as more specific than reserpine treatment, which depletes all catecholamines (Table 1).
In the next set of experiments, we determined whether replacement of agonist-induced β2AR signaling in PNMT−/− mice would restore the asthma phenotype, and, if so, the time course for restoration of the asthma phenotype. We chose systemic administration of formoterol instead of epinephrine as the ligand to restore β2AR agonist–activated signaling. The reason for this choice was several fold: (1) epinephrine has an extremely short half-life and would require infusion by osmotic minipump; however, even dissolved in 100 μM ascorbic acid to prevent oxidation, epinephrine was unstable at 37°C. Incubation of the required concentration in a water bath at 37°C resulted in significant degradation after just 3 days (data not shown); (2) formoterol has similar efficacy to epinephrine and a comparable rapid onset, but has a longer half-life, allowing for the convenience of twice-daily administration (25); and (3) formoterol is currently used in asthma therapy, and is associated with some adverse outcomes with chronic administration (26).
In order to select the dose of formoterol to use, we found several studies using 100 μg/kg/d, and a study using what was described as a “low dose” of 25 μg/kg/d of formoterol (27–30). However, in the current study, we were trying to simply “replace” β2AR signaling, and not to elicit a “drug” response. Therefore, we decided to use an even lower dose of 10 μg/kg/d formoterol.
As stated previously here, PNMT−/− mice lack the ability to produce epinephrine, yet have normal levels of norepinephrine. Administration of formoterol was able to restore cardinal features of asthma (number of days indicates minimal treatment time until significant restoration of the asthma phenotype), including: the increase in inflammatory cell infiltration (9 d; Figure 5A) and eosinophilia (12 d; Figure 5B); increased mucin production (6 d; Figures 6A and 6B); and the changes in airway responsiveness (6–19 d; Figures 7A–7C). The inability to evoke an asthma phenotype in these mice that lack the only endogenous β2AR agonist, plus the ability to restore the asthma phenotype by administering the exogenous β2AR agonist, formoterol, clearly demonstrate that agonist activation of the β2AR is required for development of the asthma phenotype in murine models.
Our present data also confirm previous pharmacological results showing that the response to allergen is not due to the effect of β2AR signaling on the process of sensitization to allergen (14). Those results showed that whether administration of β-blockers was begun before and continued during the sensitization process, or if drug treatment was not started until after the sensitization process was completed, the inhibition of asthma phenotype was identical (14). The current results show that reserpine depletion, which occurs after the sensitization phase of the model is completed, abolished development of the asthma phenotype (Figures 2C, 2D, 3C, and 4D–4E). Similarly, sensitization of PNMT−/− mice, followed by formoterol administration after the sensitization phase, completely restored the asthma phenotype (Figures 5A, 5B, 6A, 6B, and 7A–7C). Thus, these results confirm our previous pharmacological data showing that the presence or absence of β2AR signaling during sensitization has no effect on the development of any of the asthma phenotypes in response to allergen.
Another possible confounding explanation is that some overt phenotype of the PNMT−/− mice (i.e., impaired cardiac output resulting in decreased perfusion of the lungs) could account for the observed results. However, PNMT−/− mice look and breed like normal mice (31). They have normal blood pressure, body temperature, and other parameters that have been measured (32, 33). The only phenotypes reported for these mice were induced in response to manipulations, such as treadmill exercise, high-fat diets, and cold exposure. Thus, under normal conditions and diet, these mice have no reported phenotype (32–34).
We also considered the fact that depletion of the endogenous agonist could have resulted in a state of super sensitivity to subsequent agonist exposures. Indeed, not only has such a super sensitivity of response been reported after reserpine treatment, but also PNMT−/− mice that have been pithed to remove all sympathetic innervation produce an exaggerated response to the blood pressure–lowering effects of salbutamol (32). However, in testing our hypothesis of whether constitutive or agonist-induced signaling was responsible for the asthma phenotype, the development of super sensitivity would have resulted in an increased likelihood of finding a role for constitutive β2AR signaling. Thus, it could have led us to a “false positive” for the role of constitutive signaling, but our results show no evidence of any constitutive signaling, even in the presence of the possible up-regulation of receptor numbers.
These results have important therapeutic implications. From a receptor theory perspective, the results demonstrate that constitutive signaling by β2ARs is not sufficient to promote the asthma phenotype—and that chronic β2AR agonist activation is required for the pathogenesis of the asthma phenotype. The results further question the chronic use of β2AR agonists as an effective means of the chronic management of asthma. Indeed, these data would support the hypothesis that, for chronic asthma management, shutting down β2AR signaling may be a better approach (35, 36). The temporal requirement of days or weeks of β2AR agonist activation also mirrors that treatment with β2AR inverse agonists, such as nadolol, also takes chronic treatment of days or weeks to suppress the asthma phenotype in murine models (12, 13, 15) and to decrease AHR in subjects with mild asthma treated with nadolol (37, 38).
These results are consistent with the “β-paradox” phenomena—the observation that, although acute use of β2AR agonists can be beneficial due to their potent bronchodilator properties, their constant chronic use may lead to the worsening of asthma morbidity and mortality (5, 11, 39–41). Although many more studies are needed, there is increasing evidence that the detrimental effect of chronic β2AR agonist treatment may be a result of β2AR signaling via the β-arrestin pathway, as opposed to the canonical Gs-cAMP pathway (40, 42). The first clue as to the importance of β-arrestin–dependent signaling to asthma development is the observation that development of the asthma phenotype is virtually nonexistent in mice lacking β-arrestin-2 (or arrestin-3) (42). Although it remains to be definitively shown whether or not β-arrestin–dependent signaling mediates the proasthma effect of β2AR activation, if indeed it is β-arrestin signaling, and not the canonical Gs-cAMP pathway, that is required for development of asthma, it may allow a different drug development strategy. Future therapies may include β2AR agonists that are biased and preferentially activate the canonical Gs pathway, and/or the development of preferential β2AR inverse agonists that inhibit β-arrestin signaling, or the use of both in combination (40). This would result in retaining the beneficial effects of β2AR agonists, while eliminating the adverse effects observed with chronic β2AR activation. Future studies and clinical trials will eventually lead to a resolution of this β-paradox.
Supplementary Material
Acknowledgments
The authors thank Manish Taneja and Douglas Eikenburg for their expertise in optimizing the high-performance liquid chromatography protocols, and Bhupinder Singh and Douglass M. Diak for their technical assistance.
Footnotes
This work was supported by National Institutes of Health grants 1RO1A179236 (R.A.B., B.J.K., B.F.D., and J.K.L.W.) and 1R01HL084123 (J.K.L.W.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0364OC on November 29, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.American Lung Association American Lung Association. Trends in asthma morbidity and mortality. Washington, DC: American Lung Association Epidemiology & Statistics Unit Research and Program Services; 2007
- 2.Rau JL. Inhaled adrenergic bronchodilators: historical development and clinical application. Respir Care 2000;45:854–863 [PubMed] [Google Scholar]
- 3.Tattersfield AE. Current issues with beta2-adrenoceptor agonists: historical background. Clin Rev Allergy Immunol 2006;31:107–118 [DOI] [PubMed] [Google Scholar]
- 4.Aldington S, Beasley R. Asthma exacerbation: 5. Assesment and management of severe asthma in adults in hospital. Thorax 2007;62:447–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drazen JM, Israel E, Boushey HA, Chinchilli VM, Fahy JV, Fish JE, Lazarus SC, Lemanske RF, Martin RJ, Peters SP, et al. Comparison of regularly scheduled with as-needed use of albuterol in mild asthma. Asthma Clinical Research Network. N Engl J Med 1996;335:841–847 [DOI] [PubMed] [Google Scholar]
- 6.Cockcroft DW, O’Byrne PM, Swystun VA, Bhagat R. Regular use of inhaled albuterol and the allergen-induced late asthmatic response. J Allergy Clin Immunol 1995;96:44–49 [DOI] [PubMed] [Google Scholar]
- 7.Abramson MJ, Walters J, Walters EH. Adverse effects of beta-agonists: are they clinically relevant? Am J Respir Med 2003;2:287–297 [DOI] [PubMed] [Google Scholar]
- 8.Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med 2006;144:904–912 [DOI] [PubMed] [Google Scholar]
- 9.Shore SA, Drazen JM. Beta-agonists and asthma: too much of a good thing? J Clin Invest 2003;112:495–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists: is there cause for concern? Drug Saf 1997;16:295–308 [DOI] [PubMed] [Google Scholar]
- 11.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The salmeterol multicenter asthma research trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006;129:15–26 [DOI] [PubMed] [Google Scholar]
- 12.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA 2004;101:4948–4953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin R, Peng H, Nguyen LP, Dudekula NB, Shardonofsky F, Knoll BJ, Parra S, Bond RA. Changes in beta 2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulm Pharmacol Ther 2008;21:115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LP, Omoluabi O, Parra S, Frieske JM, Clement C, Ammar-Aouchiche Z, Ho SB, Ehre C, Kesimer M, Knoll BJ, et al. Chronic exposure to β-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol 2008;38:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. B2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA 2009;106:2435–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccotti L, Dickey BF, Evans CM. Assessment of intracellular mucin content in vivo. Methods Mol Biol 2012;842:279–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans KL, Bond RA, Corry DB, Shardonofsky FR. Frequency dependence of respiratory system mechanics during induced constriction in a murine model of asthma. J Appl Physiol 2003;94:245–252 [DOI] [PubMed] [Google Scholar]
- 18.Patki G, Che Y, Lau YS. Mitochondrial dysfunction in the striatum of aged chronic mouse model of Parkinson’s disease. Front Aging Neurosci 2009;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosner B. Fundamentals of biostatistics. Boston: Brooks/Cole, Cengage Learning; 2011.
- 20. Guide to receptors and channels (GRAC), 4th ed. Br J Pharmacol 2009;158:S1–S254. [DOI] [PMC free article] [PubMed]
- 21.Lands AM, Arnold A, McAuliff JP, Luduena FP, Brown TG., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature 1967;214:597–598 [DOI] [PubMed] [Google Scholar]
- 22.Goodman LS, Brunton LL, Chabner B, Knollmann BC. Goodman & Gilman’s pharmacological basis of therapeutics. New York: McGraw-Hill; 2011.
- 23.Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, et al. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol 2004;31:382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff A, Clevers H, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest 2009;119:2914–2924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore RH, Millman EE, Godines V, Hanania NA, Tran TM, Peng H, Dickey BF, Knoll BJ, Clark RB. Salmeterol stimulation dissociates beta2-adrenergic receptor phosphorylation and internalization. Am J Respir Cell Mol Biol 2007;36:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cates CJ, Cates MJ. Regular treatment with formoterol for chronic asthma: serious adverse events. Cochrane Database Syst Rev 2012;4:CD006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harcourt LJ, Schertzer JD, Ryall JG, Lynch GS. Low dose formoterol administration improves muscle function in dystrophic MDX mice without increasing fatigue. Neuromuscul Disord 2007;17:47–55 [DOI] [PubMed] [Google Scholar]
- 28.Pearen MA, Myers SA, Raichur S, Ryall JG, Lynch GS, Muscat GE. The orphan nuclear receptor, NOR-1, a target of beta-adrenergic signaling, regulates gene expression that controls oxidative metabolism in skeletal muscle. Endocrinology 2008;149:2853–2865 [DOI] [PubMed] [Google Scholar]
- 29.Leger B, Koopman R, Walrand S, Gehrig SM, Murphy KT, Lynch GS. Chronic formoterol administration reduces cardiac mitochondrial protein synthesis and oxidative capacity in mice. Int J Cardiol 2011;146:270–272 [DOI] [PubMed] [Google Scholar]
- 30.Koopman R, Gehrig SM, Leger B, Trieu J, Walrand S, Murphy KT, Lynch GS. Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic {beta}-adrenoceptor stimulation in mice. J Physiol 2010;588:4811–4823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebert SN, Rong Q, Boe S, Thompson RP, Grinberg A, Pfeifer K. Targeted insertion of the cre-recombinase gene at the phenylethanolamine n-methyltransferase locus: a new model for studying the developmental distribution of adrenergic cells. Dev Dyn 2004;231:849–858 [DOI] [PubMed] [Google Scholar]
- 32.Sun P, Bao X, Elayan H, Milic M, Liu F, Ziegler MG. Epinephrine regulation of hemodynamics in catecholamine knockouts and the pithed mouse. Ann N Y Acad Sci 2008;1148:325–330 [DOI] [PubMed] [Google Scholar]
- 33.Sharara-Chami RI, Joachim M, Mulcahey M, Ebert S, Majzoub JA. Effect of epinephrine deficiency on cold tolerance and on brown adipose tissue. Mol Cell Endocrinol 2010;328:34–39 [DOI] [PubMed] [Google Scholar]
- 34.Ziegler MG, Milic M, Sun P, Tang CM, Elayan H, Bao X, Cheung WW, O’Connor DT. Endogenous epinephrine protects against obesity induced insulin resistance. Auton Neurosci 2011;162:32–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bond RA. Is paradoxical pharmacology a strategy worth pursuing? Trends Pharmacol Sci 2001;22:273–276 [DOI] [PubMed] [Google Scholar]
- 36.Bond RA. Can intellectualism stifle scientific discovery? Nat Rev Drug Discov 2002;1:825–829 [DOI] [PubMed] [Google Scholar]
- 37.Hanania NA, Mannava B, Franklin AE, Lipworth BJ, Williamson PA, Garner WJ, Dickey BF, Bond RA. Response to salbutamol in patients with mild asthma treated with nadolol. Eur Respir J 2010;36:963–965 [DOI] [PubMed] [Google Scholar]
- 38.Hanania NA, Singh S, El-Wali R, Flashner M, Franklin AE, Garner WJ, Dickey BF, Parra S, Ruoss S, Shardonofsky F, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: an open-label pilot study. Pulm Pharmacol Ther 2008;21:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parra S, Bond RA. Inverse agonism: from curiosity to accepted dogma, but is it clinically relevant? Curr Opin Pharmacol 2007;7:146–150 [DOI] [PubMed] [Google Scholar]
- 40.Walker JK, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding beta(2)-adrenoceptor ligands in the treatment of asthma. Br J Pharmacol 2011;163:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, Yates DM, Lucas MK, Herbison GP. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet 1990;336:1391–1396 [DOI] [PubMed] [Google Scholar]
- 42.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest 2003;112:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.