Abstract
Raf-1 is a serine/threonine protein kinase that has an essential role in cell proliferation. The mechanisms that regulate Raf-1 in airway smooth muscle are not well understood. In this study, treatment with platelet-derived growth factor (PDGF) induced spatial redistribution of Raf-1 from the cytoplasm to the periphery of human airway smooth muscle cells. Moreover, a pool of Raf-1 was found in F-actin of human airway smooth muscle cells. Activation with PDGF led to an increase in the association of Raf-1 with cytoskeletal actin. Treatment of cells with the actin polymerization inhibitor latrunculin A (LAT-A), but not the microtubule depolymerizer nocodazole, inhibited the interaction of Raf-1 with actin in response to PDGF activation. Because abelson tyrosine kinase (Abl) is known to specifically regulate actin dynamics in smooth muscle, the role of Abl in modulating the coupling of Raf-1 with actin was also evaluated. Abl knockdown by RNA interference attenuated the association of Raf-1 with actin, which is recovered by Abl rescue. Treatment with LAT-A, but not nocodazole, inhibited the spatial redistribution of Raf-1 during PDGF activation. However, treatment with both LAT-A and nocodazole attenuated smooth muscle cell proliferation. Finally, Abl knockdown attenuated the redistribution of Raf-1 and cell proliferation, which were restored by Abl reexpression. The results suggest a novel mechanism that the interaction of Raf-1 with cytoskeletal actin is critical for Raf-1 redistribution and airway smooth muscle cell proliferation during activation with the growth factor.
Keywords: Raf-1, actin polymerization, tyrosine kinase, smooth muscle, cell proliferation
Clinical Relevance
Raf-1 is a serine/threonine protein kinase that has an essential role in cell proliferation. The present study suggests that the interaction of Raf-1 with actin is critical for Raf-1 activation in airway smooth muscle cells. This novel finding may provide new insights into the mechanism that controls smooth muscle cell proliferation.
Raf-1 is a serine/threonine protein kinase that has an essential role in cell proliferation, which is important for the development and homeostasis of the airways, and contributes to the pathogenesis of asthma (1). In response to stimulation with growth factors, the adapter protein Grb2, in association with the guanine exchange factor Sos, attaches to the tyrosine phosphorylated receptors through its SH2 domains. This brings the Grb2/Sos complex into the vicinity of the membrane by which it catalyzes guanine nucleotide exchange on Ras. The activated Ras induces the recruitment of Raf-1 from the cytoplasm to the membrane by poorly characterized mechanisms. Ras associates with Raf-1, which subsequently activates Raf-1. Activated Raf-1 phosphorylates MEK1/2 (MAP kinase kinase), which in turn phosphorylates and activates ERK1/2 (1, 2). It has been proposed that activated ERK1/2 translocates from the cytoplasm to the nucleus, by which ERK1/2 phosphorylates several protein kinases, nuclear transcription factors, and other proteins to promote cell proliferation (2, 3).
Although the role of Raf-1 in cell proliferation is well recognized, the mechanisms that regulate Raf-1 spatial translocation are not well understood. It has been assumed that the interactions of Raf-1 with Ras are the primary mechanism facilitating the recruitment of Raf-1 to the cell membrane (2, 3). In addition, phosphatydic acid has been implicated in the translocation of Raf-1 to the membrane. Phosphatydic acid interacts with Raf-1 in an in vitro biochemical system. Inhibition of phosphatydic acid by a pharmacological tool attenuated the translocation of green fluorescence protein–tagged Raf-1, which is rescued by the addition of phosphatydic acid (4). However, other mechanisms that regulate the spatial translocation of Raf-1 may exist.
The actin cytoskeleton has been implicated in mediating intracellular trafficking of the glucose transporter GLUT4. In adipocytes and striated muscle cells, GLUT4 undergoes spatial translocation to the plasma membrane from the cytoplasm in response to insulin activation, which may promote glucose uptake. Inhibition of actin polymerization by molecular approaches attenuates the intracellular trafficking of GLUT4 during insulin activation (5).
In nonmuscle cells such as neurons, microtubules serve as “tracks” for the movement of intracellular cargo (e.g., channels, vesicles) powered by motor proteins such as dynein and kinesin. Disruption of microtubules impairs the intracellular transport and thus excitation, repair, and regeneration of nerves (6, 7). In addition, microtubules may direct the transport of GLUT4 to the cell cortex via a kinesin motor (5).
Recent studies have shown that actin polymerization transpires in smooth muscle in response to activation with various stimuli (8–10). Actin dynamics plays an important role in regulating smooth muscle contraction and cell migration (11–13).
Abl (Abelson tyrosine kinase, C-Abl) is a nonreceptor tyrosine kinase that is able to regulate actin polymerization in various cell types including smooth muscle cells (8–12, 14). Abl has been shown to participate in the regulation of a range of cellular functions including migration and adhesion of nonmuscle cells (10, 15) and smooth muscle contraction (8, 9, 14, 16). Recent studies have demonstrated that Abl kinase has a role in the activation of ERK1/2 (a known effector of Raf-1) and smooth muscle cell proliferation (17).
The objective of this study was to evaluate whether the actin cytoskeleton and microtubules are involved in regulating Raf-1 translocation in human airway smooth muscle cells in response to the activation with platelet-derived growth factor (PDGF), a growth factor known to activate Raf-1. Because Abl specifically controls actin dynamics in smooth muscle, we also evaluated the role of Abl in this cellular process.
Materials and Methods
Cell Culture
Human airway smooth muscle (HASM) cells were obtained from the laboratory of Dr. Reynold A. Panettieri at the University of Pennsylvania (18). In addition, cells were prepared (18–22) from human airway smooth muscle tissues that were obtained from the International Institute for Advanced Medicine (details are provided in the online supplement). Human tissues were nontransplantable and consented for research. This study was approved by the Albany Medical College Committee on Research Involving Human Subjects.
Immunoblot and Immunofluorescence Analysis
Western blotting and immunostaining were performed using the methods previously described (19–22). Image analysis for protein localization was performed by modification of the method previously described (14, 20, 21, 23, 24). Detailed methods were described in online supplement.
Construction of Recombinant Lentivirus and Virus Production
To construct lentivirus encoding Abl shRNA, oligonucleotides were synthesized by Invitrogen (Carlsbad, CA). The sense target sequence of Abl shRNA was 5′-AAGCCGCTCGTTGGAACTCCA-3′ (NCBI accession number NM_005231). Oligonucleotides encoding Abl shRNA were subcloned into pFUGW lentiviral vector (25) followed by transformation into Stbl3-competent cells (Invitrogen). We also engineered inducible lentivirus, where expression of RNAi-resistant Abl mutant is 5′-AAGTCGGTCGTTGGAGCTGCA-3′ (mutated sequences are underlined), which was controlled by Tet-Op7-CMV promoter (26). Briefly, cDNA encoding Abl was cloned into pCR 8/GW/TPOP plasmids (Invitrogen) and transferred into the Gateway-compatible destination vector pSLIK using an LR-Clonase reaction kit, which resulted in the pSLIK-Abl vector. The plasmid DNA was harvested and purified using the plasmid maxiprep kits (Invitrogen). To produce viruses, 293FT cells were transfected with pFUGW encoding Abl shRNA or pSLIK-Abl plus packaging vector pCMV and envelop vector pVSV-G. Viruses were collected 48 hours after transfection. For infection, smooth muscle cells were incubated with viruses for 6 hours and cultured in the F12 growth medium for 2 to 3 days. Infection rates were nearly 100%, as evidenced by green fluorescence protein analysis (17).
Analysis of Raf-1 Association with F-actin
The fraction of F-actin in smooth muscle was collected using the method previously described (9, 21, 23). The equal amount of cytoskeletal actin was separated by SDS-PAGE and was transferred to nitrocellulose membranes. The membrane was probed with the use of Raf-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and α-actin antibody (Sigma-Aldrich, St. Louis, MO). The Raf-1/actin ratio was calculated after densitometrical analysis of immunoblots.
Far-Western Analysis
Far-Western analysis was performed by modification of the method previously described (23). Raf-1 immunoprecipitates from human lung tissues were separated by SDS-PAGE followed by membrane transfer. The membranes were incubated with 5 μg/ml purified actin (Sigma-Aldrich) at 4°C overnight and probed with use of Raf-1 antibody and actin antibody.
Assessment of Cell Proliferation
Smooth muscle cell proliferation was evaluated by counting viable cells as previously described (17).
Statistical Analysis
All statistical analysis was performed using Prism software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way ANOVA followed by Tukey’s multiple comparison test. Differences between pairs of groups were analyzed by Student-Newman-Keuls test or Dunn’s method. Values of n refer to the number of experiments used to obtain each value. P < 0.05 was considered to be significant.
Results
Treatment with PDGF Induces the Spatial Redistribution of Raf-1 in HASM Cells
We evaluated the spatial distribution of Raf-1 in smooth muscle cells in response to activation with PDGF. HASM cells were stimulated with PDGF (10 ng/ml) for 10 minutes or left unstimulated. The spatial localization of Raf-1 in cells was evaluated by immunofluorescent microscopy.
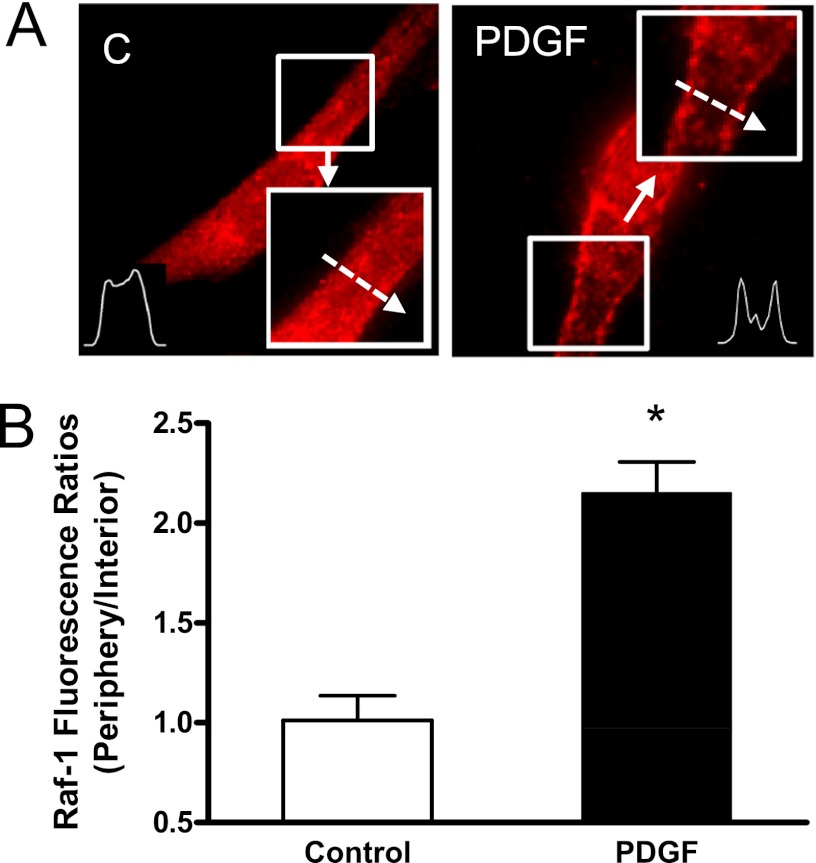
In unstimulated cells, Raf-1 was primarily distributed in the cytoplasm (Figure 1A). Stimulation with PDGF induced a Raf-1 translocation to the cell periphery. The fluorescent intensity of Raf-1 was elevated in the cell edge after PDGF stimulation. Quantitative analysis showed that the periphery/interior Raf-1 fluorescent ratios were higher in stimulated cells than in unstimulated cells (Figure 1B).
Figure 1.
Treatment with platelet-derived growth factor (PDGF) induces the spatial translocation of Raf-1 in human airway smooth muscle (HASM) cells. HASM cells were stimulated with PDGF (10 ng/ml) for 10 minutes or left unstimulated. The cellular localization of Raf-1 was evaluated by immunofluorescent microscopy. (A) Representative images illustrating the spatial redistribution of Raf-1 in response to activation with PDGF. The inserts are the 1.5× magnification of the selected area. Dashed arrows indicate a single line scan to quantify the fluorescent signal for each cell. C = control. (B) Protein distribution in cells is expressed as ratio of pixel intensity at the cell periphery/pixel intensity at cell interior. Data are means ± SE. *Significantly different between unstimulated cells and stimulated cells (P < 0.05; n = 15).
PDGF Stimulation Leads to Increases in the Association of Raf-1 with F-actin
Because the actin cytoskeleton has been implicated in GLUT4 trafficking (5), we questioned whether PDGF activation may promote the interaction of Raf-1 with F-actin, which may facilitate the translocation of Raf-1 to the membrane. To test this, fractions of cytosol and F-actin were extracted using the biochemical assay described in Materials and Methods. The distribution of Raf-1 in the cytosol and cytoskeletal actin was determined by immunoblot analysis.
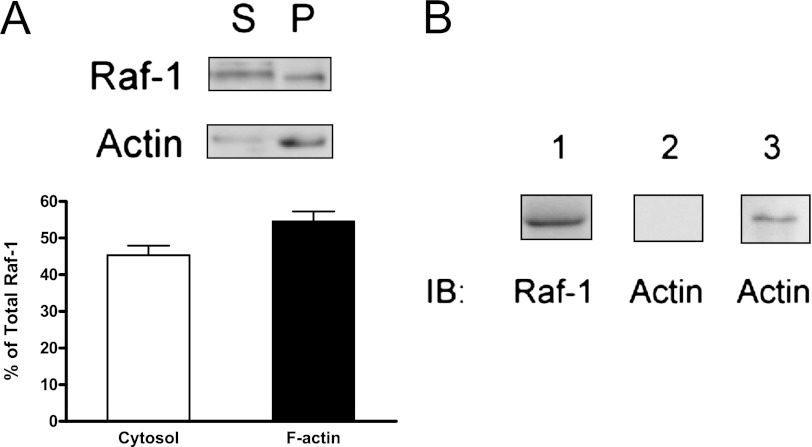
Raf-1 was found in the cytosol and cytoskeletal actin of HASM cells. Quantitative analysis showed that approximately 45% of the total Raf-1 was in the cytosol, whereas 55% of the Raf-1 was in cytoskeletal actin in unstimulated smooth muscle cells (Figure 2A). The results suggest that a pool of Raf-1 binds to cytoskeletal actin in airway smooth muscle cells.
Figure 2.
Interaction of Raf-1 with actin in HASM and in vitro. (A) Representative immunoblots illustrating the presence of Raf-1 in cytoskeletal actin (F-actin). Blots of supernatant (S) and pellet (P) fractions from HASM cells were probed with use of Raf-1 antibody and actin antibody. The amount of Raf-1 on the immunoblots from each fraction is quantified after scanning densitometry analysis. The relative amount of Raf-1 in the cytosolic fraction is expressed as follows: cytosolic Raf-1 ÷ total Raf-1 (soluble Raf-1 + cytoskeletal Raf-1) × 100. The amount of cytoskeletal Raf-1 is expressed as follows: insoluble Raf-1 ÷ total Raf-1 × 100. All values are means ± SE (n = 4). (B) Far-Western analysis of Raf-1 interaction with actin in vitro. Raf-1 immunoprecipitates were separated by SDS-PAGE and transferred to membranes. Lane 1: immobilization of Raf-1 on the membrane probed using Raf-1 antibody. Lane 2: no detection of actin in the control membrane. Lane 3: detection of actin on immobilized Raf-1 suggests the association of Raf-1 with actin in vitro. Immunoblots (IB) are representative of four identical experiments.
We also discovered that Raf-1 directly binds to actin in vitro. Raf-1 immunoprecipitates were immobilized onto nitrocellulose membranes. Immobilized Raf-1 was reacted with purified actin and detected using actin antibody. Actin was found in the membrane with immobilized Raf-1 but not in control membrane (Figure 2B).
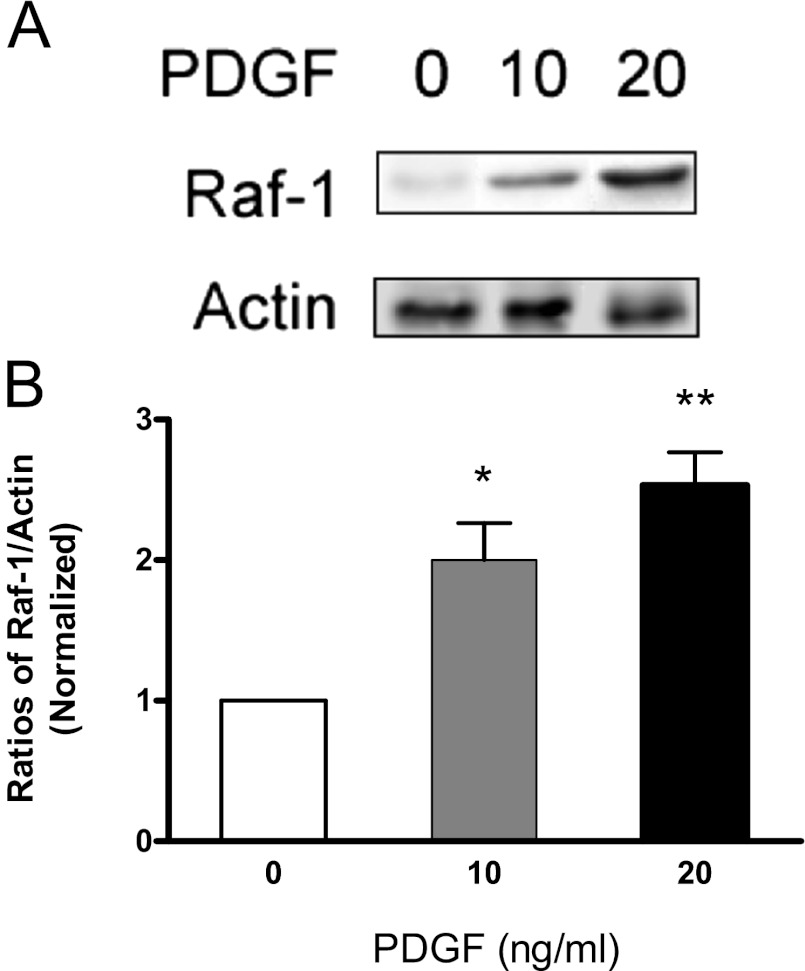
To assess whether activation with PDGF affects the interaction of Raf-1 with F-actin, HASM cells were stimulated with the growth factor or left unstimulated. The equal amount of F-actin fraction of these cells was separated by SDS-PAGE. The association of Raf-1 with F-actin was determined by immunoblot analysis. The amount of Raf-1 in the F-actin fraction was lower in unstimulated cells. In contrast, the level of Raf-1 in cytoskeletal actin was higher in PDGF-stimulated cells. The Raf-1/actin ratios were higher in stimulated cells than in control cells (Figure 3).
Figure 3.
Stimulation with PDGF results in an increase in the association of Raf-1 with F-actin. HASM cells were stimulated with different concentration of PDGF for 10 minutes. Equal amounts of F-actin from the cells were separated by SDS-PAGE and transferred to nitrocellulose membranes. The membranes were probed with Raf-1 antibody and actin antibody. (A) Representative immunoblots illustrating the effects of PDGF on the amount of Raf-1 in cytoskeletal actin. (B) The Raf-1/actin ratio in PDGF-stimulated cells is normalized to that in unstimulated cells. Asterisks indicate significantly higher ratios of Raf-1/actin in treated cells as compared with control cells (*P < 0.05; **P < 0.01). Values are means ± SE (n = 4–5).
Blockade of Actin Dynamics by Latrunculin A Inhibits the Interaction of Raf-1 with F-actin Induced by PDGF
We evaluated the effects of the actin polymerization inhibitor latrunculin A (LAT-A) on the association of Raf-1 with F-actin. Cells were pretreated with or without 1 μM LAT-A for 30 minutes. They were then stimulated with PDGF or left unstimulated. The association of Raf-1 with F-actin was evaluated by the method described above.
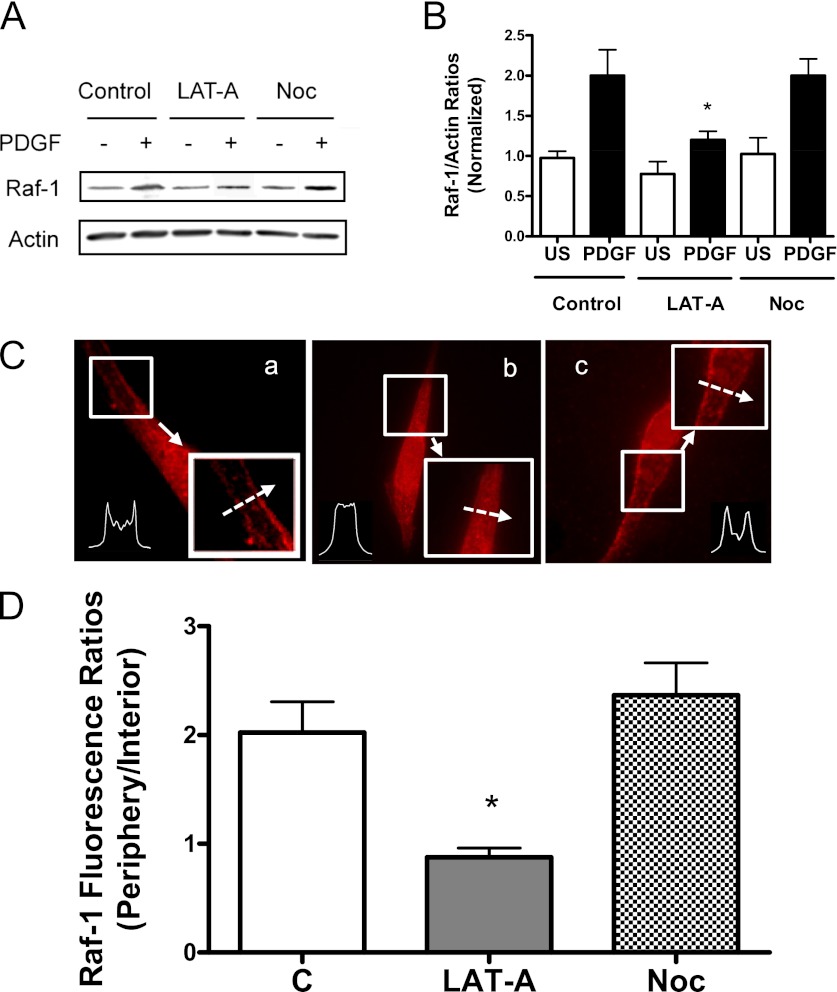
Treatment with LAT-A induces the dissociation of Raf-1 from cytoskeletal actin. The amount of Raf-1 in cytoskeletal actin in LAT-A–treated cells during PDGF activation was lower as compared with control cells (Figure 4A). The Raf-1/actin ratios during PDGF activation were significantly lower in LAT-A–treated cells than in control cells. In addition, the ratios of Raf-1/actin in LAT-A–treated cells without PDGF stimulation were similar to those in cells not treated with LAT-A and PDGF (Figure 4B).
Figure 4.
Effects of latrunculin A (LAT-A) and nocodazole (Noc) on the association of Raf-1 with F-actin and Raf-1 translocation in HASM cells. (A and B) Blots of cytoskeletal actin from unstimulated and PDGF-stimulated HASM cells in the absence or presence of LAT-A or Noc were probed with the use of Raf-1 antibody and actin antibody. (A) Immunoblots showing the effects of LAT-A and Noc on the amount of Raf-1 in F-actin. (B) The Raf-1/actin ratio in treated cells is normalized to that in cells not treated with PDGF and the inhibitors. *Significantly lower ratio of Raf-1/actin in LAT-A–treated cells as compared with control cells (P < 0.05). Values are means ± SE (n = 4). (C and D) HASM cells pretreated with LAT-A or Noc were stimulated with PDGF (10 ng/ml) for 10 minutes. The cellular localization of Raf-1 was evaluated by immunofluorescent microscopy. (C) Representative images illustrating the spatial redistribution of Raf-1 in response to various treatments. The inserts are the 1.5× magnification of the selected area. Dashed arrows indicate a single line scan to quantify the fluorescent signal for each cell. a, control; b, LAT-A; c, Noc. (D) Raf-1 distribution in cells is expressed as ratio of pixel intensity at the cell edge to pixel intensity at cell interior. Data are means ± SE (n = 16–18). *Significantly different between treated cells and control cells (P < 0.05).
Disruption of Microtubules by Nocodazole Does Not Affect the Interaction of Raf-1 with F-actin
We determined the effects of nocodazole, a microtubule depolymerizer, on the association of Raf-1 with actin in cells. Cells pretreated with 1 μM nocodazole for 30 minutes were stimulated with PDGF or left unstimulated. The interaction of Raf-1 with actin was then evaluated. The amount of Raf-1 in cytoskeletal actin in nocodazole-treated cells during PDGF activation was similar to cells not treated with nocodazole. The Raf-1/actin ratios during PDGF stimulation were comparable between cells treated with nocodazole and control cells (Figures 4A and 4B). In addition, the ratios of Raf-1/actin in nocodazole-treated cells without PDGF stimulation were not significantly different from those in control cells. The results suggest that microtubules do not regulate the coupling of Raf-1 with cytoskeletal actin in HASM cells.
Blockage of Actin Dynamics by LAT-A Inhibits Raf-1 Translocation in HASM Cells
We hypothesized that actin dynamics are important for Raf-1 translocation in smooth muscle cells. To test this, we evaluated the effects of LAT-A on Raf-1 redistribution during PDGF stimulation. Untreated cells and LAT-A–pretreated cells were stimulated with PDGF. Raf-1 redistribution was evaluated by immunofluorescence microscopy.
Actin polymerization regulates the spatial translocation of Raf-1 during PDGF activation. The fluorescent intensity of Raf-1 at the membrane was greater in untreated cells in response to PDGF activation. On the contrary, the fluorescent intensity of Raf-1 at the cell periphery was lower in cells pretreated with LAT-A (Figure 4C). The peripheral/internal Raf-1 fluorescent ratios were lower in LAT-A–treated cells as compared with control cells (Figure 4D).
To determine the role of actin polymerization in regulating the downstream effectors of Ras/Raf in airway smooth muscle, untreated cells and LAT-A–treated cells were stimulated with PDGF. Phosphorylation of MEK1/2 and ERK1/2 was evaluated by immunoblot analysis. Stimulation with PDGF led to increases in the phosphorylation of MEK12 and ERK1/2 in untreated cells. However, treatment with LAT-A attenuated the phosphorylation levels of MEK1/2 and ERK1/2 induced by PDGF (see Figure E1 in the online supplement). The results suggest that actin dynamics is important for the activation of Raf-1 signaling in airway smooth muscle cells during activation with PDGF.
Disruption of Microtubules Does Not Affect Raf-1 Translocation in HASM Cells in Response to Activation with PDGF
We then evaluated whether microtubules have a role in the redistribution of Raf-1. The fluorescent levels of Raf-1 during PDGF activation in nocodazole-treated cells were similar to those in untreated cells (Figure 4C). The peripheral/internal Raf-1 ratios were comparable between untreated cells and cells pretreated with nocodazole (Figure 4D).
Abl Knockdown and Rescue Affect the Association of Raf-1 with F-actin Induced by PDGF
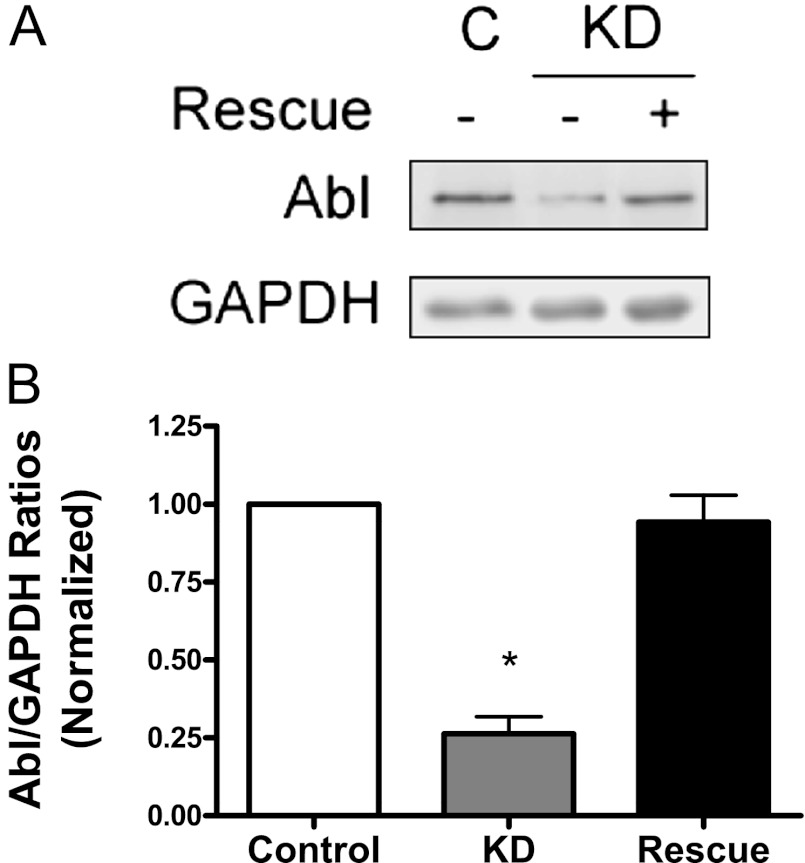
Because Abl tyrosine kinase has been shown to specifically regulate actin polymerization in smooth muscle cells during stimulation with various stimuli including PDGF (8, 9, 11, 12, 14, 17), we evaluated whether Abl Knockdown (KD) affects the interaction of Raf-1 with F-actin. We constructed recombinant lentivirus encoding Abl shRNA. We also engineered inducible lentivirus where expression of Abl was controlled by the Tet-Op7-CMV promoter (26). Cells were double infected with lentivirus encoding Abl shRNA and virus encoding inducible Abl for 3 days. Cells were either not treated with doxycyclin (for KD) or treated with doxycyclin (for rescue). Immunoblot analysis was used to determine the expression levels of Abl in these cells. The protein level of Abl in infected cells not induced with doxycyclin was lower compared with control cells. However, the expression of Abl in infected cells treated with doxycyclin was similar to control cells. The expression level of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was similar in these cells. The ratios of Abl/GAPDH were significantly lower in Abl KD cells than in control cells and in rescue cells (Figure 5). In addition, these cells are stable for approximately five passages after initial infection.
Figure 5.
Lentivirus-mediated knockdown (KD) and rescue of Abl in HASM cells. Cells were coinfected with lentiviruses encoding Abl shRNA and viruses encoding inducible Abl for 3 days. Blots of control, KD, and rescue cells were probed with the use of antibodies against Abl and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (A) Representative immunoblots illustrating the expression of Abl in KD cells and rescue cells. (B) Ratios of Abl/GAPDH protein in Abl KD cells and rescue cells were normalized to ratios obtained from control cells. Values are means ± SE (n = 4). *Significantly lower Abl/GAPDH ratios in KD cells compared with control cells and rescue cells (P < 0.05). Control, cells were uninfected; KD (knockdown), infected cells were not treated with doxycyclin; Rescue, infected cells were treated with doxycyclin (0.5 μg/ml, 24 h).
Control cells, KD cells, and rescue cells were stimulated with PDGF for 10 minutes. The effects of Abl KD and rescue on interaction of Raf-1 with F-actin were then evaluated. The amount of Raf-1 in F-actin fraction was lower in KD cells than in uninfected cells (Figure 6A). However, the level of Raf-1 in F-actin was similar between rescue cells and uninfected cells. The Raf-1/actin ratios in KD cells during activation with PDGF were significantly lower as compared with control cells and rescue cells (Figure 6B).
Figure 6.
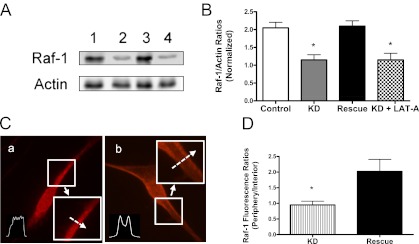
Knockdown and rescue of Abl affect the interaction of Raf-1 with actin and Raf-1 redistribution induced by PDGF. (A) Representative immunoblots illustrating the role of Abl in the association of Raf-1 with F-actin. To assess the role of Abl, control cells, KD cells, and rescue cells were stimulated with PDGF for 10 minutes. The interaction of Raf-1 with F-actin was then evaluated. To evaluate whether the effects of Abl and actin polymerization on Raf-1/actin coupling are additive, KD cells were treated with LAT-A for 30 minutes, and the coupling of Raf-1 with F-actin in response to PDGF activation was determined. The amount of Raf-1 in cytoskeletal actin in KD cells treated with LAT-A was similar to that in KD cells. 1, control; 2, KD cells; 3, rescue; and 4, KD + LAT-A. (B) The Raf-1/actin ratios in KD cells, rescue cells, and KD cells treated with LAT-A are normalized to control cells. *Significantly lower ratio of Raf-1/actin in KD cells and KD cells plus LAT-A as compared with control cells (P < 0.05). Values are means ± SE (n = 3–4). (C) Representative images illustrating the effects of Abl KD and rescue on spatial redistribution of Raf-1 induced by PDGF. The inserts are the 1.5× magnification of the selected area. Dashed arrows indicate a single line scan to quantify the fluorescent signal for each cell. a, KD; b, rescue. (D) Raf-1 distribution in cells is expressed as ratio of pixel intensity at the cell edge to pixel intensity at cell interior. Data are means ± SE (n = 17–18). *Significantly different between treated cells and control cells (P < 0.05).
To determine whether the effects of Abl and actin polymerization on Raf-1/actin coupling are additive, KD cells were treated with LAT-A for 30 minutes, and the interaction of Raf-1 with F-actin in response to PDGF activation was determined. The amount of Raf-1 in F-actin in KD cells treated with LAT-A was similar to that in KD cells (Figures 6A and 6B). The results suggest that the effects of Abl on the coupling of Raf-1 with actin are primarily mediated by actin polymerization.
Abl KD and Rescue Affect the Spatial Translocation of Raf-1
We then evaluated the effects of Abl KD and rescue on Raf-1 translocation in response to PDGF stimulation. Control cells, KD cells, and rescue cells were stimulated with PDGF for 10 minutes. Immunostaining was used to determine the spatial redistribution of Raf-1 in these cells. The fluorescent levels of Raf-1 in the membrane were lower in Abl KD cells (Figures 6C and 6D) than in control cells (Figure 4Ca). In contrast, the peripheral fluorescent intensity of Raf-1 in rescue cells (Figures 6C and 6D) was similar to that in control cells (Figure 4Ca).
We also determined the effects of Abl KD and rescue on phosphorylation of MEK1/2 and ERK1/2 by immunoblot analysis. Phosphorylation levels of PDGF-induced MEK1/2 in KD cells were lower as compared with control cells. However, phosphorylation levels of MEK1/2 in rescue cells upon PDGF activation were comparable to control cells. Similarly, phosphorylation of ERK1/2 in response to PDGF activation was diminished in KD cells and recovered in rescue cells (Figure E2).
Roles of the Actin Cytoskeleton and Microtubules in Airway Smooth Muscle Cell Proliferation
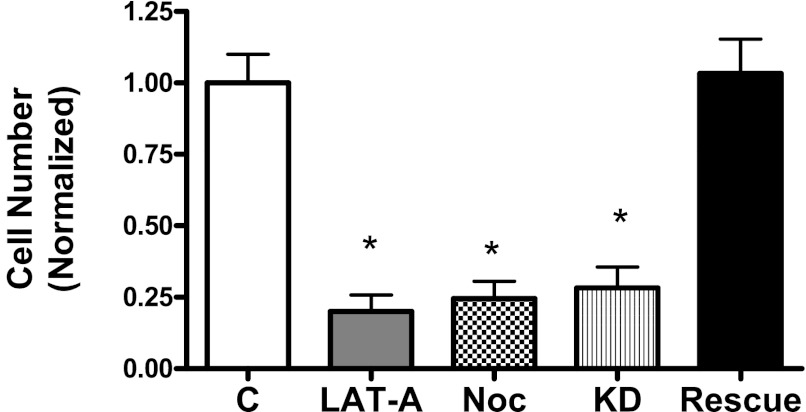
To assess the role of the actin cytoskeleton and microtubules in smooth muscle cell growth, HASM cells were cultured in the presence or absence of LAT-A or nocodazole for 2 days, and the numbers of viable cells were determined using the trypan blue exclusion test.
Treatment with LAT-A or nocodazole inhibits smooth muscle cell proliferation in response to activation with PDGF. The numbers of viable cells treated with LAT-A were reduced by 78% as compared with control cells (Figure 7). Moreover, the numbers of cells treated with nocodazole were lower than control cells (Figure 7).
Figure 7.
Effects of LAT-A, Noc, and Abl KD/rescue on smooth muscle cell proliferation. HASM cells were cultured in the presence or absence of 1 μM LAT-A or Noc for 2 days. In addition, Abl KD and rescue cells were cultured for 2 days. The numbers of viable cells were determined using the trypan blue exclusion test (*P < 0.01; n = 3).
Because Abl specifically inhibits actin dynamics in smooth muscle cells, we evaluated the effects of Abl KD and reexpression on cell proliferation. The numbers of KD cells were reduced as compared with control cells. However, the numbers of rescue cells were similar to control cells (Figure 7).
Discussion
Raf-1 is a serine/threonine protein kinase that has a role in cell proliferation. However, the mechanisms that regulate the spatial redistribution of Raf-1 (a key process for Raf-1 activation and cell proliferation) (1, 2) in smooth muscle cells are incompletely elucidated. It has been documented that Raf-1 redistribution is mediated by the interaction of Raf-1 with Ras and phosphatydic acid in nonmuscle cells (2–4). In this report, we found that PDGF activation induces an increase in the association of Raf-1 with cytoskeletal actin. Inhibition of actin polymerization by LAT-A and Abl KD attenuates the interaction of Raf-1 with F-actin and Raf-1 redistribution during PDGF stimulation. The results suggest a novel mechanism that the interaction of Raf-1 with actin is critical for Raf-1 spatial translocation and airway smooth muscle cell proliferation in response to activation with PDGF.
Although Raf-1 is proposed to regulate cell proliferation, how Raf-1 is activated in smooth muscle is not well understood. In the present study, the activation with PDGF resulted in the recruitment of Raf-1 to the cell periphery from the cytoplasm as evidenced by immunofluorescent microscopy. The recruitment of Raf-1 to Ras in the vicinity of the membrane may promote its activation, which in turn controls downstream effectors and cell proliferation (1, 2).
It has been proposed that Raf-1 redistribution is mediated by the interaction of Raf-1 with Ras and phosphatydic acid in fibroblasts, MDCK cells, and COS cells in response to activation with insulin and growth factors (4, 27). In this report, Raf-1 was able to directly bind to actin in vitro as evidenced by Far-Western analysis. In addition, approximately 55% of total Raf-1 was found in the F-actin fraction in smooth muscle cells. More importantly, PDGF stimulation led to an increase in the interaction of Raf-1 with actin. To the best of our knowledge, this is the first evidence to demonstrate that Raf-1 is able to interact with cytoskeletal actin in smooth muscle cells and that this association is regulated by activation with the growth factor.
Recent studies have shown that actin polymerization transpires in smooth muscle cells in response to activation with various stimuli, including PDGF (8–10). To assess the role of actin dynamics, we applied the pharmacological agent LAT-A to human airway smooth muscle cells. LAT-A binds to G-actin and prevents their assembly onto actin filaments (28). Treatment with LAT-A inhibited the association of Raf-1 with cytoskeletal actin during PDGF activation. To rule out the potential nonspecific effects of LAT-A, we used RNAi to inhibit the expression of Abl in smooth muscle cells. Abl is a nonreceptor tyrosine kinase that is able to specifically regulate actin polymerization in smooth muscle cells (8–12, 14). In response to external stimulation, Abl tyrosine kinase in smooth muscle cells may phosphorylate the actin-regulatory protein p130 Crk-associated substrate (8, 9, 14). Phosphorylated Crk-associated substrate may promote its association with the adapter protein CrkII, which may activate neuronal Wiskott-Aldrich Syndrome Protein and the Actin Related Protein (Arp)2/3 complex, inducing actin polymerization and branching mediated by the Arp2/3 complex (29–31). In this report, knockdown of Abl attenuated the PDGF-induced coupling of Raf-1 with actin, which was rescued by the reexpression of Abl. These results strongly suggest that actin polymerization regulates the interaction of Raf-1 with actin in human airway smooth muscle cells during proliferative response.
In adipocytes, insulin-stimulated reorganization of the actin cytoskeleton plays a role in promoting GLUT4 translocation and glucose uptake in a PI3K-dependent manner. PI3K may activate the Rac exchange factor, P-Rex1, which in turn regulates a functional actin network and GLUT4 trafficking (5). In the present study, the inhibition of actin polymerization by the pharmacological agent attenuated the PDGF-induced Raf-1 translocation and the activation of MEK1/2 and ERK1/2. Furthermore, Abl KD had similar effects, which was restored by Abl rescue. These results suggest that actin polymerization is required for Raf-1 translocation and its downstream signaling in smooth muscle cells during PDGF activation.
Because actin dynamics is necessary for the coupling of Raf-1 with actin, Raf-1 translocation and phosphorylation of MEK1/2 and ERK1/2, we propose that PDGF activation may promote the coupling of Raf-1 with cytoskeletal actin, which may facilitate the translocation and activation of Raf-1 from the cytoplasm to the membrane. Activated Raf-1 phosphorylates MEK1/2, which in turn phosphorylates ERK1/2 and activates ERK1/2, and promotes cell proliferation (1, 2).
In addition to the actin cytoskeleton, microtubules have been implicated in the trafficking of channels, vesicles, and GLT4 in neurons and adipocytes (6, 7). Our studies showed that treatment with the microtubule depolymerizer nocodazole did not affect the interaction of Raf-1 with cytoskeletal actin and Raf-1 redistribution. However, nocodazole inhibited airway smooth muscle cell proliferation. These results suggest that microtubule is not necessary for Raf-1 translocation in smooth muscle cells in the early stage of activation with the growth factor. Microtubules may regulate cell proliferation by affecting mitosis (32).
Supplementary Material
Footnotes
This work was supported by National Heart. Lung and Blood Institute grants HL-110951 and HL-113208 from the National Institutes of Health (D.D.T.) and by National Institutes of Health grants HL-097796 and ES-013508 (R.A.P.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0315OC on October 18, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ammit AJ, Panettieri RA., Jr Airway smooth muscle cell hyperplasia: a therapeutic target in airway remodeling in asthma? Prog Cell Cycle Res 2003;5:49–57 [PubMed] [Google Scholar]
- 2.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev 1999;79:143–180 [DOI] [PubMed] [Google Scholar]
- 3.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature 2001;410/6824:37–40 [DOI] [PubMed] [Google Scholar]
- 4.Rizzo MA, Shome K, Watkins SC, Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J Biol Chem 2000;275:23911–23918 [DOI] [PubMed] [Google Scholar]
- 5.Balamatsias D, Kong AM, Waters JE, Sriratana A, Gurung R, Bailey CG, Rasko JE, Tiganis T, Macaulay SL, Mitchell CA. Identification of P-Rex1 as a novel Rac1-guanine nucleotide exchange factor (GEF) that promotes actin remodeling and GLUT4 protein trafficking in adipocytes. J Biol Chem 2011;286:43229–43240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuck E, Cavalli V. Roles of membrane trafficking in nerve repair and regeneration. Commun Integr Biol 2010;3:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vacher H, Yang JW, Cerda O, Autillo-Touati A, Dargent B, Trimmer JS. Cdk-mediated phosphorylation of the Kvbeta2 auxiliary subunit regulates Kv1 channel axonal targeting. J Cell Biol 2011;192:813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res 2007;101:420–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia L, Tang DD. Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle. Am J Physiol Cell Physiol 2010;299:C630–C637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JY. Controlling Abl: auto-inhibition and co-inhibition? Nat Cell Biol 2004;6:3–7 [DOI] [PubMed] [Google Scholar]
- 11.Tang DD. p130 Crk-associated substrate (CAS) in vascular smooth muscle. J Cardiovasc Pharmacol Ther 2009;14:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang DD, Anfinogenova Y. Physiologic properties and regulation of the actin cytoskeleton in vascular smooth muscle. J Cardiovasc Pharmacol Ther 2008;13:130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 2008;295:C576–C587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Wang R, Li QF, Tang DD. Abl knockout differentially affects p130 Crk-associated substrate, vinculin, and paxillin in blood vessels of mice. Am J Physiol Heart Circ Physiol 2009;297:H533–H539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu H, Bliss JM, Wang Y, Colicelli J. RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr Biol 2005;15:815–823 [DOI] [PubMed] [Google Scholar]
- 16.Ushio-Fukai M, Zuo L, Ikeda S, Tojo T, Patrushev NA, Alexander RW. cAbl tyrosine kinase mediates reactive oxygen species- and caveolin-dependent AT1 receptor signaling in vascular smooth muscle: role in vascular hypertrophy. Circ Res 2005;97:829–836 [DOI] [PubMed] [Google Scholar]
- 17.Jia L, Wang R, Tang DD. Abl regulates smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 activation. Am J Physiol Cell Physiol 2012;302:C1026–C1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goncharova EA, Goncharov DA, Zhao H, Penn RB, Krymskaya VP, Panettieri RA., Jr beta2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein. Am J Respir Cell Mol Biol 2012;46:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q-F, Spinelli AM, Tang DD. Cdc42GAP (GTPase activating protein) regulates the activation of Cdc42 and PAK (p21-activated kinase) in tracheal smooth muscle cells upon 5-HT stimulation. Am J Respir Crit Care Med 2007;175:A348 [Google Scholar]
- 20.Li QF, Spinelli AM, Tang DD. Cdc42GAP, reactive oxygen species, and the vimentin network. Am J Physiol Cell Physiol 2009;297:C299–C309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem 2006;281:34716–34724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J 2005;388:773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of Crk-associated substrate from the vimentin network is regulated by p21-activated kinase on ACh activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2007;292:L240–L248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li QF, Tang DD. Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am J Physiol Cell Physiol 2009;297:C1424–C1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 2002;295/5556:868–872 [DOI] [PubMed] [Google Scholar]
- 26.Bajaj A, Zheng Q, Adam A, Vincent P, Pumiglia K. Activation of endothelial ras signaling bypasses senescence and causes abnormal vascular morphogenesis. Cancer Res 2010;70:3803–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leevers SJ, Paterson HF, Marshall CJ. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature 1994;369/6479:411–414 [DOI] [PubMed] [Google Scholar]
- 28.Coue M, Brenner SL, Spector I, Korn ED. Inhibition of actin polymerization by latrunculin A. FEBS Lett 1987;213:316–318 [DOI] [PubMed] [Google Scholar]
- 29.Tang DD, Zhang W, Gunst SJ. The adapter protein CrkII regulates neuronal Wiskott-Aldrich Syndrome protein, actin polymerization, and tension development during contractile stimulation of smooth muscle. J Biol Chem 2005;280:23380–23389 [DOI] [PubMed] [Google Scholar]
- 30.Tang DD, Gunst SJ. The small GTPase Cdc42 regulates actin polymerization and tension development during contractile stimulation of smooth muscle. J Biol Chem 2004;279:51722–51728 [DOI] [PubMed] [Google Scholar]
- 31.Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 2007;36:451–477 [DOI] [PubMed] [Google Scholar]
- 32.Meunier S, Vernos I. Microtubule assembly during mitosis: from distinct origins to distinct functions? J Cell Sci 2012;125:2805–2814 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.