Abstract
Background
There has been increasing interest in serial research biopsies in studies of targeted therapies. Definition of patient characteristics and optimal target tissue for safe research tumor biopsy in the era of anti-angiogenic and targeted agents is needed.
Methods
This IRB-approved retrospective study included chart and interventional radiology case review from six phase 1/2 studies at the NCI.
Results
142 of 150 protocol patients approached gave consent for research biopsies. Patients had a median age of 56 yrs (27–78), median BMI 25.8 kg/m2 (14.4–46.2), ECOG PS 0–1, and normal end-organ function. Baseline biopsies were collected in 138/142 patients (97%), and paired specimens in 96(70%). Most patients had metastatic gynecologic cancers (85%) and 78% patients had target disease below the diaphragm of median size 2.7cm (1–14.5cm). Protocol therapies included kinase inhibitors (35%), angiogenesis inhibitors (54%), and olaparib/carboplatin (11%); therapy was not interrupted for biopsies. Adverse events were all uncomplicated and were observed in four patients (liver subcapsular hematoma [1]; vasovagal syncope [2]; pneumothorax [1]). The complication rate in obese patients was similar to that in non-obese patients (3/108 vs.1/34). 67 patients (48%) were receiving bevacizumab at the time of subsequent biopsies. The complication rate in those receiving bevacizumab was not different from those without (3/67 vs 1/71). 95% of biopsies yielded useable material.
Conclusions
Serial percutaneous core needle biopsies can be obtained safely and yield material applicable for multiple translational applications. Obesity and/or concomitant anti-angiogenic therapy, and depth of disease do not increase risk or preclude successful acquisition of useful tissue.
INTRODUCTION
Advances in biotechnology and improved understanding of cancer and disease biology have shifted the cancer treatment paradigm to targeted therapy. Molecularly targeted agents offer attractive therapeutic options by putatively restoring control to oncogenic processes1. Optimal application of these new agents requires biomarkers that are predictive for response to selective agents. Activating genetic and genomic changes are readily applied as predictive biomarkers. For example, HER2 amplification is a strong predictor for trastuzumab activity2, and wild type KRAS genotype is predictive for cetuximab activity in advanced colorectal cancer3. However, many targeted agents are promiscuous with multiple potential targets and/or they modify biology that is not genetically driven, such as inhibition of vascular development or maturation. More complex analyses of target tissues appear necessary for illustration of mechanism and for biomarker discovery and validation.
The procurement of tumor tissues via serial biopsies provides a direct resource for evaluation of biologic measures and correlation to clinical outcome. Sequential research-related tumor biopsies have been applied evaluation of putative predictive biomarkers and proof of target4. It is unclear that readily obtained surrogate tissues will recapitulate the findings of tumor tissues. Peripheral blood mononuclear cells are easily obtained, and are usually in a resting state and carry a different repertoire of signaling pathways5. Thus, they would be less likely to reliably reflect biochemical events related to invasive behavior, vascular remodeling or perfusion, or those driven by somatic mutations causing constitutively activated kinases. Biochemical and/or genetic changes over time in the tumor provide stronger and more reliable evidence for biomarker utility. Criteria for safe and effective inclusion of serial tissue acquisition into trial design is necessary and increasingly relevant in drug development6. The FDA requires development and incorporation of a verified and validated biomarker for the identification of the target clinical subpopulation for registration of a targeted drug for a selective subpopulation7. Thus, incorporation of tissue acquisition into clinical trials for identification and validation of predictive biomarkers will translate into greater accuracy in selecting target patient subgroups and improve and potentially shorten the drug development process.
There are many challenges to successful incorporation of tumor tissue acquisition and analysis into clinical trial execution. These include preanalytical variables such as patient safety, selection of anatomical sites and size for biopsy, and patient suitability for percutaneous tissue acquisition, and analytical variables for tumor acquisition including minimum size of sample, and sample processing and storage. We championed inclusion of serial percutaneous core tumor biopsies for biologic endpoint analyses and successfully applied those materials in proof of principle studies of targeted agents8–11. We now examine our experience to identify parameters for successful serial biopsy ascertainment.
PATIENTS AND METHODS
Patients
Clinical data including chart review, interventional radiology procedure notes, pre-and post-procedure imaging, and laboratory records regarding specimen processing were obtained from six phase 1 and 2 studies completed by our group at the Medical Oncology Branch of the National Cancer Institute (NCI) between 2002 and 20118–11. Clinical data were abstracted from the medical and research records for all patients consented to undergo at least one core needle biopsy for research purposes. Cost of biopsy was not a decision variable in these patients. This retrospective analysis was approved by the IRB of the National Cancer Institute.
Tumor biopsies
Image-guided percutaneous biopsies were carried out in Interventional Radiology (IR) under an independent procedure informed consent. Standard local anesthesia (lidocaine) was applied per IR standard procedures and patients could elect conscious sedation after procedure consent was obtained. Initial (baseline) biopsies were done after protocol consent and prior to initiation of protocol-directed therapy. Serial passes were made using coaxial technique with an 18-gauge spring–loaded disposable core biopsy gun, inserted through a 17-gauge outer cannula under ultrasound (USN) and/or computed tomography (CT) guidance. The imaging modality and the tumor biopsy site were selected by IR for safety, best tumor visualization, and easy access. Second and third research biopsies were optional per protocol consent; biopsy intervals ranged from 2 to 6 weeks. Follow-up biopsies targeted the same lesion as the baseline lesion; interval shrinkage or disappearance of the target lesion resulted in aborted subsequent biopsies, rather than use of an alternative site.
Samples were processed in real time in the IR suite by trained members of the Molecular Signaling Section of the Medical Oncology Branch, NCI. A standard rapid freezing protocol was used in order to minimize degradation or dephosphorylation of proteins, or degradation of nucleic acids. Core biopsies were cryopreserved immediately in optimal cutting temperature (OCT) compound, and cryoblocks stored at −80°C until use. Tumor biopsy samples were sectioned, fixed, and stained with hematoxylin and eosin for quality control analysis and pathology review (K.C.). Optimal quality was defined as paired sequential biopsies that were suitable for performing the protocol-required protein array and IHC analyses. These criteria were applied to the pretreatment biopsy and included solid tissue areas containing at least 50% tumor cells and less than 25% necrosis. In subsequent samples, blocks with the greatest tumor involvement and least necrosis were selected. Application of tissue included immunohistochemistry, reverse phase protein analysis12, and mutational analysis (Azad and Yu, submitted).
Statistical analysis
All variables and patient characteristics were analyzed with a chi-squared test (Excel Microsoft, Redmond, DC).
RESULTS
Patient characteristics
Unique elements of our trials are country-wide patient accrual and lack of financial disincentive related to medical bills and patient travel costs, increasing the acceptability threshold for elective invasive procedures. A total of 142 of 150 protocol patients deemed to have a lesion safe for research biopsy gave consent (Figure 1). Four of six clinical trials included were phase 2 ovarian cancer treatment trials, skewing the patient gender distribution and tumor location. The most common histological types were metastatic gynecologic cancers (85%; Table 1) and the most common therapy was bevacizumab and sorafenib (48%). All patients had satisfactory Eastern Cooperative Oncology Group performance status 0 or 1, and normal end-organ and coagulation function at the time of biopsy. No patients were receiving aspirin or anti-coagulants at the time of biopsy. Most patients (78%) had disease below the diaphragm, in liver, mesentery, and nodal masses. This made individual body habitus an important decision element. Median BMI of the cohort was 25.8kg/m2 (range 14.4–46.2; Table 1); 36 patients (25%) were overweight (BMI 25–29.9kg/m2), and 34 (24%) were obese (BMI ≥30kg/m2).
Figure 1.
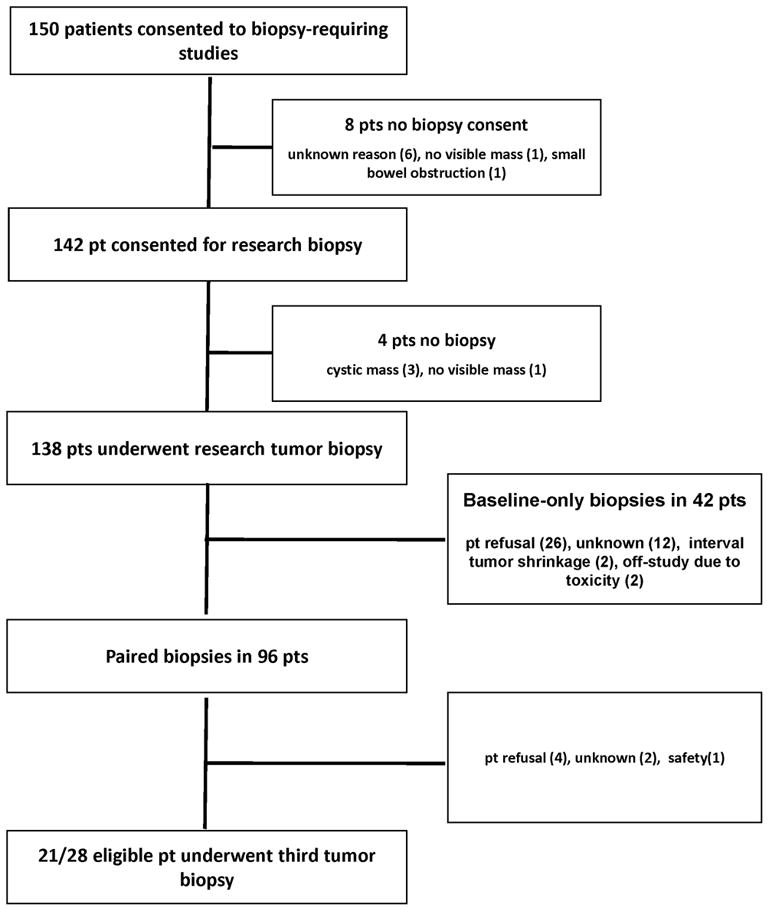
Study schema.
Table 1.
Patient Characteristics
| Total number of patients | 142 |
|
| |
| Median age at biopsy, years (range) | 55.5 (27–78) |
|
| |
| Gender, n (%) | |
| Female; Male | 132 (93%): 10 (7%) |
|
| |
| ECOG Performance Status | |
| 0 or 1; 2 or greater | 142 (100%): 0 (0%) |
|
| |
| Body Mass Index (BMI), n (%) | |
| Normal (18.5–25 kg/m2) | 72 (51%) |
| Overweight (25–29.9 kg/m2) | 36 (25%) |
| Obese patients (>30 kg/m2) | 34 (24%) |
| Median BMI (range) of all patients, kg/m2 | 25.8 (14.4–46.2) |
|
| |
| Tumor types, n (%) | |
| Ovarian cancer | 109 (77%) |
| Gynecological (non-ovarian cancer) | 12 (8%) |
| Breast | 6 (4%) |
| Sarcoma | 5 (4%) |
| Leiomyosarcoma (shoulder/mediastinal/uterine) | 4 (2/1/1) |
| Malignant fibrous histiocytoma (shoulder) | 1 |
| Melanoma | 4 (3%) |
| Colon | 2 (1%) |
| Adrenal, mesothelioma, renal cell carcinoma, and | 1 each, 4 (3%) |
| papillary thyroid cancer | |
|
| |
| Clinical trials, n (%) | |
| Phase 2 sorafenib and bevacizumab | 39 (28%) |
| Phase 1 sorafenib and bevacizumab | 28 (20%) |
| Phase 2 gefitinib | 27 (19%) |
| Phase 2 imatinib | 23 (16%) |
| Phase 1 olaparib and carboplatin | 16 (11%) |
| Phase 2 vandetanib | 9 (6%) |
Biopsy acquisition
All protocols called for at least two biopsies, and the phase I study of sorafenib and bevacizumab included a third8–11. Schedules varied with intervals between biopsies of 2 and 4 weeks (phase I sorafenib/bevacizumab), 3 weeks (olaparib/carboplatin), 4 weeks (imatinib, gefitinib), and 6 weeks (vandetanib, phase 2 sorafenib/bevacizumab). Sixty-six percent of second biopsies and 80% of third biopsies were executed within a day of the protocol-specified target date; variance accommodated IR scheduling and patient travel, with no delays due to medical reasons or drug holds. Investigational agents were not held prior to biopsy. Baseline biopsies were collected in 138 (97%) of 142 patients consented for research biopsy, and paired specimens were obtained in 96 (70%) of these patients. Baseline biopsies were aborted in 4 patients due to cystic mass with inadequate solid component (3) and safety (1: no clearly visible tumor mass on the biopsy date). Second biopsies were not attempted when baseline biopsies were not obtained. Optional second and third biopsies were carried out in approximately three quarters of patients (96/138 [70%] for the second biopsy; 21/28 [75%] for the third biopsy); attrition was due mainly to patient refusal (Figure 1). USN guidance alone was the most common technique used (86 patients), followed by CT-guidance in 39 patients (Figure 2), USN-guided biopsies with CT confirmation in 12, and method could not be ascertained in the medical record for one. Median longest diameter of the target mass size was 2.7cm (1–14.5cm), and the most common anatomical biopsy sites were abdomen/pelvis (29%), parenchymal liver (28%), and nodal (29%; Table 2).
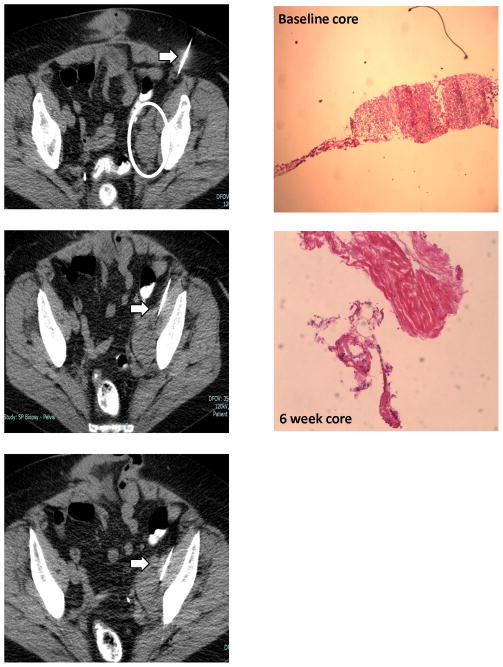
Figure 2. Illustrative case.
A 71 year old female (BMI 32.1kg/m2) with recurrent high grade ovarian cancer, treated with bevacizumab and sorafenib. A. Baseline CT (18-gauge) guided biopsy was performed for a 3.8 cm diameter left iliac lymph node mass. B. Photomicrograph shows almost 100% tumor cells in baseline biopsy with necrosis at second research biopsy at week 6 consistent with improvement of her disease at the first assessment scans at week 8.
Table 2.
Tumor Characteristics (n=138)
| Median target tumor size (longest diameter), cm (range) | 2.7 (1–14.5) |
|
| |
| Biopsy site, n | |
| Abdominal or pelvic mesenteric masses | 40 (29%) |
| Liver parenchyma | 39 (28%) |
| Lymph nodes | |
| Iliac/inguinal | 15 (11%) |
| Neck | 12 (9%) |
| Retroperitoneal | 10 (7%) |
| Axillary | 3 (2%) |
| Abdominal/chest wall | 12 (9%) |
| Lung (pleural/parenchymal) | 4 (3/1; 3%) |
| Psoas muscle, vaginal wall, adrenal | 1 each (2%) |
|
| |
| No of tumor cores, median (range) | |
| Baseline | 3 (1–6) |
| 2nd and 3rd time point | 3 (1–6) |
|
| |
| No (%) of pts with paired tumor core biopsy* | 96 (70%) |
| No (%) of pts with baseline biopsy only** | 42 (30%) |
Biopsy was aborted in 4 patients due to cystic lesion (3) and safety (1).
26 (26/138; 19 %) patients refused the second biopsy, 2 (1%) patients were off the study due to toxicities prior to second biopsy, 2 (1%) patients’ tumors with interval shrinkage were too small to be biopsied, unknown reasons in 12 (9%) patients.
Safety
Four minor complications, none requiring therapeutic intervention, were observed in a total of 255 biopsies (2%) and no major complications occurred. All events occurred during the first or second biopsies (Table 3). Two patients became vagal during the biopsy but improved immediately (right neck mass biopsy; liver mass biopsy). The patient with peritoneal mesothelioma had a small basilar pneumothorax, that was managed with observation, during the upper abdominal peritoneal mass biopsy. Nine obese patients had biopsies for abdominal/pelvic masses or deep-seated retroperitoneal lymph nodes; all were accomplished without complications (Figure 2). An uncomplicated small liver subcapsular hematoma occurred during a second biopsy at week 6 in a patient with a BMI of 32.4kg/m2. The complication rate in obese patients (1/34; 3%) was similar to the complication rate in non-obese patients (3/108; 3%). Sixty-seven patients (48%) were receiving bevacizumab at the time of their second or second/third biopsies. The complication rate in those receiving bevacizumab was not different from those without (3/67 [4%] vs 1/71 [1%]). Thirty-six of the 67 patients were either overweight or obese, and most (29/36; 81%) obese or overweight patients had tumors located below the diaphragm. The complication rate in those receiving bevacizumab was not different based on BMI (obese patients [1/19; 5%] vs. non-obese patients [2/48; 4%]; Table 3). No biopsy-related major bleeding, pain, delayed wound healing, or tumor-tracking was observed.
Table 3.
Characteristics of 67 patients on bevacizumab-based therapy by BMI
| BMI (kg/m2) | Normal (BMI 18.5–25) | Overweight (BMI 25–29.9) | Obese (BMI ≥30) |
|---|---|---|---|
|
| |||
| Number of patients (n=67) | 31* | 17 | 19 |
|
| |||
| BMI (kg/m2) | |||
|
| |||
| Median (range) | 22.5 (18.5–24.6) | 27.4 (25–29.7) | 32.4 (30.7–41.5) |
|
| |||
| Age, years | |||
| Median (range) | 59 (27–77) | 62 (42–71) | 58 (27–71) |
|
| |||
| Locations of tumor, n | |||
| Liver parenchymal | 14 | 4 | 5 |
| Deep lymph nodes | |||
| Superficial lymph nodes | 5 | 1 | 3 |
| Intra-abd/pelvic mass | 2 | 2 | 3 |
| Abdominal/chest wall | 7 | 6 | 5 |
| Lung (pleural) | 4 | 1 | |
| Other | 2 | 1 | |
| Psoas m 1 | Vaginal mass 1 | ||
|
| |||
| Median diameter of target tumor (cm; range) | 2.8 (1.2–7) | 2.5 (1.3–10) | 2.75 (1–11) |
|
| |||
| Complication | 1 minor basilar pneumothorax | 1 minor vasovagal reaction | 1 minor liver subcapsular hematoma |
No patient was under weight range
Quality of tumor biopsy samples
The median number of tumor cores taken was 3 (range 1–6) at the baseline, second, and third time points. Tumor size was measured for samples in 89 blocks from 36 patients; insufficient material remained from which to measure biopsies where size was not determined initially. The median size of those tumor samples is 3.09 mm2 (range 0.43–7.80 mm2) with the mean size 2.66 ± 1.62mm2. At least one baseline biopsy core was deemed usable upon pathology review in 131/138 (95%) of biopsied patients. These tumor core samples met the criteria for our reverse phase protein array analysis. Initial core samples lacking tumor, containing effusive lymphocyte infiltration, or >25% necrosis were deemed unsuitable. Second or third biopsies showing only inflammation or necrotic cells were obtained in 4 patients (phase I sorafenib and bevacizumab) and were associated with patient response to therapy. We have reported results of the successful translational analysis of biochemical endpoints using the paired tumor biopsies described in this report8–10.
Discussion
Identification of parameters to optimize success of acquisition of high quality tumor research biopsies is critical given the increasing need to obtain these samples for discovery and validation of predictive biomarkers, development of stratification schema for targeted therapies, and for illustration of therapeutic mechanism of action6. We demonstrated that serial research-related percutaneous core biopsies can be done safely in early-phase clinical trial patients. We recognize that this is costly in potential patient risk, time, and medical resource utilization. In our center, where procedure cost is neither a patient nor an investigator limitation, interest in, and safety and utility of core tumor biopsies could be assessed. Under an IRB-approved minimal risk mandate, we observed willingness in our patients (95%), procedural safety (98%), and useful tissue acquisition (95%) with no impact of obesity, concomitant exposure to anti-angiogenic agents, or infradiaphragmatic location. We evaluated potential risk factors for core biopsy success such as anti-angiogenic agents, obesity, and tumor location, and present clinical and tumor criteria to optimize biopsy success (Table 4).
Table 4.
Criteria to optimize success of acquisition of high quality tumor tissue biopsies
| Clinical factor | Tumor factor* |
|---|---|
| No limit up to BMI 41.5 kg/m2 | Minimum size 1.0 cm longest diameter on radiographic imaging22 |
| Tumor location limited by safety, e.g. proximity to vessels and bowel wall | Less than 25% of tissue necrosis preferred. |
| No effect of recent or ongoing bevacizumab exposure | At least 50% of tumor cells in tissue obtained is preferred unless it is recognized that signal may reflect stroma and tumor |
| No limit to number or kinds of prior treatment19 |
Usable for IHC and/or RPPA
Several factors must be taken into consideration when incorporating minimal risk invasive procedures into clinical trials. Obesity is a challenge for invasive procedures. Bleeding and poor visualization, tissue quality, and healing are common elements with even minimally invasive procedures in the obese13, 14,15. Over half of our cohort had a BMI in the obese and overweight range, though with no increased risk or loss of tissue quality uniquely in this subpopulation where deep internal lesions such as intra-abdominal masses and retroperitoneal lymph nodes were the most frequent tissue targets. The expected increased risk related to exposure to anti-angiogenic agents was not observed in our patients who were actively receiving bevacizumab and/or sorafenib at the time of their second and/or third biopsy. Our higher baseline biopsy acceptance rate and acquisition rate of paired specimen than other institutions suggests patients are willing to participate in multiple biopsies in conjunction with the experimental therapy when they understand the scientific rationale and application for the tissue and have no clinical or medical disincentive.
Applicability of the tissue is critically important since these biopsies are voluntary research samples. Thus, good quality is key as is the scientific application of the tissue. We have examined protein pathway endpoints in sequential core tumor biopsies in multiple studies to examine proof of targeted agent mechanism. Our phase II studies of imatinib and gefitinib both showed modulation of the primary drug targets, c-KIT and EGFR, respectively8, 9, albeit absent clinical benefit. Our conclusion was that the targets may not be sufficiently inhibited and/or the affected targets may be insufficiently important in ovarian cancer and its microenvironment. Protein activation tissue endpoints in our phase II study of vandetanib, purportedly primarily inhibiting EGFR and VEGFR2, allowed us to understand why the agent may have been inactive. Despite pharmacodynamic and proteomic demonstration of EGFR inhibition, no inhibition of VEGFR phosphorylation was demonstrated in paired samples10. We thus confirmed the lack of EGFR as a reliable target in ovarian cancer and could not qualify the dual target of EGFR and VEGFR2 as successful. In contrast, serial biopsies from our sorafenib and bevacizumab study (Azad et al, submitted), demonstrated on-target action of both agents in tissue proteomics and IHC studies. This application of research tissue biopsies can yield key information with which to make rational, data-driven clinical trial steps.
Despite the importance of this question, there are limited assessments in the literature. Dowlati et al reported research biopsies in 107 early clinical trial patients at their institution from 1989 – 2000, describing liver as the most common biopsied location (73%), with only 5 patients sampled in the abdominopelvic regions16. Brown and coworkers demonstrated biopsies can be performed in irradiated tissues without excess risk in their analysis of 29 published studies of 2,160 patients17. Few studies report on sequential tumor biopsies. A retrospective chart review of 155 patients enrolled in 45 institutional phase I studies, that included a mandatory or optional biopsy for correlative studies, was reported by El-Osta et al18. Approximately 60% of patients underwent sequential tumor core biopsies with a 1.4% major complication rate. Their most common biopsy sites were superficial lymph node (19.9%) and liver (16.4%) with abdominopelvic and retroperitoneal lesions in 54 patients (14.9%). Gomez-Roca et al reported 155 phase I trial patients. They demonstrated 84% acceptance and 69% tissue acquisition success, 30% of which had unacceptable tissue quality19. Only 43% of patients had paired tumor core biopsies and their most frequent locations were liver (38%) and lung (19%) in 89 patients. There were 10% minor complications with 2 of 9 complications in patients on anti-angiogenic agents, although the rate of complication did not differ based on anti-angiogenic therapy. Authors agreed ongoing and future trials including research biopsies should record and report biopsy-associated adverse events to provide data on safety and toxicity.
There still remains the ethical concern of performing elective invasive procedures for research tissue collection where patients undergo a potentially harmful procedure with no direct benefit. Many investigators and IRBs consider risks of such biopsies to be low enough to warrant their use in clinical trials if they are scientifically justified and performed with fully informed discussion of risks, rationale, and requirements of the study and treatment alternatives. Peppercorn et al20 examined ethics concerns related to correlative endpoint research biopsies and concluded with support for their mandatory application in the context of those criteria. They concluded that there is a need both for incorporation of tumor tissue acquisition and for ongoing discussion of this important research issue. Canadian doctors and IRBs were described as anticipating more biopsy-associated anxiety related to potential risks than was found in the patients21. The patient perception of research biopsy benefit to their care, procedure anxiety, experience of the interventional radiologist and institutional experience, and financial costs were other key elements. The most important issues in research-related biopsies are careful risk assessment and ensuring proper informed consent. The relative patient-specific risk should be addressed after multi-disciplinary consensus on the appropriate course of action.
Our study reports the lack of added patient jeopardy with recognized potential risk factors such as obesity, antiangiogenic agents, and deep intra-abdominal tumor location. We demonstrate research-related core tumor biopsies are feasible and safe in early-phase clinical trial patients with these and other risk factors. Our data suggest that in an experienced center, percutaneous research biopsies in potentially high-risk patients do not confer additional harm beyond IRB determined acceptable levels of risk for medically unnecessary procedures. This experience helps initiate a framework from which to minimize the previously perceived necessary selection biases for patients undergoing research biopsies.
Acknowledgments
This work was supported by the Intramural Program of the Center for Cancer Research, NCI, and the Center for Interventional Oncology, Radiology and Imaging Sciences, NIH, USA.
Footnotes
All authors except Dr. Wood have no financial disclosures, Dr. Wood has research funding from Cooperative Research and Development Agreement with Philips Healthcare.
References
- 1.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I clinical trial design in cancer drug development. J Clin Oncol. 2000;18:684–692. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 2.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 3.Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–1765. doi: 10.1056/NEJMoa0804385. [DOI] [PubMed] [Google Scholar]
- 4.Lee JM, Han JJ, Altwerger G, Kohn EC. Proteomics and biomarkers in clinical trials for drug development. J Proteomics. 2011;74:2632–2641. doi: 10.1016/j.jprot.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji J, Kinders RJ, Zhang Y, et al. Modeling pharmacodynamic response to the poly(ADP-Ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells. PLoS One. 2011;6:e26152. doi: 10.1371/journal.pone.0026152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sargent DJ, Conley BA, Allegra C, Collette L. Clinical trial designs for predictive marker validation in cancer treatment trials. J Clin Oncol. 2005;23:2020–2027. doi: 10.1200/JCO.2005.01.112. [DOI] [PubMed] [Google Scholar]
- 7.http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm.
- 8.Posadas EM, Kwitkowski V, Kotz HL, et al. A prospective analysis of imatinib-induced c-KIT modulation in ovarian cancer: a phase II clinical study with proteomic profiling. Cancer. 2007;110:309–317. doi: 10.1002/cncr.22757. [DOI] [PubMed] [Google Scholar]
- 9.Posadas EM, Liel MS, Kwitkowski V, et al. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109:1323–1330. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annunziata CM, Walker AJ, Minasian L, et al. Vandetanib, designed to inhibit VEGFR2 and EGFR signaling, had no clinical activity as monotherapy for recurrent ovarian cancer and no detectable modulation of VEGFR2. Clin Cancer Res. 2010;16:664–672. doi: 10.1158/1078-0432.CCR-09-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Azad NS, Posadas EM, Kwitkowski VE, et al. Combination targeted therapy with sorafenib and bevacizumab results in enhanced toxicity and antitumor activity. J Clin Oncol. 2008;26:3709–3714. doi: 10.1200/JCO.2007.10.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espina V, Wulfkuhle J, Calvert VS, Liotta LA, Petricoin EF., 3rd Reverse phase protein microarrays for theranostics and patient-tailored therapy. Methods Mol Biol. 2008;441:113–128. doi: 10.1007/978-1-60327-047-2_8. [DOI] [PubMed] [Google Scholar]
- 13.Kadar N. Laparoscopic pelvic lymphadenectomy in obese women with gynecologic malignancies. J Am Assoc Gynecol Laparosc. 1995;2:163–167. doi: 10.1016/s1074-3804(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 14.Eltabbakh GH, Shamonki MI, Moody JM, Garafano LL. Hysterectomy for obese women with endometrial cancer: laparoscopy or laparotomy? Gynecol Oncol. 2000;78:329–335. doi: 10.1006/gyno.2000.5914. [DOI] [PubMed] [Google Scholar]
- 15.Holtz G. Laparoscopy in the massively obese female. Obstet Gynecol. 1987;69:423–424. [PubMed] [Google Scholar]
- 16.Dowlati A, Haaga J, Remick SC, et al. Sequential tumor biopsies in early phase clinical trials of anticancer agents for pharmacodynamic evaluation. Clin Cancer Res. 2001;7:2971–2976. [PubMed] [Google Scholar]
- 17.Brown AP, Wendler DS, Camphausen KA, Miller FG, Citrin D. Performing nondiagnostic research biopsies in irradiated tissue: a review of scientific, clinical, and ethical considerations. J Clin Oncol. 2008;26:3987–3994. doi: 10.1200/JCO.2008.16.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Osta H, Hong D, Wheler J, et al. Outcomes of research biopsies in phase I clinical trials: the MD anderson cancer center experience. Oncologist. 2011;16:1292–1298. doi: 10.1634/theoncologist.2011-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez-Roca CA, Lacroix L, Massard C, et al. Sequential research-related biopsies in phase I trials: acceptance, feasibility and safety. Ann Oncol. 2011 doi: 10.1093/annonc/mdr383. [DOI] [PubMed] [Google Scholar]
- 20.Peppercorn J, Shapira I, Collyar D, et al. Ethics of mandatory research biopsy for correlative end points within clinical trials in oncology. J Clin Oncol. 2010;28:2635–2640. doi: 10.1200/JCO.2009.27.2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agulnik M, Oza AM, Pond GR, Siu LL. Impact and perceptions of mandatory tumor biopsies for correlative studies in clinical trials of novel anticancer agents. J Clin Oncol. 2006;24:4801–4807. doi: 10.1200/JCO.2005.03.4496. [DOI] [PubMed] [Google Scholar]
- 22.Loubeyre P, Copercini M, Dietrich PY. Percutaneous CT-guided multisampling core needle biopsy of thoracic lesions. AJR Am J Roentgenol. 2005;185:1294–1298. doi: 10.2214/AJR.04.1344. [DOI] [PubMed] [Google Scholar]