Abstract
Dysfunction of the cystic fibrosis transmembrane regulator (CFTR) leads to chronic inflammation and infection of the respiratory tract. The role of CFTR for cells of the pulmonary immune system is only partly understood. The present study analyzes the phenotype and immune stimulatory capacity of lung dendritic cells (DCs) from CFTR knockout (CF) mice. Total numbers of conventional DCs, plasmacytoid DCs, and CD103-positive DCs were lower in CF mice compared with wild-type (WT) control mice, as was the expression of major histocompatibility complex class II molecules (MHCII), CD40, and CD86. After pulmonary infection with respiratory syncytial virus, DC numbers increased in WT mice but not in CF mice, and the T cell–stimulatory capacity of CF DCs was impaired. The culture of CF lung DCs with bronchoalveolar lavage fluid (BALF) from WT mice increased the expression of MHCII, CD40, and CD86. The supplementation of CF BALF with sphingosine-1–phosphate (S1P), a mediator of immune cell migration and activation that is decreased in CF BALF, rescued the reduced expression of MHCII and CD40 in WT lung DCs and human blood DCs. These findings suggest that DCs are impaired in the CF lung, and that altered S1P affects lung DC function. These findings provide a novel link between defective CFTR and pulmonary innate immune dysfunction in CF.
Keywords: cystic fibrosis, dendritic cells, sphingolipids
Cystic fibrosis (CF) lung disease is characterized by chronic infections and inflammation (1). The mechanisms for the increased susceptibility of the CF lung to infections and to inflammation even in the absence of infection are only partly understood (2). The dysregulated cytokine secretion of CF epithelial cells plays an important role in creating the inflammatory milieu (3). It has become increasingly clear that the cystic fibrosis transmembrane regulator (CFTR) also plays a role in lung immune cells, and that the dysfunction of the CFTR affects immune cell responses (4–11). The dysfunction of pulmonary immune cells in CF could result from the lack of their own CFTR function, or may be induced by the altered milieu created by defective CFTR function in epithelial cells (5, 8).
The dysfunction or lack of CFTR expression in macrophages, neutrophils, and dendritic cells (DCs) results in an inflammatory phenotype (4, 5, 8–11). In addition, antigen presentation is affected in CF. The major histocompatibility complex class I (MHC I) presentation pathway is altered in CF epithelial cells (12). Proliferation in response to Pseudomonas aeruginosa antigens is impaired in CF lymphocytes (13). Moreover, the reduced IL-10 that is characteristic of the CF lung milieu increases the cleavage of costimulatory molecule B7 on human macrophages (14).
Clinically relevant immune cell abnormalities in CF may be restricted to the lung, because chronic infections outside the respiratory tract are uncommon (15). Consistent with this finding is the normal presentation via the CD1 pathway in blood-derived CF DCs (16). Interestingly, decreased MHCII expression has been seen on CF neutrophils (12), aberrant, Th2-biased cytokine secretion has been reported for CF lymphocytes of patients with pulmonary P. aeruginosa infection (17), and CF monocytes and neutrophils were shown to be abnormally stimulated (7). Bone marrow–derived DCs from CF mice show delayed maturation (11). Pulmonary DCs, crucial in orchestrating innate and adaptive immune responses in the lung, have not been studied in CF.
The present study analyzes murine CF lung DCs to assess their contributions to the innate immune dysfunction in the CF lung. Although CF murine models generally do not mimic the severe CF lung disease observed in humans (18), Cftrtm1UNC mice backcrossed on a C57BL/6 background exhibit increased pulmonary inflammatory response and increased susceptibility to lung infections with CF-related bacterial and viral pathogens, including respiratory syncytial virus (RSV) (19). We found that the numbers, maturation, activation, and T cell–stimulatory capacity were altered in CF lung DCs at baseline and after infection with RSV. The culture of CF DCs in bronchoalveolar lavage fluid (BALF) from wild-type (WT) mice improved their activation and maturation, suggesting that factors in the pulmonary milieu contribute to the CF DC phenotype. Based on our previous observations that CFTR dysfunction causes abnormalities in sphingolipid metabolism (20), we found that sphingosine-1–phosphate (S1P), a critical mediator for immune cell trafficking and DC activation (21–23), decreased in CF BALF. We demonstrate that CF BALF supplemented with S1P normalizes the maturation of CF DCs. These findings highlight abnormalities in pulmonary DCs in CF that could affect susceptibility and the clearance of pathogens from the CF lung, and indicate that decreased concentrations of S1P could comprise a contributing factor.
Materials and Methods
Mice
CFTR knockout mice (Cftrtm1UNC; CF mice) and WT C57BL/6 mice (WT mice) were used at 12–20 weeks of age, and were maintained on a diet composed of filtered liquid (1:1 mixture of water and PEG3350; Paddock Laboratories, Minneapolis, MN) and solid food 5061 (LabDiet, Elkridge, MD). All procedures were approved by the Institutional Animal Care and Use Committee of Weill Cornell Medical College (Institutional Animal Care and Use Committee protocol number 2009-0039).
Cells
DCs were isolated from lung single-cell suspensions from WT and CF mice, using magnetic beads. Details are presented in the online supplement.
CFTR Expression in DCs
CFTR protein expression was determined in CD11c+ cells or lungs from WT mice by Western blot analysis, and CFTR mRNA was analyzed in purified conventional DCs (cDCs) and plasmacytoid DCs (pDCs) via real-time RT-PCR. Details are presented in the online supplement.
Allostimulatory Capacity of DCs
The allostimulatory capacity of DCs was assessed by a mixed leukocyte reaction (MLR). Details are presented in the online supplement.
Infection with RSV
To assess the effects of infection in the respiratory tract with a viral pathogen relevant to CF, CF or WT mice were infected with RSV strain A2. Details are presented in the online supplement.
Coculture of DCs with BALF
To evaluate whether the phenotype of DCs from CF mice is affected by factors in the milieu of the respiratory tract, DCs from CF or WT mice were cultured in the presence of BALF from CF or WT mice. Details are presented in the online supplement.
S1P Analysis
The concentration of S1P in BALF was analyzed by mass spectrometry. Details are presented in the online supplement.
Statistical Analysis
The results are presented as means ± SEMs. Significance was calculated according to a nonpaired, two-tailed Student t test for two-group comparisons, and according to ANOVA for multiple comparisons of more than two groups. Statistical significance was determined at P < 0.05.
Results
DCs Express CFTR and Are Decreased in CF Lungs
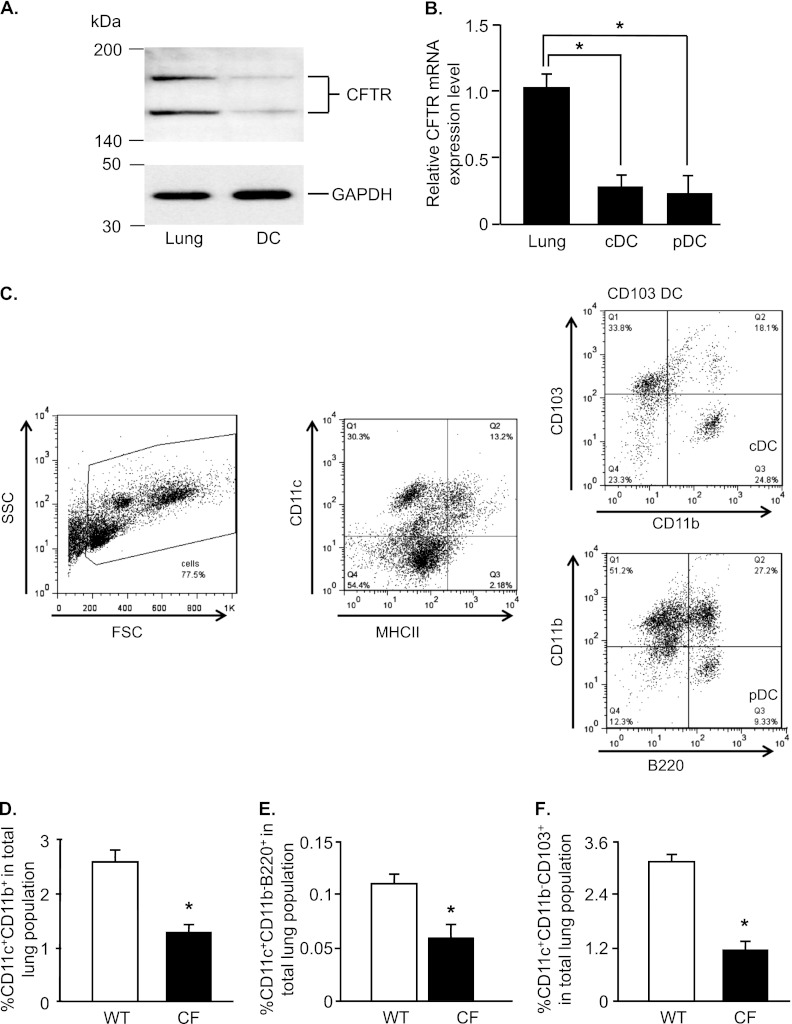
CFTR protein was detected in purified lung DCs by Western blot analysis (Figure 1A). The size of the CFTR protein bands was 150 and 170 kD in DCs and whole lung, respectively. CFTR expression was lower in DCs compared with whole lung. To evaluate whether the two major DC subsets (cDCs and pDCs) expressed CFTR, cDCs and pDCs were isolated from the lung single-cell suspensions of WT mice, and CFTR mRNA expression was quantified by real-time RT-PCR (Figure 1B). The cDCs and pDCs expressed CFTR mRNA at comparable concentrations, but the expression in both DC subsets was lower compared with the expression in whole lung (3.5-fold for cDCs, and 4.4-fold for pDCs; P < 0.05).
Figure 1.
Cystic fibrosis transmembrane regulator (CFTR) expression in dendritic cells (DCs), and decreased percentage of DCs in CFTR knockout (CF) mice. DCs (CD11c+) were isolated from the lungs of wild-type (WT) C57BL/6 mice by magnetic beads, and were further separated into conventional DCs (cDCs; CD11c+CD11b+) or plasmacytoid DCs (pDCs; CD11c+CD11b−B220+) by fluorescence-activated cell sorting. (A) Western blot analysis of CFTR protein expression in DCs and all lung cells. (B) CFTR mRNA expression in cDCs, pDCs, and all lung cells by real-time RT-PCR, normalized to the expression of glyceraldehyde 3–phosphate dehydrogenase mRNA. Data are presented as the means ± SEMs of n = 5 mice/group for one of two independent experiments. (C) Diagram of gating strategy of cDCs and pDCs by flow cytometry. The percentages of cDCs (D), pDCs (E), and CD103-positive (CD103+) DCs (F) from CF mice and WT control mice were quantified by flow cytometry. Data are expressed as the means ± SEMs of the percentages of stained cells of all lung cells. Data shown are representative of six independent experiments, each with n = 4–5 mice/group. *P < 0.05. SSC, side scatter; FSC, forward scatter; MHCII, major histocompatibility complex class II molecules; Q, quadrant.
Percentages of cDCs and pDCs were evaluated in single-cell lung suspensions by flow cytometry, as shown in a diagram detailing the gating strategy of cDCs and pDCs according to flow cytometry (Figure 1C). The percentage of cDCs accounted for approximately 2.5% of total lung cells in WT mice, and was 2-fold lower in CF mice (Figure 1D; P < 0.05). Likewise, the pDC and CD103-positive (CD103+) DC populations were 1.8-fold and 2.7-folder lower in CF mice compared with WT mice, respectively (Figures 1E and 1F, P < 0.05).
Altered Maturation of DCs in CF Mice
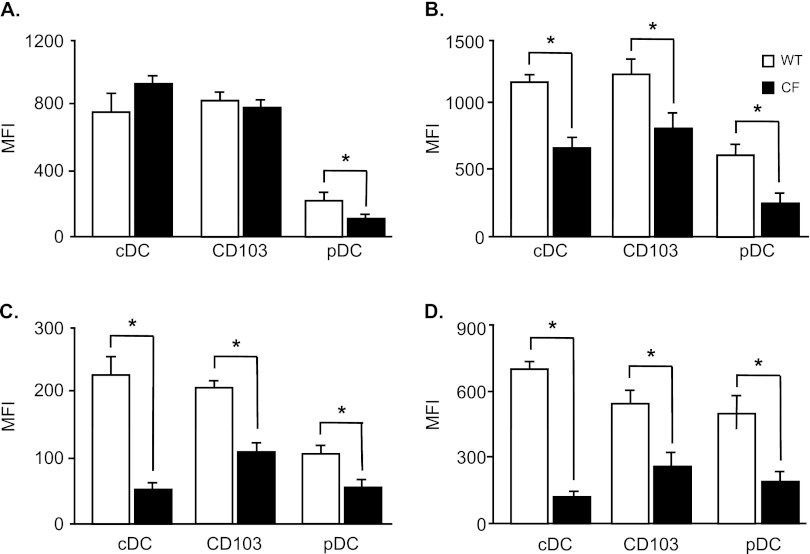
To evaluate the maturation and activation of lung DCs, the surface expressions of MHCI, MHCII, CD40, and CD86 were analyzed in cDCs, pDCs, and CD103+ DCs by flow cytometry. The expression of MHCI was decreased in pDCs of CF mice compared with WT control mice (P < 0.05), but was similar in cDCs (Figure 2A). The expressions of MHCII (Figure 2B), CD40 (Figure 2C), and CD86 (Figure 2D) in cDCs, pDCs, and CD103+ DCs were lower in cells from CF mice compared with WT control mice (P < 0.05; all comparisons). In contrast to lung DCs, the expression of MHCII was increased in splenic cDCs, pDCs, and CD103+ DCs from CF mice compared with control mice (see Figure E1A in the online supplement; P < 0.05), whereas the expression of CD40 was reduced (P < 0.05; Figure E1B). The expression of CD86 was no different between WT and CF splenic DCs (Figure E1C).
Figure 2.
Impaired activation and maturation of DCs from CF mice. The cDCs, pDCs, and CD103+ DCs from CF and WT mice were analyzed for their surface expression of MHCI (A), MHCII (B), CD40 (C), and CD86 (D) by flow cytometry. Data represent the means ± SEMs of mean fluorescence intensity (MFI) from six independent experiments, each with n = 4–5 mice/group.*P < 0.05.
Decreased Allostimulatory Capacity of CF DCs
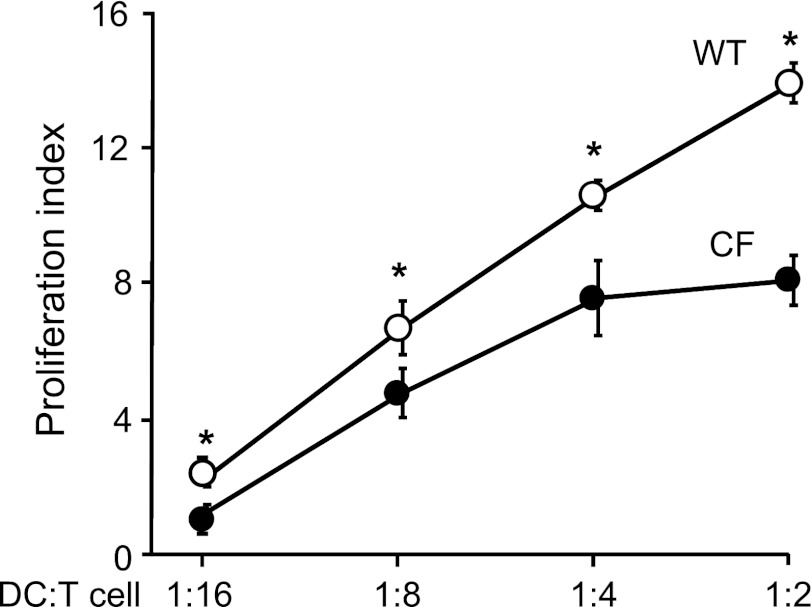
To assess whether the T cell–stimulatory potency of CF lung DCs was affected, we analyzed their allostimulatory capacity via MLR. Allogeneic splenic T cells from BALB/c mice proliferated less when cocultured with DCs from CF mice, compared with DCs from WT mice (P < 0.05; Figure 3). This suggests that DCs from CF mice are impaired in the initiation of T-cell responses.
Figure 3.
Allostimulatory capacity of DCs from CF mice. DCs from CF and WT mice were cocultured with allogeneic T cells purified from spleens of BALB/c mice labeled with carboxyfluorescein succinimidyl ester at ratios of 1:2, 1:4, 1:8, and 1:16 for 5 days. The proliferation index (mean fluorescence intensity of proliferated cells to nonproliferated cells) was determined by flow cytometry. Data shown are representative of results from six independent experiments, each with n = 4–5 mice/group. *P < 0.05.
DCs and T-Cell Response to Pulmonary Infection with RSV
To evaluate how CF lung DCs respond to infection with a viral pathogen relevant to CF lung disease, mice were infected with RSV. Six days after infection, both cDCs (Figure 4A) and pDCs (Figure 4B) from WT mice increased in number (1.8-fold and 2.1-fold, respectively; P < 0.05). In contrast, the number of cDCs from CF mice did not increase (Figure 4A). The number of pDCs in CF mice increased threefold (Figure 4B, P < 0.05). These data suggest that the overall RSV-induced increase in DCs is blunted in CF mice, because cDCs constitute the majority of lung DCs.
Figure 4.
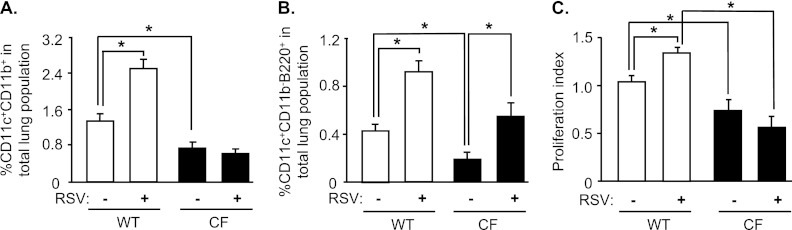
Effects of pulmonary infection with respiratory syncytial virus (RSV) in DCs of CF mice. CF and WT mice were infected with the RSV strain A2 (106 plaque-forming units/mouse) via intranasal installation. The percentages of cDCs (A) and pDCs (B) from CF and WT mice were quantified by flow cytometry after 6 days. Data are expressed as the means ± SEMs of the percentages of stained cells of all lung cells. (C) Allostimulatory capacity of DCs from WT and CF mice after infection with RSV was assessed by coculture with allogeneic T cells purified from spleens of BALB/c mice labeled with CFSE at a ratio of 1:2 for 5 days. The proliferation index (mean fluorescence intensity of proliferated cells to nonproliferated cells) was determined by flow cytometry. Data shown represent the results from six independent experiments, each with n = 4–5 mice/group. *P < 0.05.
We next evaluated the T cell–stimulatory capacity of lung DCs after RSV infection. Again, the proliferation of T cells stimulated by DCs from uninfected CF mice was lower compared with that of T cells stimulated by WT DCs (P < 0.05; Figure 4C). DCs from WT mice that had been infected with RSV showed increased T-cell stimulation compared with uninfected WT control mice (P < 0.05; Figure 4C). In contrast, the allostimulatory capacity of DCs from CF mice infected with RSV was not increased compared with that of DCs from uninfected CF mice (Figure 4C). Similar results were obtained when lung DCs were harvested 3 days after infection with RSV (Figure E2). This suggests that the capacity of DCs from CF mice to stimulate T cells is impaired after infection with RSV.
IL-4 and IFN-γ were decreased in lung CD4 and CD8 T cells from CF mice, compared with WT control mice at baseline (Figure E3; P < 0.05). After infection with RSV, the secretion of IL-4 from CD4 T cells of both WT and CF mice increased (P < 0.5; Figure E3A). In contrast, IFN-γ was only increased in CF CD4 T cells but not in WT CD4 T cells (Figure E3B). Both IL-4 and IFN-γ increased in CD8 T cells from WT mice after RSV infection (P < 0.05; Figure E3C), but not in CD8 T cells from CF mice (Figure E3D). These data suggest that Th1 and Th2 cytokine responses in CF lung T cells are decreased at baseline, and are dysregulated in CD8 but not CD4 T cells after infection with RSV.
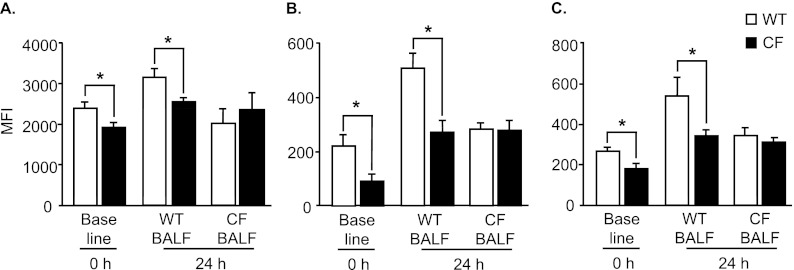
CF Pulmonary Milieu Affects Maturation and Activation of DCs
To determine whether the decreased maturation and activation of CF DCs was secondary to factors in the milieu of the CF lung or if it was primarily caused by a lack of CFTR in DCs, the expression of MHCII, CD40, and CD86 was evaluated in CF and WT DCs cultured in WT or CF BALF (Figures 5A–5C). The lower baseline expression of MHCII in CF DCs was maintained when the cells were cultured with WT BALF (P < 0.05; Figure 5A). Interestingly, when WT DCs and CF DCs were cultured in CF BALF, the expression of MHCII was comparable between WT and CF DCs (Figure 5A). A similar pattern was seen for the expression of CD40 (Figure 5B) and CD86 (Figure 5C) in DCs from WT and CF mice when cultured in WT or CF BALF. This suggests that the decreased surface expression of MHCII, CD40, and CD86 in DCs is influenced, at least in part, by factors in the milieu of the CF lung.
Figure 5.
Profiles of DCs from WT and CF mice in vitro cultured in bronchoalveolar lavage fluid (BALF). CF and WT DCs were cultured in vitro in BALF from either WT or CF mice for 24 hours. The surface expressions of MHCII (A), CD40 (B), and CD86 (C) in DC were assessed by flow cytometry. Data represent the means ± SEMs of mean fluorescence intensity from three independent experiments, each with n = 4–5 mice/group. *P < 0.05.
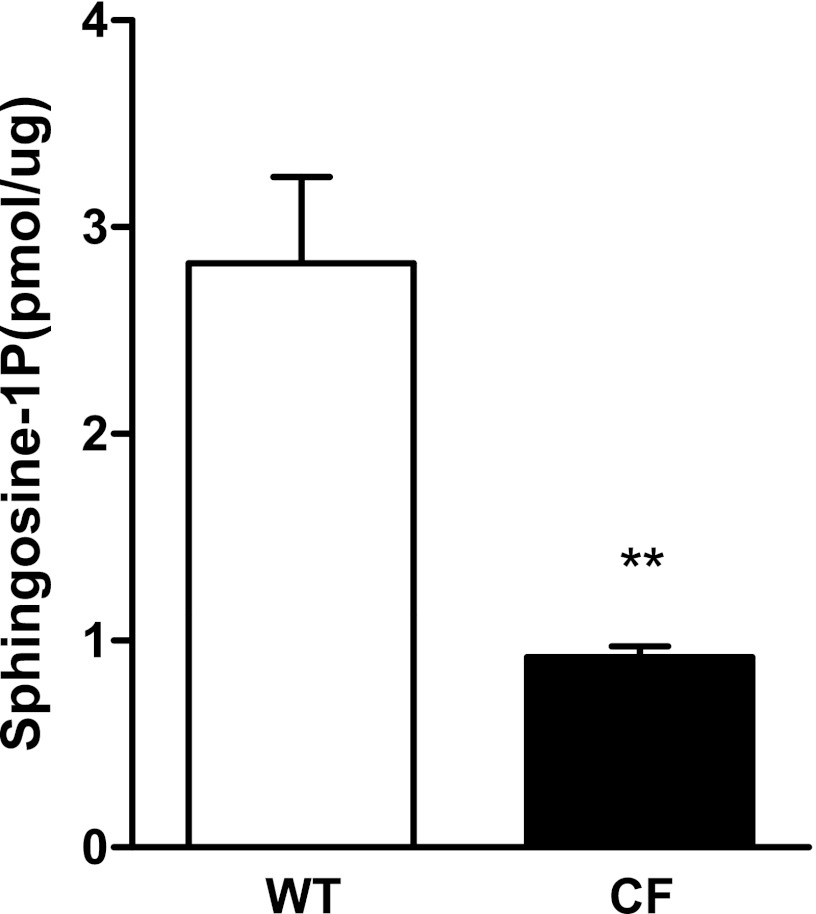
To assess whether altered sphingolipid metabolism in CF could contribute to the DC phenotype, we analyzed BALF of the CF and WT mice for S1P, a sphingolipid mediator involved in immune-cell trafficking and activation. Interestingly, S1P, normalized to the BALF protein concentration (37.5 ± 3.4 μg/ml in WT mice; 41.1 ± 2.9 μg/ml in CF mice), was decreased in CF BALF (P < 0.01; Figure 6). These concentrations are comparable to those analyzed previously in the BALF of C57BL/6 mice by an enzymatic method and thin liquid chromatography (24, 25).
Figure 6.
Sphingosine-1–phosphate (S1P) in BALF of CF mice. S1P concentrations in BALF from either WT or CF mice were analyzed by high-performance liquid chromatography–mass spectrometry and normalized for protein content, each with n = 10 mice/group. **P < 0.05.
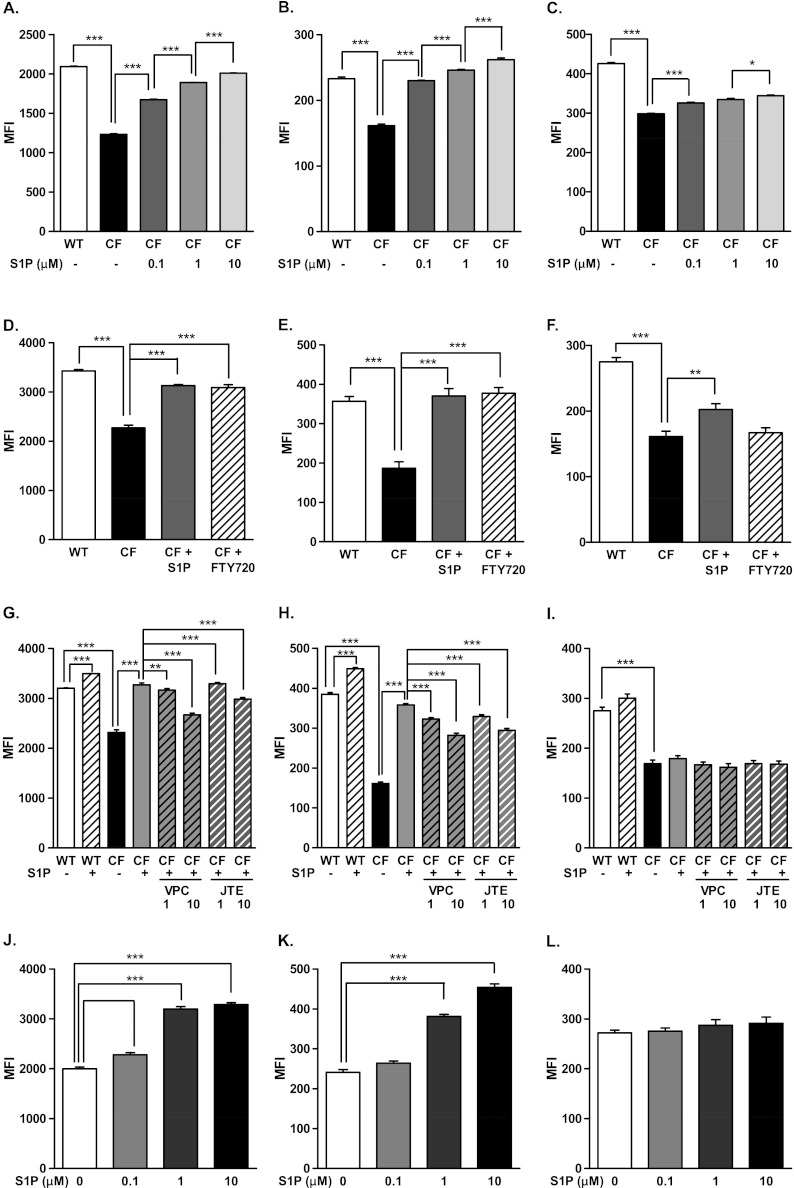
To evaluate whether decreased S1P could contribute to the CF lung DC phenotype, DCs isolated from WT mice were cultured in WT BALF, CF BALF, or CF BALF supplemented with 0.1, 1.0, and 10.0 μM S1P for 24 hours. The expression of MHCII (Figure 7A), CD40 (Figure 7B), and CD86 (Figure 7C) in DCs cultured in CF BALF were decreased compared with those cultured in WT BALF (P < 0.001; all comparisons). A dose-dependent increase was evident in the expression of MHCII (P < 0.001; Figure 7A) and CD40 (P < 0.001; Figure 7B) with S1P supplementation to the levels seen in WT BALF. The expression of CD86 also increased with S1P supplementation, but to a lesser extent (Figure 7C). Similarly, the expressions of human leukocyte antigen (HLA)-DR (P < 0.05; Figure E4A), CD40 (P < 0.05; Figure E4B), and CD86 (P < 0.05; Figure E4C) were reduced in normal human peripheral blood–derived DCs cultured in CF BALF compared with WT BALF. The supplementation of S1P reversed the decreased expression of HLA-DR (P < 0.05; Figure E4A), CD40 (P < 0.05; Figure E4B), and CD86 (P < 0.05; Figure E4C).
Figure 7.
Effects of S1P on maturation and activation of DCs cultured in vitro in CF BALF. Lung DCs from WT mice were cultured in CF BALF supplemented with 0.1, 1.0, and 10.0 μM S1P (A–C), or 20 nM of the S1P analogue FTY720 or 1 μM S1P (D–F), or 1 and 10 nM of the S1P receptor inhibitors JTE013 (JTE) or VPC23019 (VPC) (G–I) for 24 hours. CF lung DCs were cultured in CF BALF supplemented with 0.1, 1.0, and 10.0 μM S1P (J–L) for 24 hours. The surface expression of MHCII (left panels), CD40 (middle panels), and CD86 (right panels) was assessed by flow cytometry. Data represent the means ± SEMs of mean fluorescence intensity. *P < 0.05. **P < 0.01. ***P < 0.001.
The addition of the S1P analog FTY720 at a low dose (20 nM) increased the expression of MHCII (P < 0.001; Figure 7D) and CD40 (P < 0.001; Figure 7E), but failed to increase CD86 (Figure 7G), as did the addition of 1 μM S1P (26). The addition of S1P receptor (S1PR)2 blocker JTE013 and S1PR1/3 blocker VPC23019 reversed the S1P-induced increased expression of MHCII (P < 0.001; Figure 7G) and CD40 (P < 0.001; Figure 7H), but not CD86 (Figure 7I), suggesting that the effect of S1P on the expression of MHCII and CD40 could be mediated through S1P receptors. Likewise, S1P increased the expression of MHCII (P < 0.001; Figure 7J) and CD40 (P < 0.001; Figure 7K), but not CD86 (Figure 7L), in CF DCs cultured in CF BALF.
To evaluate other factors in the CF milieu that could affect the maturation and activation of DCs, we quantified neutrophil elastase (NE) and Type 1 IFN in BALF. As expected, elevated concentrations of IFN-α/β (P < 0.001; Figure E5A) and NE (P < 0.001; Figure E5B) were seen in CF BALF. Interestingly, when NE or IFN-α/β was added to WT BALF, the expression of MHCII (Figure E5C) was only slightly decreased by NE (P < 0.05) and unchanged with IFN-α/β. The expressions of CD40 (Figure E5D) and CD86 (Figure E5E) were not altered.
Discussion
The role of CFTR in cells of the pulmonary immune system is not completely understood. Pulmonary immune cells are affected by the altered milieu created by defective CFTR function in respiratory epithelial cells, but may also be primarily affected by their own defective CFTR expression. We observed phenotypic and functional abnormalities in CF lung DCs, including an overall decreased number, reduced maturation and activation, and impaired T cell–stimulatory capacity that were not seen in systemic DCs. Infections outside the respiratory tract are not characteristic of CF, and despite some reports of depressed B and T cell responses (27, 28), systemic immune responses appear not to be impaired in CF (29).
Lung immune responses, in contrast, are affected. A recent analysis of multiple gene expression studies identified altered MHCI presentation as one of the consistent differences between a variety of CF and non-CF epithelial cells (12). We show that CFTR is expressed in lung DCs at similar concentrations in their two major subfractions (cDCs and pDCs). cDCs comprise the majority of lung DCs (30), and pDCs are characterized by their capacity to produce Type 1 interferons (31). It has been suggested that the balance between the total numbers of cDCs and pDCs maintains tolerance for inhaled antigens, and that pDCs mediate the suppression of Th2 immunity (32). The numbers of both cDCs and pDCs were decreased in CF mice, which may cause a shift in the T-helper cell balance, and could thus be responsible for changes in the type of immune responses to inhaled antigens. Various studies have described a Th2-favoring imbalance in the CF lung (17, 33). CD103+ DCs, which comprise another lung DC subset, are closely associated with airway epithelium and can prime Th2 differentiation (34). Lower numbers of CD103+ DCs in CF lungs indicate a reduced DC population that could migrate across the airway epithelia for antigen and pathogen capture, transport, and presentation.
Lower numbers of DCs in CF mice suggest defective migration or recruitment to the lung. DCs, widely distributed throughout the conducting airways and the peripheral lung, are derived from CD34 bone marrow progenitors (35). The lower overall number could thus be a reflection of the decreased recruitment of monocytes. The half-life of a lung DC is only approximately 2 days (36). Interestingly, the expression of MHCI was not altered in CF DCs compared with WT DCs. The lower expression of MHCII, CD40, and CD86 in CF lung DCs indicates decreased activation and maturation, leading to the impaired proliferation of allogeneic T cells. Interestingly, this phenotype was not seen in splenic DCs. The contrasting difference of phenotypes in systemic and lung DCs suggests a lung-specific alteration in DC activation. After the infection of CF mice with RSV, numbers of lung DCs did not increase, and their allogeneic stimulatory capacity was impaired. CF mice react with increased inflammation and decreased viral clearance to RSV infection (19). RSV is known to trigger exacerbations of CF lung disease (37–39) and to facilitate colonization with P. aeruginosa (40). Both cDCs and pDCs are typically activated and increase in number in response to RSV infection (41, 42). Lung CD8 T-cell IL-4 and INF-γ responses to RSV infection were also compromised in CF mice, indicating a potential role for T cells in CF lung disease.
Inflammatory mucosal conditions have been associated with DC activation. CD40 and CD86 are up-regulated in human lung myeloid DCs during chronic obstructive pulmonary disease, and intestinal DCs are up-regulated in inflammatory bowel disease (43, 44). In contrast, the CF lung DC phenotype is characterized by the decreased expression of MHCII, CD40, and CD86. The decreased expression of these markers is partly dependent on factors present in airway epithelial fluid. Inflammatory cytokines, which are known to be altered in the BALF of CF mice (5), affect the activation of DCs (45). The decreased concentrations of IL-10 in the CF lung have been shown to lead to an increase in the expression of CD80 and CD86 (14), contrary to the phenotype seen in CF lung DCs. One factor abundantly present in CF airway fluid that could inhibit the maturation of pulmonary DCs is NE. Both purified NE and CF sputum from adult patients with CF were able to down-regulate CD40, CD80, and CD86 but not MHCII in murine bone marrow–derived DCs (46). The decreased expression of CD86 supposedly occurred via enzymatic cleavage by NE. NE, at the concentrations found in CF BALF, did not affect the expression of MHCII and CD40, and only slightly decreased CD40. Type 1 IFN signaling, recently shown to be activated in the CF lung (47), could also affect the activation and maturation of DCs. We found Type 1 IFN to be increased in CF BALF, but the supplementation of WT lavage with IFN-α/β at concentrations present in CF BALF did not affect the expression of the activation and maturation markers in lung DCs. Only the supplementation of CF BALF with S1P corrected both MHCII and CD40 expression in murine lung DCs and human blood DCs. The role of sphingolipids in CF lung disease is incompletely understood. Most of the focus has centered on CF-related changes in membrane-bound ceramides (48). Our study points to a role for sphingolipid mediators. S1P is involved in the positioning and trafficking of lymphocytes as well as the activation of DCs. Critical for these activators are a S1P gradient between blood and tissue compartments and the presence of S1P receptors (21, 22). Lung DCs express all known S1P receptors (23). We do not know the exact mechanism by which decreased S1P affects the maturation and activation of CF lung DCs. A receptor-mediated mechanism is likely responsible. Both S1PR1/3 and S1PR2 inhibition exerted a similar effect on MHCII and CD40 expression. Because different intracellular GTPases are activated by S1PR1/3 or S1PR2 ligation, these findings suggest that not only one receptor or intracellular signaling pathway is involved (49–51).
CFTR is involved in the cellular uptake of S1P (52), and sphingolipid synthesis is increased in CF epithelial cells (20). It is tempting to speculate that CFTR could be involved in the secretion of S1P, which would explain the lower concentrations in BALF despite increased de novo sphingolipid synthesis. Future studies need to analyze S1P transport in CFTR-deficient cells. Increased concentrations of S1P in BALF were recently reported in patients with idiopathic pulmonary fibrosis, which may affect the epithelial-to-mesenchymal transition in pulmonary fibrosis (53). A 10-fold or 100-fold increase of S1P above the concentrations found in WT BAL showed a dose-dependent increase in expression of CD40 and MHCII, without reaching a ceiling. Decreased S1P in the BALF could not only affect DC function but also DC recruitment to the lung, which could be reflected by the decreased overall numbers of DCs in CF lungs. S1P has been shown to inhibit chemotaxis and the transendothelial migration of neutrophils, and equally relevant to CF lung disease, to antagonize IL-8–mediated neutrophil chemotaxis (54). Increased S1P in S1P lyase knockout mice is not only associated with lymphopenia, but also with neutrophilia and impaired neutrophil trafficking (55). Low S1P in CF may thus not only affect DC recruitment but also neutrophil recruitment through a common mechanism that has so far not been studied in the CF lung.
Overall, the present study demonstrates that lung DCs are functionally affected in CF mice, at least partly because of low S1P in the lung milieu. Alterations in DC function may play a role in the susceptibility to CF, and in chronic infections in CF.
Supplementary Material
Footnotes
This study was supported in part by National Institutes of Health grant R21 HL077557 and Cystic Fibrosis Foundation grant XU09F0.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0021OC on December 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ratjen F, Doring G. Cystic fibrosis. Lancet 2003;361:681–689 [DOI] [PubMed] [Google Scholar]
- 2.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med 2012;18:509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med 2004;169:645–653 [DOI] [PubMed] [Google Scholar]
- 4.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol 2011;186:6990–6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in CFTR−/− mice. Am J Respir Cell Mol Biol 2008;40:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bubien J. CFTR may play a role in regulated secretion by lymphocytes: a new hypothesis for the pathophysiology of cystic fibrosis. Pflugers Arch 2001;443:S36–S39 [DOI] [PubMed] [Google Scholar]
- 7.del Fresno C, Garcia-Rio F, Gomez-Pina V, Soares-Schanoski A, Fernandez-Ruiz I, Jurado T, Kajiji T, Shu C, Marin E, Gutierrez del Arroyo A, et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol 2009;182:6494–6507 [DOI] [PubMed] [Google Scholar]
- 8.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol 2006;8:933–944 [DOI] [PubMed] [Google Scholar]
- 9.Painter RG, Bonvillain RW, Valentine VG, Lombard GA, LaPlace SG, Nauseef WM, Wang G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J Leukoc Biol 2008;83:1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Y, Krause A, Hamai H, Harvey BG, Worgall TS, Worgall S. Proinflammatory phenotype and increased caveolin-1 in alveolar macrophages with silenced CFTR mRNA. PLoS ONE 2010;5:e11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Tertilt C, Krause A, Quadri L, Crystal R, Worgall S. Influence of the cystic fibrosis transmembrane conductance regulator on expression of lipid metabolism–related genes in dendritic cells. Respir Res 2009;10:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton TH, Stanton BA. A novel approach to analyze gene expression data demonstrates that the {Delta}F508 mutation in CFTR downregulates the antigen presentation pathway. Am J Physiol Lung Cell Mol Physiol 2010;298:L473–L482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorensen RU, Chase PA, Stern RC, Polmar SH. Influence of cystic fibrosis plasma on lymphocyte responses to Pseudomonas aeruginosa in vitro. Pediatr Res 1981;15:14–18 [DOI] [PubMed] [Google Scholar]
- 14.Soltys J, Bonfield T, Chmiel J, Berger M. Functional IL-10 deficiency in the lung of cystic fibrosis (Cftr−/−) and IL-10 knockout mice causes increased expression and function of B7 costimulatory molecules on alveolar macrophages. J Immunol 2002;168:1903–1910 [DOI] [PubMed] [Google Scholar]
- 15.Lyczak JB, Cannon CL, Pier GB. Lung infections associated with cystic fibrosis. Clin Microbiol Rev 2002;15:194–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rzemieniak SE, Hirschfeld AF, Victor RE, Chilvers MA, Zheng D, Van Den Elzen P, Turvey SE. Acidification-dependent activation of CD1d-restricted natural killer T cells is intact in cystic fibrosis. Immunology 2010;130:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartl D, Griese M, Kappler M, Zissel G, Reinhardt D, Rebhan C, Schendel DJ, Krauss-Etschmann S. Pulmonary TH2 response in Pseudomonas aeruginosa–infected patients with cystic fibrosis. J Allergy Clin Immunol 2006;117:204–211 [DOI] [PubMed] [Google Scholar]
- 18.Bragonzi A. Murine models of acute and chronic lung infection with cystic fibrosis pathogens. Int J Med Microbiol 2010;300:584–593 [DOI] [PubMed] [Google Scholar]
- 19.Colasurdo GN, Fullmer JJ, Elidemir O, Atkins C, Khan AM, Stark JM. Respiratory syncytial virus infection in a murine model of cystic fibrosis. J Med Virol 2006;78:651–658 [DOI] [PubMed] [Google Scholar]
- 20.Hamai H, Keyserman F, Quittell LM, Worgall TS. Defective CFTR increases synthesis and mass of sphingolipids that modulate membrane composition and lipid signaling. J Lipid Res 2009;50:1101–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hla T, Venkataraman K, Michaud J. The vascular S1P gradient: cellular sources and biological significance. Biochim Biophys Acta 2008;1781:477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera J, Proia RL, Olivera A. The alliance of sphingosine-1–phosphate and its receptors in immunity. Nat Rev Immunol 2008;8:753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idzko M, Panther E, Corinti S, Morelli A, Ferrari D, Herouy Y, Dichmann S, Mockenhaupt M, Gebicke-Haerter P, Di Virgilio F, et al. Sphingosine 1–phosphate induces chemotaxis of immature dendritic cells and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J 2002;16:625–627 [DOI] [PubMed] [Google Scholar]
- 24.Nishiuma T, Nishimura Y, Okada T, Kuramoto E, Kotani Y, Jahangeer S, Nakamura SI. Inhalation of sphingosine kinase inhibitor attenuates airway nflammation in asthmatic mouse model. Am J Physiol Lung Cell Mol Physiol 2008;294:L1085–L1093 [DOI] [PubMed] [Google Scholar]
- 25.Edsall LC, Spiegel S. Enzymatic measurement of sphingosine 1\x{2013}phosphate. Anal Biochem 1999;272:80–86 [DOI] [PubMed] [Google Scholar]
- 26.Muller HC, Hocke AC, Hellwig K, Gutbier B, Peters H, Schonrock SM, Tschernig T, Schmiedl A, Hippenstiel S, N'Guessan PD, et al. The sphingosine-1 phosphate receptor agonist FTY720 dose dependently affected endothelial integrity in vitro and aggravated ventilator-induced lung injury in mice. Pulm Pharmacol Ther 2011;24:377–385 [DOI] [PubMed] [Google Scholar]
- 27.Emilie D, Crevon M, Chicheportiche R, Auffredou M, Barot-Ciobraru R, Lenoir G, Dayer J, Galanaud P. Cystic fibrosis patients’ B-lymphocyte response is resistant to the in vitro enhancing effect of corticosteroids. Eur J Clin Invest 1990;20:620–626 [DOI] [PubMed] [Google Scholar]
- 28.Knutsen AP, Slavin RG. In vitro T cell responses in patients with cystic fibrosis and allergic bronchopulmonary aspergillosis. J Lab Clin Med 1989;113:428–435 [PubMed] [Google Scholar]
- 29.Bals R, Weiner DJ, Wilson JM. The innate immune system in cystic fibrosis lung disease. J Clin Invest 1999;103:303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity 2007;26:741–750 [DOI] [PubMed] [Google Scholar]
- 31.Liu YJ. IPC: professional Type 1 interferon–producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol 2005;23:275–306 [DOI] [PubMed] [Google Scholar]
- 32.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MAM, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004;200:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brazova J, Sediva A, Pospisilova D, Vavrova V, Pohunek P, Macek J, Bartunkova J, Lauschmann H. Differential cytokine profile in children with cystic fibrosis. Clin Immunol 2005;115:210–215 [DOI] [PubMed] [Google Scholar]
- 34.Nakano H, Free ME, Whitehead GS, Maruoka S, Wilson RH, Nakano K, Cook DN. Pulmonary CD103+ dendritic cells prime Th2 responses to inhaled allergens. Mucos Immunol 2012;5:53–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Webb TJ, Sumpter TL, Thiele AT, Swanson KA, Wilkes DS. The phenotype and function of lung dendritic cells. Crit Rev Immunol 2005;25:465–491 [DOI] [PubMed] [Google Scholar]
- 36.Holt PG, Haining S, Nelson DJ, Sedgwick JD. Origin and steady-state turnover of Class II MHC-bearing dendritic cells in the epithelium of the conducting airways. J Immunol 1994;153:256–261 [PubMed] [Google Scholar]
- 37.Hiatt PW, Grace SC, Kozinetz CA, Raboudi SH, Treece DG, Taber LH, Piedra PA. Effects of viral lower respiratory tract infection on lung function in infants with cystic fibrosis. Pediatrics 1999;103:619–626 [DOI] [PubMed] [Google Scholar]
- 38.Wang EE, Prober CG, Manson B, Corey M, Levison H. Association of respiratory viral infections with pulmonary deterioration in patients with cystic fibrosis. N Engl J Med 1984;311:1653–1658 [DOI] [PubMed] [Google Scholar]
- 39.Armstrong D, Grimwood K, Carlin JB, Carzino R, Hull J, Olinsky A, Phelan PD. Severe viral respiratory infections in infants with cystic fibrosis. Pediatr Pulmonol 1998;26:371–379 [DOI] [PubMed] [Google Scholar]
- 40.Van Ewijk BE, Wolfs TF, Aerts PC, Van Kessel KP, Fleer A, Kimpen JL, Van der Ent CK. RSV mediates Pseudomonas aeruginosa binding to cystic fibrosis and normal epithelial cells. Pediatr Res 2007;61:398–403 [DOI] [PubMed] [Google Scholar]
- 41.McWilliam AS, Napoli S, Marsh AM, Pemper FL, Nelson DJ, Pimm CL, Stumbles PA, Wells TNC, Holt PG. Dendritic cells are recruited into the airway epithelium during the inflammatory response to a broad spectrum of stimuli. J Exp Med 1996;184:2429–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med 2006;203:1153–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Freeman CM, Martinez FJ, Han MK, Ames TM, Chensue SW, Todt JC, Arenberg DA, Meldrum CA, Getty C, McCloskey L, et al. Lung dendritic cell expression of maturation molecules increases with worsening chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009;180:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vuckovic S, Florin THJ, Khalil D, Zhang MF, Patel K, Hamilton I, Hart DNJ. CD40 and CD86 upregulation with divergent CMRF44 expression on blood dendritic cells in inflammatory bowel diseases. Am J Gastroenterol 2001;96:2946–2956 [DOI] [PubMed] [Google Scholar]
- 45.McWilliam AS, Nelson D, Thomas JA, Holt PG. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med 1994;179:1331–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roghanian A, Drost EM, MacNee W, Howie SEM, Sallenave JM. Inflammatory lung secretions inhibit dendritic cell maturation and function via neutrophil elastase. Am J Respir Crit Care Med 2006;174:1189–1198 [DOI] [PubMed] [Google Scholar]
- 47.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. Induction of Type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 2012;46:6–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wojewodka G, De Sanctis JB, Radzioch D. Ceramide in cystic fibrosis: a potential new target for therapeutic intervention. J Lipids 2011;2011:674968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1–phosphate receptor2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 2007;27:1312–1318 [DOI] [PubMed] [Google Scholar]
- 50.Mou F, Praskova M, Xia F, Van Buren D, Hock H, Avruch J, Zhou D. The MST1 and MST2 kinases control activation of Rho family GTPases and thymic egress of mature thymocytes. J Exp Med 2012;209:741–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du J, Zeng C, Li Q, Chen B, Liu H, Huang X, Huang Q. LPS and TNF-α induce expression of sphingosine-1–phosphate receptor–2 in human microvascular endothelial cells. Pathol Res Pract 2012;208:82–88 [DOI] [PubMed] [Google Scholar]
- 52.Boujaoude LC, Bradshaw-Wilder C, Mao C, Cohn J, Ogretmen B, Hannun YA, Obeid LM. Cystic fibrosis transmembrane regulator regulates uptake of sphingoid base phosphates and lysophosphatidic acid: modulation of cellular activity of sphingosine 1–phosphate. J Biol Chem 2001;276:35258–35264 [DOI] [PubMed] [Google Scholar]
- 53.Milara J, Navarro R, Juan G, Peir T, Serrano A, Ramn M, Morcillo E, Cortijo J. Sphingosine-1–phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax 2012;67:147–156 [DOI] [PubMed] [Google Scholar]
- 54.Kawa S, Kimura S, Hakomori SI, Igarashi Y. Inhibition of chemotactic motility and trans-endothelial migration of human neutrophils by sphingosine 1–phosphate. FEBS Lett 1997;420:196–200 [DOI] [PubMed] [Google Scholar]
- 55.Allende ML, Bektas M, Lee BG, Bonifacino E, Kang J, Tuymetova G, Chen W, Saba JD, Proia RL. Sphingosine-1–phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J Biol Chem 2010;286:7348–7358 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.