Abstract
Infants born with intrauterine growth retardation (IUGR) are at increased risk of adverse pulmonary outcomes at birth, including meconium aspiration and persistent pulmonary hypertension. Preterm infants with IUGR are at especially high risk of developing bronchopulmonary dysplasia (BPD), a disease hallmarked by alveolar hypoplasia. Although vitamin A supplementation has been shown to decrease the incidence of BPD or death in preterm very low birth weight infants, its potential to reduce BPD or death in preterm infants with IUGR remains unknown. We used a well-characterized rat model of caloric restriction to mimic IUGR and determine the impact of IUGR on lung development. We hypothesized that retinoic acid treatment would preserve alveolar formation through increases in key signaling molecules of the retinoic acid signaling pathway. Our results showed that alveolar hypoplasia caused by caloric restriction can be reversed with refeeding, and that retinoic acid prevents the alveolar hypoplasia coincident with the increased expression of elastin and retinoic acid receptor–α and decreased transforming growth factor–β activity in developing rat lungs. These findings suggest that alveolar hypoplasia attributable to caloric restriction is reversible, and raises the possibility that retinoic acid therapy may prove a useful strategy to prevent adverse pulmonary sequelae such as BPD in preterm infants with IUGR.
Keywords: intrauterine growth retardation, bronchopulmonary dysplasia, vitamin A, RARα, TGF-β
Clinical Relevance
This study highlights the role of vitamin A and its analogues during lung development in the context of caloric restriction during prenatal and postnatal life. These findings may affect the treatment and prevention of bronchopulmonary disease in preterm newborn infants with intrauterine growth retardation. This study also expands the knowledge base of crosstalk between vitamin A and transforming growth factor–β signaling pathways.
Intrauterine growth retardation (IUGR) describes fetal growth that is less than normal for the growth potential of an infant. Decreased fetal growth rates reflect an adaptation to an unfavorable intrauterine environment, and may result in permanent alterations in metabolism, growth, and organ development (1, 2). Although growth restriction of early onset during gestation is often attributable to genetic disorders, congenital malformation syndromes, congenital infections, inborn errors of metabolism, and intrauterine drug exposures, growth restriction during late gestation is usually related to impaired uteroplacental function or nutrient deficiency. Infants with IUGR can experience adverse pulmonary sequelae, including meconium aspiration syndrome and persistent pulmonary hypertension of the newborn (2), placing them at high risk for poor respiratory transition immediately after birth.
Preterm infants who are small for their gestational age (and who may often have experienced IUGR) run an additional risk for chronic respiratory disorders such as respiratory distress syndrome and bronchopulmonary dysplasia (2, 3). As these infants grow, they are more likely to develop wheezing and respiratory infections, compared with age-matched infants born at appropriate weights (4). Contrary to the common wisdom that intrauterine stress enhances lung maturation, IUGR stress paradoxically increases the incidence of respiratory distress (3). Alveolar abnormalities in these infants are known to extend into adulthood, but the mechanisms by which the fetal lung is reprogrammed because of compromised maternal uteroplacental function or nutrient delivery during embryonic and early postnatal development remain unknown.
Vitamin A and its analogues are lipophilic signaling molecules that play an important role in alveolar development, maintenance, and regeneration (reviewed by Maden and Hind [5]). Vitamin A is obtained from the diet as retinyl esters, whereas cells of the developing embryo obtain retinoic acid (derived from vitamin A) through the blood, where it circulates as retinol bound to retinoic acid–binding protein. In the cell cytoplasm, retinol is bound to cellular retinoic acid–binding protein (Crabp1), which facilitates its enzymatic conversion to retinoic acid. Retinoic acid enters the cell nucleus and activates gene transcription by binding to two classes of transcription factors, retinoic acid receptors (RARs) and retinoic X receptors (RXRs). These transcription factors form heterodimers and directly bind to promoter sequences of retinoic acid–responsive genes. Retinoic acid signaling during alveolar development involves the observation that lung Crabp1 concentrations peak during alveolar formation (6). Elegant studies in mice and rats demonstrated the rescue of alveolar formation and regeneration with retinoic acid treatment in genetically induced and dexamethasone-induced alveolar hypoplasia (7, 8), or in chemically induced emphysema (9). Finally, human studies have shown that vitamin A supplementation in extremely low birth weight preterm infants reduces the incidence of bronchopulmonary dysplasia or death, compared with placebo-treated control subjects (10). Whether treatment with vitamin A or its analogues provides similar benefits in preventing the alveolar hypoplasia produced by caloric restriction during the critical period of lung development has not been studied, to the best of our knowledge.
The present investigation used a well-characterized rat model of intrauterine and early postnatal caloric restriction to study the impact of these restrictions on postnatal alveolar formation. We observed that refeeding after caloric restriction improved lung alveolar phenotypes, and we hypothesized that treatment with retinoic acid would preserve alveolar formation in calorie-restricted postnatal rat lungs. Specifically, we tested whether the daily administration of retinoic acid would prevent alveolar hypoplasia in newborn rat pups born to calorie-restricted mothers and reared by calorie-restricted dams. Our results are the first to demonstrate that treatment with all-trans retinoic acid (ATRA) preserves alveolar formation in a caloric restriction model, with associated increases in the expression of lung elastin and RARα and decreased transforming growth factor–β (TGF-β) activity.
Materials and Methods
Animals
Timed-pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Hollister, CA). Animals were maintained under Institutional Animal Care and Use Committee–approved protocols in a vivarium barrier facility by the Division of Laboratory Animal Medicine at the University of California at Los Angeles on a 12-hour/12-hour light/dark cycle, and were allowed to eat standard rat chow (composition: 63.9% carbohydrates, 4.5% fat, 14.5% protein, 10% ash, and 7–8% fiber and crude fiber) and drink water ad libitum (normal caloric intake, ∼ 22 g). Half of the pregnant dams received food ad libitum during the first 11 days of gestation (Embryonic Days 0–11), and were then switched to a 50% calorie-restricted diet of 11 g/day, as previously described (11, 12) (Figure 1A). Please refer to the online supplement for further details.
Figure 1.
Schematic representation of experimental design. (A) Caloric restriction and refeeding. Male and female pups born to ad libitum (Ad lib)–fed control or 50% calorie-restricted dams were culled to equal numbers and cross-fostered to mothers receiving either ad libitum or 50% restricted intake to generate four experimental groups: (1) control, (2) intrauterine caloric restriction (ICR), (3) intrauterine and postnatal caloric restriction (IPCR), and (4) postnatal caloric restriction (PCR). Offspring were weaned from their mothers and allowed ad libitum intake starting on Postnatal Day 21 (P21). (B) Retinoic acid treatment. Female pups born to control or 50% calorie–restricted dams were culled to equal numbers and matched to similarly fed mothers. Pups were injected daily with all-trans retinoic acid (ATRA) or cottonseed oil vehicle from P4–P21 to generate four experimental groups: (1) oil control, (2) retinoic acid (RA) control, (3) oil IPCR, and (4) RA IPCR (n = 6 per group in both A and B). *Calorie-restricted groups, common to both experimental strategies. E, Embryonic Day.
Retinoic Acid Treatment
In separate experiments, control and intrauterine and postnatal caloric restriction (IPCR) rat pups were injected with either ATRA (500 μg/kg intraperitoneal, as previously described) (13) or cottonseed oil (vehicle control) daily from Postnatal Days 4–21, to generate four experimental groups: (1) oil control, (2) retinoic acid control, (3) oil IPCR, and (4) retinoic acid IPCR (Figure 1B). The daily dose of ATRA in newborn rats (∼ 1.7 IU/g/d, intraperitoneally) approximates the dosing calculated from clinical studies in newborn human infants (average range, 1.7–4.2 IU/g/d, intramuscularly) (10).
Lung Tissue Dissection and Processing
Lung tissue was prepared from arbitrarily selected neonatal (Postnatal Day 2), weanling (Postnatal Day 21), and adult (Postnatal Day 50) offspring in the caloric restriction and refeeding model, and from Postnatal Day 21 offspring in the retinoic acid treatment model. Please refer to the online supplement for further details.
mRNA and Protein Analysis
Whole-lung mRNA was extracted with TRIzol and chloroform (Invitrogen Corp., Carlsbad, CA), as described by the manufacturer. The quantitative polymerase chain reaction analysis of gene expression was performed as previously described (14, 15).
Total protein was extracted and used in Western blot and ELISA analyses. Please refer to the online supplement for further details.
Immunofluorescent Histochemistry
Slides were cleared in CitriSolv and rehydrated through a graded series of alcohols with antigen retrieval, as described by the manufacturer (DAKO, Inc., Carpinteria, CA). RARα, phosphorylated Smad2 (pSmad2), and proliferating cell nuclear antigen (PCNA) expression and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining were captured and estimated using the Image J program, version 1.37 (National Institutes of Health, Bethesda, MD), by converting signal-positive immunofluorescence images into a binary composite, highlighting all pixels specific for the corresponding color channel (Cy3), as described previously (16, 17). Please refer to the online supplement for further details.
Hart’s Elastin Stain
Lung-tissue slides were cleared in CitriSolv, rehydrated through graded alcohols, and incubated overnight in Hart’s solution (9:1 solution of Weigert’s iron resorcin fuchsin and 1% HCl). Please refer to the online supplement for further details.
Lung Morphometric Analysis
Lung morphometric analysis was performed using radial alveolar counts to assess alveolar numbers, as previously described (18–20). Please refer to the online supplement for further details.
Statistical Analysis
Data were analyzed using the Microsoft Excel 2003 statistical package (Microsoft Corporation, Redmond, WA). Two-group comparisons were evaluated using two-tailed unpaired Student t tests, and multiple-group comparisons used one-way ANOVA with a Bonferroni correction. Data are expressed as means ± SEMs where appropriate.
Results
Lung Growth Is Preserved during Caloric Restriction
Animal models describing the effects of compromised nutrient delivery to a developing fetus include experimental perturbations of maternal placental–fetal blood flow (21, 22) and caloric restriction during either prenatal or early postnatal stages (23, 24). Few studies have explored the combined effects of caloric restriction during embryonic and early postnatal development on lung morphogenesis. The IPCR model presented here provides a reproducible degree of growth restriction that may be comparable to that in preterm newborn human infants with moderate to severe IUGR. Lung and whole-body weights of offspring were measured on Postnatal Days 2, 21, and 50. The results showed that both whole-body and lung weights decreased after caloric restriction, and significantly improved with refeeding. Compared with body weights, lung weights dropped less during caloric restriction, as reflected by increased lung/body weight ratios when compared with ad libitum–fed controls or refed animals (Figure E1 in the online supplement).
Caloric Restriction Impairs Alveolar Formation, and Refeeding Restores Alveolar Formation
To determine the phenotypic impact of caloric restriction on alveolar development, we examined histological lung sections and performed morphometric analyses of alveolar spaces. On Postnatal Day 2, results showed that intrauterine caloric restriction (ICR) resulted in a 30% reduction (P < 0.05) in saccular numbers, as confirmed by the measurement of radial alveolar counts. On Postnatal Day 21, IPCR and postnatal caloric restriction (PCR) reduced alveoli by more than 50% (P < 0.05) (Figures 2A and 2B). The mechanisms responsible for reduced lung growth in this model involve a combination of decreased cell proliferation and increased cell apoptosis (Figures 2C and 2D). Refeeding with ad libitum intake (ICR on Postnatal Day 21; IPCR and PCR on Postnatal Day 50) subsequently increased alveolar numbers to normal levels, suggesting that alveolar formation is dependent on adequate nutrient delivery, and that alveolar hypoplasia after caloric restriction may be reversible, provided nutritional restoration occurs during the window of the early postnatal phase of development.
Figure 2.
Lung histological analysis with caloric restriction and refeeding. (A) Representative photomicrographs of hematoxylin and eosin–stained lung sections from designated groups on P2, P21, and P50 (magnification, ×4; insets, ×20 magnification; scale bar, 100 μM). (B) Radial alveolar count (RAC) analysis for designated groups on P2, P21, and P50. Immunofluorescence staining for (C) proliferating cell nuclear antigen (PCNA) and (D) terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells (arrows), with quantitation for designated groups on P21 (magnification, ×20; scale bar represents 100 μM). Data are presented as mean ± SEM (n = 6 rats per group). *P < 0.05, compared with control (Con) values.
Caloric Restriction Decreases, and Refeeding Up-Regulates, Elastin and Crabp1 Expression
With the finding that refeeding can reverse alveolar hypoplasia in the context of caloric restriction during lung development, we determined the impact of calorie restriction and refeeding on a limited number of lung morpho-regulatory genes that may be involved in this process. Results showed that concentrations of elastin mRNA from whole-lung homogenates trended downward with caloric restriction (ICR on Postnatal Day 2; IPCR on Postnatal Day 21), with significant decreases for PCR on Postnatal Day 21 (P < 0.05), followed by significant up-regulation by 3-fold for ICR (Postnatal Day 21 versus Postnatal Day 2; P < 0.05), and by 2-fold for IPCR (Postnatal Day 50 versus Postnatal Day 21; P < 0.05) with refeeding (Figure 3A). Concentrations of Crabp1 mRNA also decreased with caloric restriction (IPCR and PCR on Postnatal Day 21), and were significantly up-regulated by 3-fold for IPCR (Postnatal Day 21 versus Postnatal Day 2; P < 0.05) and PCR (Postnatal Day 50 versus Postnatal Day 21; P < 0.05) with refeeding (Figure 3B). Importantly, we confirmed the specificity of gene induction through the inclusion of two stable housekeeping genes, β-actin and β-2 microglobulin, to establish that the observed mRNA responses did not result from nonspecific changes attributable to refeeding, as previously described (25) (data not shown). These findings suggest that structural proteins such as elastin and signaling molecules central to retinoic acid developmental pathways are affected by nutrient-related effects during alveolar formation.
Figure 3.
Lung elastin and cellular retinoic acid–binding protein (Crabp1) mRNA concentrations. Quantitative polymerase chain reaction shows fold induction for (A) lung elastin and (B) lung Crabp1 mRNA from whole-lung homogenates of designated groups on P2, P21, and P50. Data are presented as mean ± SEM (n = 6 rats per group). *P < 0.05, compared with control values.
Retinoic Acid Preserves Lung Growth in Calorie-Restricted Rats
To determine whether treatment with retinoic acid improves alveolar development after caloric restriction, we focused on the IPCR subgroup, because these animals hold the greatest relevance to critically ill human newborns with intrauterine growth restriction who continue to exhibit compromised nutrient intake in a neonatal intensive care setting. We used a modified experimental strategy based on previous reports (7) to deliver ATRA or cottonseed oil vehicle control via daily intraperitoneal injection to pups from Postnatal Days 4–21 (Figure 1B). Animals were killed on Postnatal Day 21, and results showed that although no change was evident in total body weights, ATRA injections in the IPCR subgroup resulted in a small but statistically significant increase in lung weight and lung/body weight ratios (13% and 14%, respectively; P < 0.05), compared with cottonseed oil control pups (Figures E2A and E2B).
Retinoic Acid Preserves Alveolar Formation
Comparisons of histological lung sections qualitatively showed that treatment with ATRA increased the number of alveoli in both control and calorie-restricted rat pups, compared with cottonseed oil control pups (Figure 4A). Similarly, morphometric analyses to calculate radial alveolar counts confirmed a statistically significant increase (P < 0.05) in alveolar numbers in both control and calorie-restricted rat pups in response to ATRA (Figure 4B). These findings demonstrate that retinoic acid preserves alveolar formation in calorie-restricted rats, and recapitulate previously published observations that the administration of ATRA also enhances alveolar formation in control pups (26).
Figure 4.
Lung histological analysis with caloric restriction and retinoic acid treatment. (A) Representative photomicrographs of hematoxylin and eosin–stained lung sections from designated groups on P21 (magnification, ×10; scale bar represents 100 μM). (B) Radial alveolar count analysis for designated groups on P21.
Retinoic Acid Preserves Lung Secondary Crest Formation
To examine the effects of retinoic acid on alveolar secondary crest formation, we performed Western blot analyses and Hart’s elastin staining on lung tissue. Results showed both quantitative and qualitative differences in the amounts and distribution patterns of lung elastin. ATRA treatment significantly increased elastin protein concentrations (P < 0.05) in calorie-restricted rat lungs, compared with cottonseed oil control rats (Figure 5A). Furthermore, Hart’s elastin stain showed that elastin expression was abnormally distributed to several areas lacking secondary crests within alveoli in calorie-restricted lungs, whereas ATRA treatment preserved elastin distribution closer to that found in control rats (Figure 5B). These data are consistent with previous reports describing decreased elastin expression in IUGR rats (27) and studies concluding that retinoic acid signaling is important for alveolar secondary crest formation (28, 29).
Figure 5.
Lung elastin expression with caloric restriction and retinoic acid treatment. (A) Representative Western blots with quantification via densitometry for lung elastin protein expression from designated groups on P21, normalized to control protein vinculin. (B) Hart’s stain indicates elastin distribution in lung tissue from designated groups on P21 (magnification, ×20; scale bar represents 100 μM). Arrows point to elastin in the alveolar septal wall. RA, retinoic acid; Con, control; IPCR, intrauterine and postnatal caloric restriction. Data are presented as mean ± SEM (n = 6 rats per group). *P < 0.05, versus corresponding comparison group as indicated.
Retinoic Acid Treatment Is Associated with Increased Lung RARα Expression and Decreased TGF-β Signaling Pathway Activity
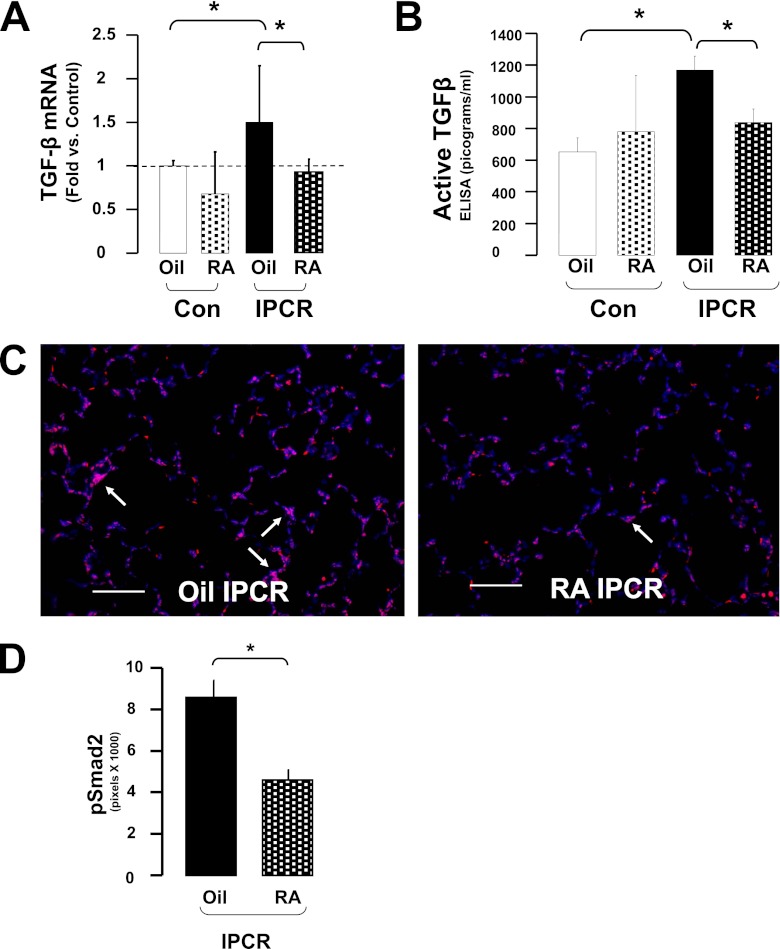
Finally, to identify the molecular pathways potentially involved in preventing alveolar hypoplasia through retinoic acid treatment, we investigated key signaling molecules that comprise, or interact with, the retinoic acid signal transduction pathway. Immunofluorescence and ELISA analyses showed that the expression of RARα was increased by ATRA in calorie-restricted lungs (Figures 6A–6C). Western blotting showed no differences in protein expression of RARβ or RARγ (data not shown). Lastly, quantitative polymerase chain reactions showed that TGF-β expression increased by 50% (P < 0.05) with caloric restriction, and was significantly decreased by 40% (P < 0.05) after treatment with ATRA (Figure 7A). These findings were confirmed through ELISA analyses showing increased active TGF-β with caloric restriction and a subsequent decrease after ATRA treatment (Figure 7B), as well as immunofluorescence indicating a nearly 50% reduction (P < 0.05) of pSmad2 expression after ATRA treatment (Figures 7C and 7D). These results underscore that ATRA treatment during caloric restriction is associated with the up-regulation of RARα and the down-regulation of TGF-β activity.
Figure 6.
Lung retinoic acid receptor–α (RARα) expression. (A) Representative photomicrographs from immunofluorescence staining for RARα protein expression (scale bar: 100 mM), with (B) quantification of RARα-positive pixels and (C) ELISA analysis. *P < 0.05, versus corresponding comparison group as indicated.
Figure 7.
Lung transforming growth factor–β (TGF-β) activity. (A) Quantitative polymerase chain reaction shows fold induction for lung TGF-β mRNA from whole-lung homogenates of designated groups on P21. (B) ELISA analysis for active TGF-β activity. (C) Representative photomicrographs from immunofluorescence staining for phosphorylated Smad2 (pSmad2) protein expression. (D) Quantification of pSmad2-positive pixels (magnification, ×20; scale bars, 100 μM). Arrows point to pSmad2-positive staining in alveolar wall. Blue staining in C represents 4′,6-diamidino-2-phenylindole nuclear stain. Data are presented as mean ± SEM (n = 6 rats per group). *P < 0.05, compared with control values.
Discussion
The impact of caloric restriction on lung function in humans and animals has long been recognized. A recent study reported that school-aged children born with IUGR demonstrated poorer lung function compared with age-matched control children (4). A large, longitudinal, placebo-controlled, double-blind, cluster-randomized trial in a chronically undernourished population of Nepali children also demonstrated that maternal supplementation with vitamin A before, during, and after pregnancy improved lung function in their offspring (30). Our study sought to determine whether treatment with the vitamin A analogue retinoic acid might provide similar benefits by involving molecular mechanisms critical for lung development, using a rat model of intrauterine and early postnatal caloric restriction. Our results showed that treatment with ATRA preserved alveolar formation, and was associated with the increased expression of RARα and the decreased expression of TGF-β activity. To our knowledge, this is the first report describing a beneficial effect of retinoic acid on preventing the alveolar hypoplasia caused by caloric restriction during lung development.
Alveolar formation is the final stage of a defined developmental process involving the transition from a rudimentary lung bud to a mature gas-exchange system. In humans, alveolar formation begins prenatally and is ready for functionality by 35 weeks of gestation, with continued growth into early childhood. In contrast, alveolar development in rodents is a postnatal event occurring between Days 5 and 21, making the rodent an ideal surrogate model for studying mechanisms of development that focus on alveolar maturation. Thus, the alveoli of rodents born at term approximate the alveolar development of human preterm infants born between 24 and 26 weeks of gestational age (13). Any perturbation during this complex process, such as exposure to hyperoxia, inflammation secondary to infection, or compromised nutrient delivery, can result in permanent alterations in alveolar structure and function, as seen with bronchopulmonary dysplasia in preterm newborns, or with chronic obstructive pulmonary disease and emphysema in adults.
Previous animal models of intrauterine growth retardation have included large animals such as fetal sheep, and have relied on strategies to reduce placental blood flow surgically via umbilical–placental embolization (22) or the removal of placental implantation sites (31). These ischemic approaches result in lungs with fewer and enlarged alveoli, and with thickening of the interalveolar septae secondary to increased extracellular matrix deposition. A fetal rat model of uteroplacental insufficiency via uterine artery ligation has also been developed, which shows similar phenotypic changes in lung architecture secondary to ischemic–reperfusion injury (21). To date, the most widely studied experimental approach to determine the impact of caloric restriction on lung structure and function has been limited to postnatal perturbations of food intake in young rats and mice (32–35). Collectively, these studies show that food restriction during late gestation or at the early postnatal stage in rodents who have been weaned from their mothers elicits structural and functional changes in lung growth and development, primarily through decreased connective tissue elastin content. A recent study by Rehan and colleagues, focusing exclusively on prenatal maternal food restriction, showed that elastin content actually increased in pups who were calorie-restricted in utero and then fed ad libitum by control dams postnatally (36). These findings are consistent with results from our study that also showed nearly threefold increases in elastin mRNA expression, specifically in the prenatal calorie-restricted ICR group after refeeding on Postnatal Day 21 (Figure 4A). The study by Rehan and colleagues was not designed to include postnatal caloric restriction, which may explain why no decrease was evident in elastin content, as was seen in postnatal calorie-restricted groups in our study and in other postnatal caloric restriction studies (32–35).
The findings of our present study demonstrate that developing embryos and early postnatal rats undergoing lung morphogenesis preserved lung growth over somatic growth during caloric restriction, and show that alveolar hypoplasia can be almost completely reversed with refeeding. These observations suggest that the underlying mechanisms responsible for alveolar development remain intact and provide justification for therapeutic approaches that can drive alveolar maturation. Our results show that treatment with retinoic acid was able to partly preserve alveolar formation with an increased expression of RARα, a key regulator of the retinoic acid signaling pathway. These data are consistent with an extensive body of literature that highlights the role of retinoic acid in alveolar development. Crabp1 concentrations in alveolar septae are known to peak on Postnatal Day 9 (5), and studies using rat lung cells in culture showed that Crabp1 protein expression was induced by ATRA (6). In addition, one isoform of RARα (RARα1), two isoforms of RARβ (RARβ2 and RARβ4), and one isoform of RARγ (RARγ2) reach peak expression on Postnatal Day 4 (37), and perturbations in expression levels can lead to a dysregulation of alveolar formation (38, 39). Finally, a subset of lipid-laden pulmonary lipofibroblasts is known to store retinoids and produce elastin during alveolar formation and in response to retinoic acid (40). The observed increases of both RARα and elastin after retinoic acid treatment in our study thus implicate these molecules as key participants in secondary crest formation, a critical step during alveolar development (41).
TGF-β is a growth factor expressed in both lung mesenchyme and epithelium, and is known to regulate branching morphogenesis and alveolar formation during lung development (42). Our results showed that lung TGF-β mRNA concentrations significantly increased with caloric restriction, but remained normal after treatment with retinoic acid. Our results also showed that pSmad2 protein expression was diminished after retinoic acid treatment, possibly implicating the involvement of the TGF-β signaling pathway, whose activation is known to inhibit alveolar development (43). These data are consistent with previous reports in which transgenic murine embryos deficient in retinoic acid lacked the development of a lung bud because of the hyperactivation of TGF-β (42). The data also recapitulate observations from a preterm lamb model of mechanical ventilation in which daily vitamin A treatment decreased pSmad2 (and therefore TGF- β activity), while preserving alveolar formation (43). These findings provide further evidence of crosstalk between the retinoic acid and TGF-β signaling pathways that may represent another mechanism to explain the effects of caloric restriction on alveolar development.
A few limitations of our study merit consideration. First, we are cognizant of the obvious differences in rats versus humans, and we emphasize that our experimental design serves only as a surrogate model for caloric restriction in humans. Nevertheless, this study was designed to mimic decreased nutrient delivery in a developing organism during both intrauterine and early postnatal life, as seen in critically ill human preterm or term newborns who cannot be optimally fed in an intensive care environment. Next, retinoic acid is only one component of a complex system of several nutrients and secretory factors including other vitamins, fats, and proteins (e.g., vitamin D, phosphatidyl choline, and surfactant) that may also affect alveolar development and function. Moreover, retinoic acid signaling may interact with numerous other converging molecular pathways, including matrix metalloproteinases, transcription factors (NK2 homeobox 1 and GATA-binding factor 6), and growth factors (fibroblast growth factor, vascular endothelial growth factor, and sonic hedgehog), as reviewed elsewhere (44), and retinoic acid signaling may involve the influence of cortisol, glucocorticoids, and insulin (45). Furthermore, our study did not specifically account for changes or differences in the composition of maternal milk between calorie-restricted versus control conditions, although previous studies of our model confirmed that early calorie-restricted rat pups consume lower quantities of milk (46). Finally, our study did not specifically test the physiological consequences of caloric restriction or vitamin A treatment on pulmonary function, whereas earlier studies reported on a loss of connective tissue components and subsequent reduction in tissue elastic forces after caloric restriction (33).
Finally, clinical implications of our study may be especially relevant to infants with IUGR who are born prematurely. Whereas previous studies have shown that vitamin A supplementation reduces the risk of bronchopulmonary dysplasia or death in a general population of preterm, very low birth weight infants, our study provides compelling evidence that the beneficial effects of vitamin A therapy may be of greatest significance in preterm infants with IUGR who cannot be adequately refed. Thus, vitamin A may serve as a therapeutic substitution until refeeding is possible. Interestingly, our results were also consistent with previous reports that vitamin A treatment increased alveolar formation in control animals (26), although the clinical implications of these observations remain unknown.
In conclusion, we have used an established rat model of caloric restriction to provide novel evidence that the mechanism by which caloric restriction induces alveolar hypoplasia partly involves the decreased expression of key retinoic acid–signaling molecules. Furthermore, we have shown that treatment with retinoic acid can prevent alveolar hypoplasia by restoring the normal expression of RARα and by decreasing TGF-β signaling pathway activity. These findings provide a potential rationale for future human studies to determine whether vitamin A therapy has a greater potential to prevent adverse pulmonary sequelae in preterm or term infants with IUGR.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health grant KO8 HL076538, a University of California at Los Angeles Faculty Research Grant, and a University of California at Los Angeles Pediatric Translational Research Seed Grant (V.A.L.), and by National Institutes of Health grants R01 HD41230, HD25024, HD46979, and HD33997 (S.U.D.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0229OC on October 18, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol 2002;31:1235–1239 [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg A. The IUGR newborn. Semin Perinatol 2008;32:219–224 [DOI] [PubMed] [Google Scholar]
- 3.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med 1999;340:1234–1238 [DOI] [PubMed] [Google Scholar]
- 4.Kotecha SJ, Watkins WJ, Heron J, Henderson J, Dunstan FD, Kotecha S. Spirometric lung function in school-age children: effect of intrauterine growth retardation and catch-up growth. Am J Respir Crit Care Med 2010;181:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci 2004;359:799–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dirami G, Massaro GD, Clerch LB, Ryan US, Reczek PR, Massaro D. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol 2004;286:L249–L256 [DOI] [PubMed] [Google Scholar]
- 7.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L955–L960 [DOI] [PubMed] [Google Scholar]
- 8.Whitney D, Massaro GD, Massaro D, Clerch LB. Gene expression of cellular retinoid–binding proteins: modulation by retinoic acid and dexamethasone in postnatal rat lung. Pediatr Res 1999;45:2–7 [DOI] [PubMed] [Google Scholar]
- 9.Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J 2004;23:20–27 [DOI] [PubMed] [Google Scholar]
- 10.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, et al. Vitamin A supplementation for extremely-low-birth-weight infants: National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 1999;340:1962–1968 [DOI] [PubMed] [Google Scholar]
- 11.Garg M, Thamotharan M, Pan G, Lee PW, Devaskar SU. Early exposure of the pregestational intrauterine and postnatal growth-restricted female offspring to a peroxisome proliferator–activated receptor–{gamma} agonist. Am J Physiol Endocrinol Metab 2010;298:E489–E498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth–restricted offspring. J Biol Chem 2008;283:13611–13626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massaro GD, Massaro D. Formation of pulmonary alveoli and gas-exchange surface area: quantitation and regulation. Annu Rev Physiol 1996;58:73–92 [DOI] [PubMed] [Google Scholar]
- 14.Londhe VA, Belperio JA, Keane MP, Burdick MD, Xue YY, Strieter RM. CXCR2/CXCR2 ligand biological axis impairs alveologenesis during dsRNA-induced lung inflammation in mice. Pediatr Res 2005;58:919–926 [DOI] [PubMed] [Google Scholar]
- 15.Londhe VA, Nguyen HT, Jeng JM, Li X, Li C, Tiozzo C, Zhu N, Minoo P. NF-κB induces lung maturation during mouse lung morphogenesis. Dev Dyn 2008;237:328–338 [DOI] [PubMed] [Google Scholar]
- 16.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Xiao J, Li C, Minoo P. Conditional deletion of epithelial IKKbeta impairs alveolar formation through apoptosis and decreased VEGF expression during early mouse lung morphogenesis. Respir Res 2011;12:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Li C, Minoo P. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am J Respir Cell Mol Biol 2011;44:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burri PH, Dbaly J, Weibel ER. The postnatal growth of the rat lung: I. Morphometry. Anat Rec 1974;178:711–730 [DOI] [PubMed] [Google Scholar]
- 19.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 2—intrauterine and early postnatal lung growth. Thorax 1982;37:580–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooney TP, Thurlbeck WM. The radial alveolar count method of Emery and Mithal: a reappraisal 1—postnatal lung growth. Thorax 1982;37:572–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien EA, Barnes V, Zhao L, McKnight RA, Yu X, Callaway CW, Wang L, Sun JC, Dahl MJ, Wint A, et al. Uteroplacental insufficiency decreases p53 serine-15 phosphorylation in term IUGR rat lungs. Am J Physiol Regul Integr Comp Physiol 2007;293:R314–R322 [DOI] [PubMed] [Google Scholar]
- 22.Maritz GS, Cock ML, Louey S, Suzuki K, Harding R. Fetal growth restriction has long-term effects on postnatal lung structure in sheep. Pediatr Res 2004;55:287–295 [DOI] [PubMed] [Google Scholar]
- 23.Gruppuso PA, Boylan JM, Anand P, Bienieki TC. Effects of maternal starvation on hepatocyte proliferation in the late gestation fetal rat. Pediatr Res 2005;57:185–191 [DOI] [PubMed] [Google Scholar]
- 24.Equils O, Singh S, Karaburun S, Lu D, Thamotharan M, Devaskar SU. Intra-uterine growth restriction downregulates the hepatic Toll like receptor–4 expression and function. Clin Dev Immunol 2005;12:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin R, Tian F, Frankenberger B, de Angelis MH, Stoeger T. Selection and evaluation of stable housekeeping genes for gene expression normalization in carbon nanoparticle–induced acute pulmonary inflammation in mice. Biochem Biophys Res Commun 2010;399:531–536 [DOI] [PubMed] [Google Scholar]
- 26.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol 1996;270:L305–L310 [DOI] [PubMed] [Google Scholar]
- 27.Joss-Moore LA, Wang Y, Yu X, Campbell MS, Callaway CW, McKnight RA, Wint A, Dahl MJ, Dull RO, Albertine KH, et al. IUGR decreases elastin mRNA expression in the developing rat lung and alters elastin content and lung compliance in the mature rat lung. Physiol Genomics 2011;43:499–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi W, Chen F, Cardoso WV. Mechanisms of lung development: contribution to adult lung disease and relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009;6:558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burri PH. The postnatal growth of the rat lung: 3. Morphology. Anat Rec 1974;180:77–98 [DOI] [PubMed] [Google Scholar]
- 30.Checkley W, West KP, Jr, Wise RA, Baldwin MR, Wu L, LeClerq SC, Christian P, Katz J, Tielsch JM, Khatry S, et al. Maternal vitamin A supplementation and lung function in offspring. N Engl J Med 2010;362:1784–1794 [DOI] [PubMed] [Google Scholar]
- 31.Lipsett J, Tamblyn M, Madigan K, Roberts P, Cool JC, Runciman SI, McMillen IC, Robinson J, Owens JA. Restricted fetal growth and lung development: a morphometric analysis of pulmonary structure. Pediatr Pulmonol 2006;41:1138–1145 [DOI] [PubMed] [Google Scholar]
- 32.Massaro D, Alexander E, Reiland K, Hoffman EP, Massaro GD, Clerch LB. Rapid onset of gene expression in lung, supportive of formation of alveolar septa, induced by refeeding mice after calorie restriction. Am J Physiol Lung Cell Mol Physiol 2007;292:L1313–L1326 [DOI] [PubMed] [Google Scholar]
- 33.Sahebjami H, MacGee J. Effects of starvation on lung mechanics and biochemistry in young and old rats. J Appl Physiol 1985;58:778–784 [DOI] [PubMed] [Google Scholar]
- 34.Sahebjami H, Domino M. Effects of repeated cycles of starvation and refeeding on lungs of growing rats. J Appl Physiol 1992;73:2349–2354 [DOI] [PubMed] [Google Scholar]
- 35.Das RM. The effects of intermittent starvation on lung development in suckling rats. Am J Pathol 1984;117:326–332 [PMC free article] [PubMed] [Google Scholar]
- 36.Rehan VK, Sakurai R, Li Y, Karadag A, Corral J, Bellusci S, Xue YY, Belperio J, Torday JS. Effects of maternal food restriction on offspring lung extracellular matrix deposition and long term pulmonary function in an experimental rat model. Pediatr Pulmonol 2012;47:162–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hind M, Corcoran J, Maden M. Temporal/spatial expression of retinoid binding proteins and RAR isoforms in the postnatal lung. Am J Physiol Lung Cell Mol Physiol 2002;282:L468–L476 [DOI] [PubMed] [Google Scholar]
- 38.Massaro GD, Massaro D, Chan WY, Clerch LB, Ghyselinck N, Chambon P, Chandraratna RA. Retinoic acid receptor–beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics 2000;4:51–57 [DOI] [PubMed] [Google Scholar]
- 39.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 2000;23:162–167 [DOI] [PubMed] [Google Scholar]
- 40.McGowan SE, Doro MM, Jackson SK. Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol 1997;273:L410–L416 [DOI] [PubMed] [Google Scholar]
- 41.Okabe T, Yorifuji H, Yamada E, Takaku F. Isolation and characterization of vitamin-A–storing lung cells. Exp Cell Res 1984;154:125–135 [DOI] [PubMed] [Google Scholar]
- 42.Chen F, Desai TJ, Qian J, Niederreither K, Lu J, Cardoso WV. Inhibition of TGF beta signaling by endogenous retinoic acid is essential for primary lung bud induction. Development 2007;134:2969–2979 [DOI] [PubMed] [Google Scholar]
- 43.Albertine KH, Dahl MJ, Gonzales LW, Wang ZM, Metcalfe D, Hyde DM, Plopper CG, Starcher BC, Carlton DP, Bland RD. Chronic lung disease in preterm lambs: effect of daily vitamin A treatment on alveolarization. Am J Physiol Lung Cell Mol Physiol 2010;299:L59–L72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galambos C, Demello DE. Regulation of alveologenesis: clinical implications of impaired growth. Pathology 2008;40:124–140 [DOI] [PubMed] [Google Scholar]
- 45.Devaskar SU, deMello DE. Cell-specific localization of glucose transporter proteins in mammalian lung. J Clin Endocrinol Metab 1996;81:4373–4378 [DOI] [PubMed] [Google Scholar]
- 46.Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res 2012;90:1169–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.