Abstract
The regeneration of alveolar epithelial cells is a critical aspect of alveolar reorganization after lung injury. Although alveolar Type II (AT2) cells have been described as progenitor cells for alveolar epithelia, more remains to be understood about how their progenitor cell properties are regulated. A nuclear, chromatin-bound green fluorescence protein reporter (H2B-GFP) was driven from the murine surfactant protein–C (SPC) promoter to generate SPC H2B-GFP transgenic mice. The SPC H2B-GFP allele allowed the FACS-based enrichment and gene expression profiling of AT2 cells. Approximately 97% of AT2 cells were GFP-labeled on Postnatal Day 1, and the percentage of GFP-labeled AT2 cells decreased to approximately 63% at Postnatal Week 8. Isolated young adult SPC H2B-GFP+ cells displayed proliferation, differentiation, and self-renewal capacity in the presence of lung fibroblasts in a Matrigel-based three-dimensional culture system. Heterogeneity within the GFP+ population was revealed, because cells with distinct alveolar and bronchiolar gene expression arose in three-dimensional cultures. CD74, a surface marker highly enriched on GFP+ cells, was identified as a positive selection marker, providing 3-fold enrichment for AT2 cells. In vivo, GFP expression was induced within other epithelial cell types during maturation of the distal lung. The utility of the SPC H2B-GFP murine model for the identification of AT2 cells was greatest in early postnatal lungs and more limited with age, when some discordance between SPC and GFP expression was observed. In adult mice, this allele may allow for the enrichment and future characterization of other SPC-expressing alveolar and bronchiolar cells, including putative stem/progenitor cell populations.
Keywords: alveolar Type II cells, SPC, epithelial, GFP, differentiation
The adult mammalian lung can be divided into several regions, such as the proximal cartilaginous airways, distal bronchioles, and gas-exchanging alveolar spaces that are hypothesized to be maintained by distinct populations of epithelial stem/progenitor cells. Stem/progenitor cells in the lung often cells carry out daily functional roles (e.g., surfactant secretion) while being largely quiescent, and they are stimulated by lung injury to proliferate and give rise to more specialized lung cells (1). Injury models and lineage tracing approaches have shown that basal cells act as stem cells for maintaining proximal airways (2, 3), whereas club cells (Clara) act as stem/progenitor cells for club cells and ciliated cells in proximal and distal bronchioles (4). Pulmonary neuroendocrine cells (PNECs), found in the bronchioles, also exhibit a proliferative response after lung injury (5). Finally, subsets of club cells, including variant club cells and bronchioalveolar stem cells (BASCs), respond to distal bronchiolar injury (6, 7). BASCs have an ability to give rise to bronchiolar and alveolar lineages in vitro, and their plausible contribution to alveolar epithelia in vivo after injury was recently demonstrated (8–10).
A wide-ranging literature has defined alveolar epithelial cells and characterized their functional properties. The alveoli are specialized terminal structures lined by two distinct types of epithelial cells, alveolar Type I (AT1) and alveolar Type II (AT2) cells. AT1 cells cover approximately 96% of the gas-exchange surface (11). Traditionally, AT1 cells have been accepted as terminally differentiated, yet new reports indicate that AT1 cells have proliferative potential and phenotypic plasticity (12). AT2 cells are most widely known as the source of pulmonary surfactant, a lipid and protein complex that reduces surface tension and plays a role in lung host defense (13). Studies for more than 30 years have suggested that the surfactant protein-C (SPC)–expressing AT2 cells comprise the progenitor cells that proliferate and differentiate into AT1 cells after various types of lung injury (14–16) (17) and in the developing lung (18). BASCs (10) and cells with club cell secretory protein (CCSP) promoter activity (19, 20), have also been implicated as stem/progenitor cells functioning in alveolar development or regeneration. Finally, alveolar cells expressing integrin α6β4, but not the club cell marker (CCSP) or the AT2 cell marker (SPC), have been identified as a multipotent progenitor population that maintains AT2 cells after severe lung injury (21).
A number of strategies have been developed to isolate AT2 cells using FACS, based on cell-surface markers (10, 22–26). Transgenic mice have been generated in which green fluorescence protein (GFP) was targeted to AT2 cells under the control of the human SPC promoter (27–29). These animals exhibited either a limited expression of GFP only in some AT2 cells (27), or a broad expression of GFP in both bronchiolar and AT2 cells (29). Previous studies also demonstrated the importance of species-specific promoter use for proper AT2 cell expression (30, 31). Further detailed analysis of the murine SPC gene promoter revealed the particular sequences needed for AT2 cell–specific expression (32). More recently, the creation of SPC-reverse tetracycline transactivator (rtTA) and SPC-Cre knock-in mice that are useful for analyzing AT2 cells in vivo has also been reported (8, 21). AT2 cells in primary cultures may be expanded under carefully defined conditions (33–41), yet their ability to self-renew in culture systems designed for putative lung stem/progenitor cells has remained limited (10). Therefore, establishing additional methods to track, isolate, and propagate AT2 cells may allow for a further appreciation of the molecular mechanisms that regulate AT2 cell progenitor cell functions in vivo and in vitro.
Here we present a useful reporter mouse for the identification of AT2 cells and other SPC-expressing cells, in which the histone H2B-GFP fusion is driven from the murine SPC promoter, using a bacterial artificial chromosome (BAC; hereafter, SPC H2B-GFP). We demonstrate the expression of GFP in early postnatal AT2 cells and with age in other distal lung epithelial cell types. The highest concordance of GFP and SPC expression was observed on Postnatal Day 1, with approximately 97% of AT2 cells expressing GFP, whereas the percentage of GFP-labeled AT2 cells decreased to approximately 63% at Postnatal Week 8. FACS-isolated SPC H2B-GFP–expressing cells demonstrated proliferation and a capacity for differentiation into multiple lineages in a three-dimensional Matrigel culture system. Microarray analysis of GFP+ cells identified a cell-surface marker, CD74, which can be used for the threefold enrichment of, and positive selection schemes in, AT2 cells.
Materials and Methods
Transgenic Murine Strains
The SPC H2B-GFP reporter construct is a BAC transgene based on the BAC RP23-247J9. The BAC involves approximately 180 kb, of which 107 kb occur upstream of the first coding exon of the SPC gene. The BAC was modified by the insertion of a cassette in frame into the first coding exon, using the method of recombineering (42). The insertion consisted of the human histone H2B gene fused to the coding sequence for enhanced green flourescence protein (EGFP), followed by the SV40 polyadenylation signal sequence (43). Please see the online supplement for more detailed information. The PCR-based genotype screening of tail snips was performed with the primers Plasmid (PL) EGFP F 5′-CGCACCATCTTCTTCAAGGACGAC-3′, PL EGFP R 5′-AACTCCAGCAGGACCATGTGATCG-3′, R26F2 5′-AAAGTCGCTCTGAGTTGTTAT-3′, and R523 5′-GGAGCGGGAGAAATGGATATG-3′. We obtained β-actin–driven DsRed transgenic mice (B6.Cg-Tg(ACTB-DsRed*MST)1Nagy/J) from Jackson Laboratories (Bar Harbor, ME). All murine work was approved by the Animal Care and Use Committee of Boston Children’s Hospital, accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and performed in accordance with relevant institutional and national guidelines and regulations.
Flow Cytometry
Primary lung cells were isolated from 1- to 12-week-old wild-type SPC H2B-GFP or β-actin-DsRed mice, as previously described (10), using pan CD45-allophycocyanin (APC), CD31-APC, CD74-fluorescein isothiocyanate (PharMingen, San Diego, CA), and EpCAM-phycoerythrin-cyanine dye (BioLegend, San Diego, CA) with 7-aminoacrinomycin D (Molecular Probes, Eugene, OR) or 4′,6-diamidino-2-phenylindole (Sigma Chemical Co., St. Louis, MO) staining to eliminate dead cells. In the intracellular staining for CD74 (PharMingen), proSP-C (Chemicon, Billerica, MA), and CCSP (Seven Hills Bioreagents, cincinnati, OH), cells were fixed with BD Cytofix/Cytoperm (PharMingen), according to the manufacturer’s instructions. Rat IgG2b and rabbit IgG (PharMingen) were used for isotype controls. Cell sorting was performed with a Cytomation MoFlo (Beckman Coulter, Inc., Brea, CA) or a BD FACS Aria (BD Bioscience, San Jose, CA), and data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Gene Expression Analysis
CD31−CD45−GFP+ and CD31−CD4−GFP− cell fractions were FACS-isolated from 6-week-old SPC H2B-GFP mice. Please see the online supplement for more detailed information.
Cell Culture
Murine lung fibroblasts (MLgs; American Type Culture Collection, Manassas, VA) were added to growth factor–reduced Matrigel (BD Bioscience) at 2 × 106 cells/ml. Sorted cells were resuspended in the MLg containing Matrigel, which was prediluted at a ratio of 1:1 with medium (1 × 104 cells/ml) and added to a 24-well Transwell filter inserts (Corning, Corning, NY) in a 24-well tissue culture plate containing 410 μl of medium. Dulbecco’s Modified Eagle’s Medium/F12 (Invitrogen, Carlsbad, CA) was supplemented with 10% FBS, penicillin/streptomycin, 1 mM HEPES, and insulin/transferrin/selenium (Sigma Chemical Co.) for all cultures. Cultures were incubated at 37°C in a humidified incubator (5% CO2), and the medium was replaced every other day for up to 14 to 21 days. For serial passages, cultures were dissociated in Dispase (BD Bioscience) to generate a single-cell suspension.
Histology and Immunofluorescent Staining
Cultured colonies were fixed with 4% paraformaldehyde in PBS for 30 minutes at room temperature. After rinsing with PBS, fixed colonies were immobilized with Histogel (Thermo Scientific, Billerica, MA) for embedding in paraffin.
Statistical Analysis
Data are representative of three or more independent experiments unless otherwise noted, and were analyzed as mean ± SD. Significant differences were determined by a paired t test between two groups.
Please see the online supplement for more information.
Results
Characterization of SPC H2B-GFP Mice
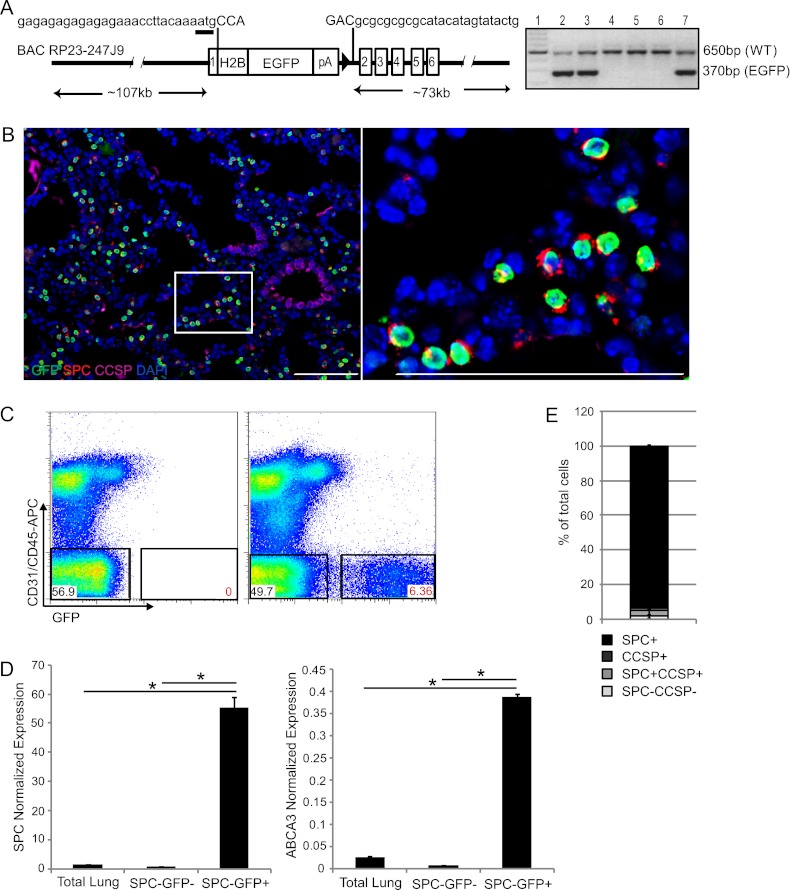
We speculated that a unique BAC transgenic approach may have utility in marking AT2 cells. To generate SPC H2B-GFP mice, a chimeric H2B-EGFP gene (henceforth, GFP) (43) was targeted to the ATG site in the SPC locus on a BAC transgene (Figure 1A). Four female and six male founder mice were identified and mated to produce offspring. Three lines of transgenic mice from different founders were used for further characterization, and exhibited similar features. Immunofluorescence (IF) with antibodies for CCSP, SPC, and GFP on sectioned lungs from 1-week-old SPC H2B-GFP mice showed a restricted expression of GFP in cells expressing SPC (Figure 1B and Figure E1A in the online supplement). No expression was detected in cells lacking SPC staining (Figure E1B). Notably, some SPC-positive cells appeared to be GFP-negative. To assess the incidence of GFP-labeled cells, FACS analysis was performed in lungs from wild-type and SPC H2B-GFP mice. GFP-positive cells, which represented 6 to 8% of the total lung cells, were readily detected in 1-week-old SPC H2B-GFP mice, whereas no GFP expression was detectable in wild-type littermates (Figure 1C).
Figure 1.
Restricted expression of green fluorescence protein (GFP) in alveolar Type II (AT2) cells in neonatal surfactant protein–C (SPC) H2B-GFP mice. (A) Diagram of the SPC H2B-GFP construct (left) shows the organization of the transgenic construct, based on bacterial artificial chromosome (BAC) RP23-247J9. The sequences at the insertion site are shown. Lower case text corresponds to the SPC gene. Upper case text corresponds to the border of the inserted sequence. The translational start site is underlined. Solid triangle represents the remaining flippase recognition target site after Flp recombinase–mediated recombination to remove the Zeocin cassette. Also included is a representative analysis of the PCR genotype from six potential transgenic mice (lanes 2–7) and a DNA ladder (lane 1) (right). A 370-bp product is produced from the GFP gene. EGFP, enhanced green fluorescence protein; pA, poly Adenylation. (B) Four-color immunofluorescence (IF) with antibodies for GFP (green), SPC (red), club cell secretory protein (CCSP) (purple), and 4′,6-diamidino-2-phenylindole (DAPI) (blue) in sectioned lungs of 1-week-old SPC H2B-GFP mice. Boxed area is magnified. Scale bars, 100 μm. (C) Representative FACS plots show GFP expression in lungs from 1-week-old wild-type (WT) mice (left) and SPC H2B-GFP mice (right). The percentage of total lung cells represented by each population is indicated. (D) RT-PCR (quantitative PCR) analysis for validating the expression of AT2 cell marker SPC (left) and ATP-binding cassette sub-familly A member 3 (ABCA3) (right) in freshly sorted total lung cells, CD31−CD45−GFP− cells, and GFP+ cells from 1-week-old SPC H2B-GFP mice. The y axis shows the relative quantification of mRNA abundance normalized to glyceraldehyde 3–phosphate dehydrogenase (GAPDH) (*P < 0.001). (E) Differential cell counts of SPC-expressing and/or CCSP-expressing cells within freshly isolated CD31−CD45−GFP+ cells from 1-week-old SPC H2B-GFP mice. We examined more than 104 cells per lung (n = 3). APC, allophycocyanin; PECy7, phycoerythrin-cyanine dye.
After excluding hematopoietic (CD45-positive) and endothelial (CD31-positive) lineages, GFP-positive cells were isolated to confirm the enrichment of AT2 cells. SPC and ATP-binding cassette sub-familly A member 3 (ABCA3) were highly up-regulated in CD31-negative, CD45-negative, and GFP-positive cells (henceforth, GFP+ cells), as assessed by quantitative RT-PCR. Expression levels of SPC were 36.3-fold and 69.8-fold higher in GFP+ cells, compared with total lung cells and GFP− cells (i.e., CD31-negative, CD45-negative, and GFP-negative), respectively (P = 1.2E-5 and 1.41E-8, respectively) (Figure 1D). The abundance of ABCA3 was 14.4-fold and 43.8-fold enriched in GFP+ cells compared with total lung cells and GFP− cells, respectively (P = 3.54E-5 and 2.04E-12, respectively) (Figure 1D). The cytospin staining and immunostaining of freshly sorted GFP+ cells were performed with antibodies for SPC and CCSP to further validate the purity of GFP-labeled cells. Greater than 90% (93.5% ± 0.7%, n = 3) of GFP+ cells were uniformly SPC-positive and CCSP-negative, indicating the high purity of AT2 cells within GFP+ populations (Figure 1E). A small percentage of GFP+ cells (3.2% ± 2.1%) were SPC-positive and CCSP-positive, suggesting that BASCs were also labeled by GFP. Therefore, the SPC H2B-GFP allele marks the majority of AT2 cells in 1-week-old mice, with some limitations.
Heterogeneity of GFP+ Cells In Vitro
To better understand the properties of GFP+ cells, we used a Matrigel-based three-dimensional culture system from a recent report on the growth of lung epithelial progenitor cells in the presence of stromal cells (29, 44). CD31−CD45−GFP+ cells were sorted from the lungs of 6-week-old SPC H2B-GFP mice (Figure 2A). Isolated GFP+ cells were mixed with lung fibroblast cells (MLgs) and plated in Matrigel. All GFP-positive cells expressed epithelial cell adhesion molecule (EpCAM), confirming the enrichment of epithelial cells within the GFP+ fraction (Figure 2A). Spherical colonies were observed within 4 days, and heterotypic colonies subsequently formed within 21 days in culture (Figure 2B). The majority of colonies (78.8% ± 9.1%, n = 3) expressed bright GFP and displayed a clustered morphology, whereas 21.2% ± 4.1% of colonies showed a distinct “mixed” morphology and contained cells with weak GFP expression or the loss of GFP expression (Figures 2B and 2C).
Figure 2.
Colony-forming ability of GFP+ cells in three-dimensional (3D) culture. (A) Representative FACS plots show GFP in 6-week-old SPC H2B-GFP mice. Left: CD31/CD45-negative, GFP+ cells were isolated for use in cultures. Right: Analysis showed that all GFP+ cells were positive for the epithelial cell marker epithelial cell adhesion molecule (EpCAM). Notably, EpCAM was not used for cell sorting. Only CD31/CD45− GFP+ cells were used for cultures. (B) Representative fluorescence images of primary colonies from GFP+ cells after 21 days in culture (left) and magnified colonies (top right, alveolar-like colonies; bottom right, mixed colonies). Magnification, ×10; the GFP channel is imaged. (C) Proportion of colonies that exhibited the indicated GFP intensity in primary cultures from GFP+ cells. (D and E) Hematoxylin-and-eosin staining shows alveolar-like (D) and mixed (E) structures, along with IF for GFP (green), SPC (purple), CCSP (red), and DAPI (blue) in paraffin-embedded primary colonies from GFP+ cells. (F and G) IF for antigen Ki67 (red), GFP (green), and DAPI (blue) on alveolar-like (F) and mixed (G) colonies. Scale bar, 100 μm. APC, allophycocyanin;
For additional phenotypic analyses, IF was performed on sectioned colonies after 14 days of culture. Hematoxylin-and-eosin and IF staining showed that the clustered structures consisted of small cuboidal, epithelial cells that expressed SPC with bright GFP (Figure 2D). Based on the presence of SPC expression in the clustered morphology colonies, we refer to them as “alveolar-like.” Mixed-morphology colonies comprised two cell types: CCSP-positive cells with weak GFP or a loss of GFP expression, and SPC-positive cells with bright GFP expression (Figure 2E). IF analysis for antigen Ki67 revealed that both the bright GFP-expressing, SPC-expressing cells and the weak GFP-expressing, CCSP-expressing cells were proliferative (Figures 2F and 2G). Equal numbers of GFP+ cells from 6-week-old SPC H2B-GFP mice and EpCAM-positive DsRed+ epithelial cells from 6-week-old β-actin DsRed mice were cocultured. All individual colonies (n = 51) were either green or red, suggesting either that each colony was derived from a single cell, or that GFP-positive cells selectively reassociated (Figure 3A).
Figure 3.
Heterogeneity of GFP+ cells, as revealed by culture. (A) Analysis of colonies derived from GFP+ cells. Representative phase-contrast and fluorescence images of GFP+ cells (green) from 6-week-old SPC H2B-GFP mice and EpCAM+ cells (red) from 6-week-old β-actin DsRed mice after 14 days of coculture. Top left, phase; top right, DsRed channel; bottom left, GFP channel; bottom right, merge. Note that no mixing of red and green fluorescent cells was observed within individual colonies. (B) Incidence of colony formation from GFP+ cells after serial passaging. (C) Representative phase-contrast (left) and GFP fluorescence (right) images of tertiary colonies from GFP+ cells after 14 days of culture. Magnification, ×10. (D) Proportion of colonies that exhibited indicated morphology after serial passaging shows increased mixed colonies with passages. (E) Quantitative PCR analysis of the expression of lung markers in freshly isolated cells and cultured colonies from GFP+ cells, normalized to GAPDH (*P < 0.001). (F) Representative FACS plots show strategy of separating CD31−CD45−GFPlow and CD31−CD45−GFPhigh cells from SPC H2B-GFP mice. (G) Proportion of colonies with indicated morphology from CD31−CD45−GFPlow and CD31−CD45−GFPhigh cells. All data are representative of two independent experiments.
To examine the self-renewal capacity of cultured GFP+ cells, primary colonies were dissociated by enzymatic digestion after 14 days of culture, and sorted by EpCAM to separate epithelial cells from stromal cells for serial passaging. EpCAM-positive cells were replated for secondary and subsequent colonies with fresh MLgs/Matrigel. The colony-forming efficiency of replated cells was maintained without diminution over at least three passages, indicative of self-renewal capacity (Figure 3B). Interestingly, the incidence of mixed-morphology colonies from GFP+ cells significantly increased with passaging (Figures 3C and 3D).
To better characterize these colonies, we performed quantitative PCR with lung lineage genes. The abundance of SPC was 4.7-fold significantly enriched in freshly isolated GFP+ cells, compared with the cultured cells (P = 2.36E-5), and the concentration of CCSP was fourfold higher in these cultures compared with freshly isolated GFP+ cells (P = 1.97E-4) (Figure 3E). No expression of the ciliated cell marker forkhead box J1 (FOXJ1) or the AT1 cell marker T1α was detected (not shown).
Because the culture results from the GFP+ cells supported the hypothesis that GFP+ cells from 6-week-old mice are heterogeneous, we compared the differentiation potential in three-dimensional Matrigel cultures of GFP-labeled cells, separated according to GFP intensity (Figures 3F and 3G). Whereas GFPhigh cells generated greater than 90% (95.1% ± 4.1%, n = 2) alveolar-like colonies with bright GFP expression, GFPlow cells produced heterotypic colonies comprising a similar ratio of alveolar-like and mixed colonies with weak/negative GFP, suggesting that the GFPlow population contained cells with a broader differentiation potential (Figure 3G).
Alteration of SPC H2B-GFP Expression with Age
The observed heterogeneity in cultured GFP+ cells prompted us to perform further IF analysis on lung tissue sections of SPC H2B-GFP mice at various ages. During the postnatal period, the percentage of GFP-labeled AT2 cells decreased from approximately 97% on Postnatal Day 1 to approximately 63% at Postnatal Week 8 (Figure 4A). To follow GFP-labeled cells after birth, we scored the proportion of labeled SPC-positive AT2 cells, CCSP-positive club cells, CCSP/SPC–double positive BASCs, and CCSP/SPC-negative cells in SPC H2B-GFP murine lung tissue sections (representative images are provided in Figures E2–E4). In addition to the GFP+, SPC-positive AT2 cells found in the alveoli, GFP-labeled cells were detected within bronchioles, beginning at 1 week of age. Interestingly, bronchiolar GFP+ cells were preferentially localized to bronchioalveolar duct junctions (BADJs) and exhibited the coexpression of SPC and CCSP, as previously seen in BASCs (10) (Figures 4B, 4D, and 4G).
Figure 4.
Characterization of GFP-labeled cells in postnatal and adult lungs. (A) Bar graph shows the percentage of SPC+ cells with SPC immunoreactivity in alveolar regions of variously aged SPC H2B-GFP mice. Twenty alveolar regions from four lung sections per lung and three mice for each age were scored by IF with antibodies for SPC and GFP. P, postnatal. (B) IF in sectioned lungs from 8-week-old SPC H2B-GFP mice shows GFP expression in terminal bronchiolar cells, involving CCSP (purple), SPC (red), and GFP (green). Boxed areas are magnified in C–E. Scale bar, 100 μm. (C) Terminal bronchioles retain weak GFP expression. (D) GFP-labeled cells coexpress SPC and CCSP in bronchioalveolar duct junctions. (E and F) IF with antibodies for GFP, SPC, and CCSP (E) and for GFP and calcitonin gene-related peptide (red) (F) in serial sections shows that GFP-labeled cells reside around pulmonary neuroendocrine cells. (G) Bar graph shows the proportion of GFP-labeled cells with the indicated patterns of CCSP and/or SPC immunoreactivity at various ages in SPC H2B-GFP mice. Twenty alveolar regions, 20 bronchioles, and 20 terminal bronchioles with 4–8 lung sections per lung from three mice for each age group were scored by IF with antibodies for SPC, CCSP, and GFP. (H–I) Quantitative PCR for assessing the expression of lung markers SPC (H) and CCSP (I) in GFP− versus GFP+ cells from variously aged SPC H2B-GFP mice. The y axis shows relative quantifications normalized to GAPDH. The data are representative of two independent experiments.
Starting at 4 weeks of age, additional cell types expressed the SPC H2B-GFP transgene. Weak expression of GFP was observed in CCSP-positive club cells in the terminal bronchioles of 8-week-old mice (Figures 4B, 4C, and 4G). Costaining for GFP and the PNEC marker calcitonin gene-related peptide on serial sectioned lung tissue showed that CCSP-positive cells near PNECs were also labeled by GFP (Figures 4B, 4E, and 4F). In the alveoli, CCSP-negative, SPC-negative, GFP-labeled cells expressed aquaporin 5 (data not shown). GFP-labeled cells were never observed in the mesenchymal or endothelial compartments, regardless of age. Quantitative PCR analysis with lung lineage markers in freshly sorted GFP− and GFP+ cells from lungs of different ages confirmed that the GFP-labeled population contained more differentiated cells in addition to AT2 cells with age. SPC concentrations were increased in GFP− cells (Figure 4H), whereas the abundance of CCSP was increased in GFP+ cells from 6-week-old mice (Figure 4I). Thus, IF and quantitaive PCR validated the in vitro findings, and showed that SPC H2B-GFP transgene-expressing cells are heterogeneous in vivo.
Identification of Surface Markers for AT2 Cells
Previous studies have shown that AT2 cells are enriched in the CD31-negative, CD45-negative, and Stem cell antigen 1–negative population of adult murine lungs, providing a strategy for the FACS isolation of this population (10, 29). However, with these protocols a positive selection marker for AT2 cells is lacking. Hence we performed transcriptional profiling in isolated GFP− versus GFP+ cells, using the Affymetrix microarray platform to identify new surface markers for detecting AT2 cells. GFP− and GFP+ cells were freshly isolated from lungs of 6-week-old SPC H2B-GFP mice by FACS (Figure 5A), and were verified by quantitative PCR for the expression of lung lineage genes. AT2 markers SPC and ABCA3 were 28.6-fold and 19.1-fold highly up-regulated in GFP+ cells (P = 1.07E-6 and 2.61E-7, respectively), whereas club cell marker CCSP and ciliated cell marker FOXJ1 were 9.1-fold and 12.0-fold up-regulated in GFP− cells, respectively (P = 1.63E-9 and 2.78E-5, respectively) (Figure 5B), suggesting the enrichment of AT2 cells within the GFP+ populations used for the array.
Figure 5.
(A) Representative FACS plots show the strategy of isolating GFP− and GFP+ cells from 6-week-old wild-type mice (top) and SPC H2B-GFP mice (bottom) for transcriptional profiling. The percentage of total lung cells represented by each population is indicated. Histograms show the percentage of cells negative or positive for GFP in the CD31−CD45− population. (B) Validation of gene expression for lung lineage markers in GFP− and GFP+ cells by quantitative PCR. The y axis shows relative quantifications normalized to GAPDH (*P < 0.001). (C and D) Probe intensity signals of gene expression profiles from SPC-GFP− and SPC-GFP+ cells correspond to the genes exhibiting the highest-fold differential expression between rat AT1 and AT2 cells, compared with the mean of 10 sets of randomly selected probe sets. (C) Genes up-regulated in rat AT2 cells. (D) Genes up-regulated in rat AT1 cells. (E) Analysis of microarray gene expression for selected genes expressed in distal tip cells during branching morphogenesis. Gene expression differences between SPC-GFP− and SPC-GFP+ cells (depicted as fold change [log2] of GFP+ gene expression relative to GFP− expression level) are reported for the genes listed. P < 0.05. FOXJ1, forkhead box J1.
Microarray data analysis using GeneSpring GX software (Agilent Technologies, Santa Clara, CA) revealed that among 45,101 probes presented on the Affymetrix Mouse Genome 430 version 2.0 arrays, approximately 10% of probes were differentially expressed in either GFP+ cells or GFP− cells. Because we were interested in AT2 cell isolation, we further analyzed probes up-regulated in the GFP+ population. We identified 2,281 genes (3,801 probes) as up-regulated (> 2-fold) in the GFP+ cell population via GeneSpring fold change analysis (summarized in Table E1). To assess the specificity of gene expression profiles of AT2 cells, we first compared genes that were up-regulated in rat AT2 cells with our murine dataset. Gonzalez and colleagues reported 48 genes expressing the highest-fold differences between freshly isolated AT1 cells and AT2 cells (45). The comparison of 31 of these genes after interspecies gene conversion showed that 15 genes (34 probes) up-regulated in AT2 cells, compared with AT1 cells, were highly enriched in SPC-GFP+ cells compared with SPC-GFP− cells (Figure 5C and Figure E5A). Sixteen genes (29 probes) that were up-regulated in AT1 cells compared with AT2 cells were down-regulated in SPC-GFP+ cells, compared with SPC-GFP− cells (Figure 5D and Figure E5B). Functional annotation clustering in DAVID (the Database for Annotation, Visualization and Integrated Discovery; http://david.niaid.nih.gov) revealed a distinct gene expression signature in GFP+ cells, including genes that are reported to be highly expressed in distal tip progenitor cells during branching morphogenesis (46), such as Etv5, Elf5, Spry2, Shh, Id2, Foxp1, and Foxp2 (Figure 5E). In addition, 85 genes known to encode membrane-localized proteins were up-regulated in GFP+ cells compared with GFP− cells (Table E2), suggesting that at least some of these membrane-localized proteins may serve as novel surface markers for detecting AT2 cells.
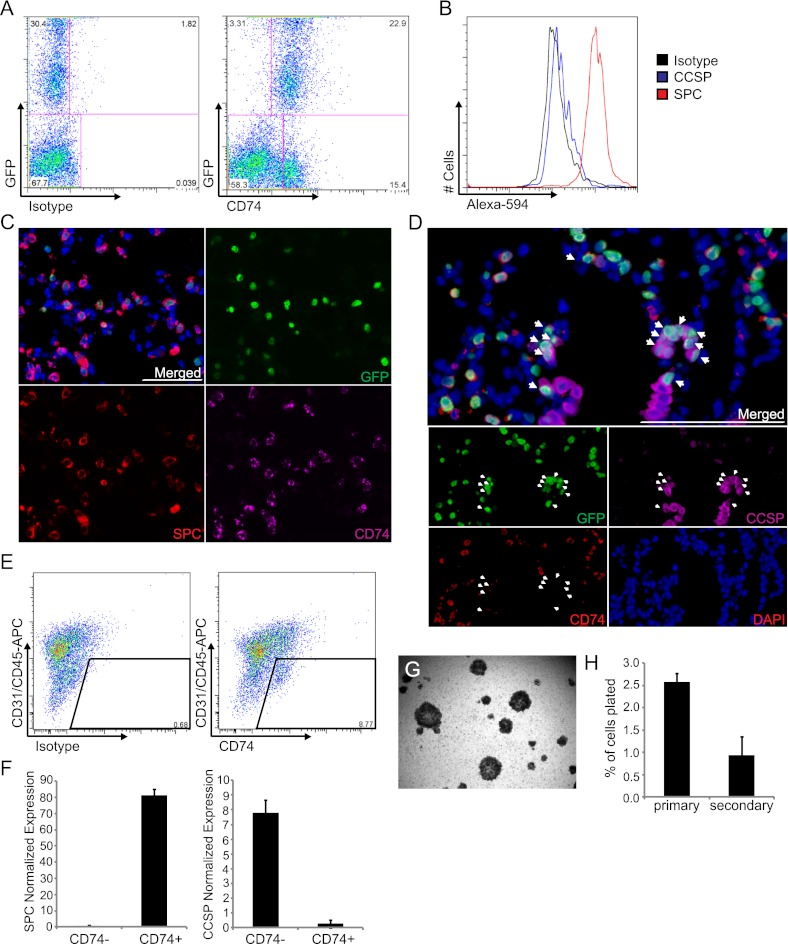
Focusing on cell-surface genes for which commercial antibodies are available, we tested CD36, CD66a (Ceacam1), CD71 (Tfrc), CD74, and CD138 (Sdc1) as possible surface markers for AT2 cells. Isolated lung cells from 6-week-old SPC H2B-GFP mice were stained with antibodies for each protein and analyzed by FACS. Most of the cells positive for CD36, CD66a, CD71, or CD138 staining presented within the CD31-positive, CD45-positive fractions (not shown), eliminating their utility as AT2 cell markers. CD74 was uniformly expressed within the CD31-negative, CD45-negative, GFP-positive fractions. Almost all GFP-positive cells expressed CD74 (Figure 6A), and the further fractionation of GFP-positive, CD74-positive cells revealed high levels of intracellular SPC expression and no detectable CCSP expression, indicative of CD74 expression in AT2 cells (Figure 6B). Regarding CD31-negative, CD45-negative cells, 28.3% were GFP-positive in 6-week-old SPC H2B-GFP mice, whereas 91.8% of CD74-positive, CD31-negative, and CD45-negative cells were GFP-positive (data not shown). CD74 provided 3.2-fold enrichment for AT2 cells. Fewer than 20% of GFP-negative cells (15.4% ± 3.8%) exhibited CD74 immunoreactivity (Figure 6A). CD31-positive, CD45-positive cells contained CD74-expressing cells likely to be macrophages or cells from other hematopoietic lineages (Figure E6A).
Figure 6.
CD74 is a surface marker of AT2 cells. (A) Representative FACS plots from detection of GFP and CD74 on lung cells from 6-week-old SPC H2B-GFP mice. Left: Isotype control; no CD74 antibody was used in the sample. Right: Results with CD74 antibody. The percentage of total lung cells represented by each population is indicated. (B) SPC (red), CCSP (blue), and isotype control (black) expression in fixed GFP+CD74+ cell fractions from 6-week-old SPC H2B-GFP mice. (C and D) Four-color IF for CD74 (red), SPC or CCSP (purple), GFP (green), and DAPI (blue) in lung tissue sections from 6-week-old (C) and 8-week-old (D) SPC H2B-GFP mice. Arrowheads indicate bronchiolar GFP+ cells. Scale bar, 100 μm. (E) Representative FACS plots isolate CD31−CD45−CD74− and CD74+ cells from 6-week-old wild-type mice. Left: Isotype control; no CD74 antibody was used in the sample. Right: Results with CD74 antibody. (F) Validation of gene expressions of lung lineage markers SPC (left) and CCSP (right) in sorted CD31−CD45−CD74− and CD31−CD45−CD74+ cells by quantitative PCR. The y axis shows relative quantifications normalized to GAPDH (*P < 0.001). (E–H) Data are representative of two independent experiments. (G) Representative phase-contrast images of CD31−CD45−CD74+ cells from 6-week-old wild-type mice after 21 days in culture. (H) Incidence of colony formation from CD31−CD45−CD74+ cells after passages.
The IF analysis of lung tissue sections from 6-week-old SPC H2B-GFP mice showed a restricted expression of CD74 in AT2 cells and hematopoietic cells. Costaining for GFP, SPC, and CD74 showed a predominant colocalization of CD74 with SPC and GFP (Figure 6C). Additional costaining for GFP, CCSP, and CD74 revealed that terminal bronchiolar cells exhibiting GFP expression lacked immunoreactivity for CD74, suggesting the specificity of CD74 in AT2 cells and not in BASCs (Figure 6D). Rare CD74-expressing cells were not labeled by SPC or GFP around blood vessels or alveolar regions (Figure E6B), consistent with FACS data indicating that the CD31/CD45-positive population contains CD74-positive cells. To confirm CD74 expression in AT2 cells, quantitative PCR for SPC and CCSP was performed in sorted CD31−CD45−CD74− and CD31−CD45−CD74+ cells from 6-week-old wild-type mice (Figure 6E). The abundance of SPC expression was 21.7-fold up-regulated in CD31−CD45−CD74+ cells compared with CD31−CD45−CD74− cells. CCSP expression was 8.7-fold down-regulated in CD31−CD45−CD74+ cells compared with CD31−CD45−CD74− cells (P = 2.41E-5 and 1.74E-5, respectively) (Figure 6F).
Isolated CD31−CD45−CD74+ cells were subjected to 3D Matrigel culture to define the properties of CD74-expressing AT2 cells. In contrast to the heterogeneity of GFP+ cells observed in culture, CD31−CD45−CD74+ cells produced only alveolar-like colonies (Figure 6G). Importantly, when primary colonies were dissociated after sorting by EpCAM for replating, a progressive decrease in colony-forming efficiency occurred (Figure 6H), suggesting that CD74 marks a cell population with limited self-renewal and differentiation potential. These data reveal that CD74 is a positive selection cell surface marker useful in enriching threefold for AT2 cells, in combination with negative selection markers for hematopoietic and endothelial cells, as supported by an earlier report that CD74 is highly expressed in AT2 cells (47).
Discussion
This study presents a detailed characterization of a new transgenic murine line designed to allow the rapid identification, direct visualization, and ready enrichment of AT2 cells and other SPC-expressing cells from developing and postnatal lungs. Using SPC H2B-GFP mice, we isolated GFP+ cells and demonstrated their potential for self-renewal and the production of progeny with characteristics of bronchiolar or alveolar cells, using the three-dimensional Matrigel culture system. In vivo analysis of SPC H2B-GFP mice provided evidence that epithelial maturation in the distal lung includes the induction of the SPC-H2B GFP transgene in subsets of bronchiolar and alveolar epithelial cells that are not AT2 cells. Using microarray analysis of GFP-expressing cells followed by functional characterization, we defined CD74 as a positive selection marker for AT2 cells.
The expression pattern of GFP in early postnatal SPC H2B-GFP mice is similar to the expression pattern of endogenous SPC, making this a useful strain for the analysis of AT2 cells at young ages. GFP was highly expressed in almost all AT2 cells throughout the alveolar space in the developing lung. The most homogenous expression of GFP in SPC+ AT2 cells was observed at 1 day after birth. Up to 6 weeks after birth, approximately 80% of GFP-labeled cells stained specifically with SPC. It remains unclear why certain bronchiolar cells expressed GFP with age in SPC H2B-GFP mice. Aberrant transgene expression from position-effect variegation may have led to the observed expression patterns. However, the consistency of results across three independent founder lines makes this unlikely. In addition, the inclusion of proximal sequences for the regulation of gene expression within the BAC construct decreases the possibility of nonspecific expression of GFP. Previous studies in transgenic lines with a murine SPC promoter provided a plausible explanation that species differences, including their chromatin environments, may account for the aberrant bronchiolar expression obtained from the human SPC promoter, rather than position effects or the copy number of transgenes (30, 32). Finally, a GFP+ cell in SPC H2B-GFP mice may no longer be expressing SPC, but rather its precursor cell expressed SPC, and GFP is simply observed before protein decay. This is unlikely, given that we have confirmed the coexpression of SPC and GFP by immunostaining. However, lineage tracing techniques would be needed to formally rule out this possibility.
SPC H2B-GFP mice will be useful in the identification of new lineage or surface markers and gene expression programs in the developing and adult distal lung. Although the surface marker profiling and gene expression programs described here will require further analysis, this study provides gene expression data that potentially distinguish AT2 cells from other lung cell types. We found 85 new cell surface markers that were up-regulated in SPC-expressing cells according to bioinformatics analysis (Table E2), and among them, we confirmed that CD74 can be used to isolate AT2 cells when combined with the exclusion of CD45/CD31-positive cells. The selection of CD74+, CD45−, and CD31− cells provided 3-fold enrichment for AT2 cells relative to FACS, without the use of CD74. We cannot rule out the possibility that some AT2 cells are not marked by CD74. Although some macrophages and dendritic cells in the lung have been reported to express CD74 weakly (47), the future use of CD74 in FACS strategies in combination with hematopoietic and endothelial cell markers will be helpful in discriminating AT2 cells from other lung epithelial cells (e.g., AT1 cells or bronchiolar cells) and from lung stromal cells.
Our studies reinforce the importance of cell-isolation methods and culture conditions for in vitro assays of AT2 cells or other lung epithelial cells. Although recent work has revealed a successful expansion of lung epithelial progenitor cells by using the three-dimensional Matrigel culture system, purified AT2 cells from previously described SPC-GFP transgenic mice (29) or cultured alveolar-like colonies (44) failed to expand further in culture, or did not self-renew (10). In contrast, GFP+ cells from SPC H2B-GFP mice developed proliferative, alveolar-like structures expressing SPC in the three-dimensional Matrigel culture setting (Figure 2). Serial passages revealed the self-renewal capacity of GFP+ cells under the same conditions (Figure 3B). Interestingly, distinct phenotypic colonies, referred to as mixed colonies, were observed more than alveolar colonies with the passage of GFP+ cells (Figures 3C–3E). Similar colonies from EpCAM-positive fractions have been reported as multipotent epithelial colony-forming units giving rise to bronchiolar and alveolar lineages (44).
The fortuitous expression of GFP in SPC H2B-GFP mice provides a new opportunity for the further enrichment and characterization of multiple lung stem/progenitor cell populations. Interestingly, GFP+ bronchiolar cells in young adults were only detected in proposed stem/progenitor cell niches such as the BADJs for BASCs, or adjacent to PNECs for variant club cells (6, 7, 10). Furthermore, with age, the SPC H2B-GFP transgene was detected more readily outside these stem-cell regions, and was present in epithelial cells that are thought to be the progeny of BASCs or variant club cells. GFP was observed in CCSP-positive (SPC-negative) bronchiolar cells and AT1 cells. Further supporting this idea, our microarray analysis indicated that GFP+ cells express genes known to be highly expressed in multipotent progenitor cells of the fetal lung distal tip cells that contribute to both bronchiolar and alveolar lineages during lung development (46). Chapman and colleagues showed that a subpopulation of alveolar epithelial cells expressing α6β4 serves as a multipotent progenitor, although in contrast to our results, the α6β4-positive alveolar epithelial progenitors did not express SPC (21). Although the knock-in mice that were used in the studies by Chapman and colleagues (21) and Rock and colleagues (8) provide useful resources to appreciate the properties of AT2 cells in vivo, the SPC H2B-GFP strain allows for the direct visualization of fluorescent cells without relying on a color reporter allele, providing increased sensitivity. For example, certain bronchiolar cells may normally express SPC at undetectable concentrations that were visualized by the strong nuclear H2B-GFP signal. Future studies, including lineage tracing approaches not possible with SPC H2B-GFP mice alone, will be needed to further explore the differentiation potential of AT2 cells and other SPC-expressing cells in vivo. Thus, existing SPC-Cre knock-in mice and now SPC H2B-GFP mice offer complementary new tools for examining the lung stem-cell community.
It remains to be determined whether SPC H2B-GFP GFP+ cells contain a single multipotent stem/progenitor cell type that gives rise to both bronchiolar and alveolar lineages in culture, or if GFP+ cells contain distinct populations that are separately committed to either a bronchiolar or an alveolar lineage. A subset of cells with stem/progenitor cell activity labeled by SPC H2B-GFP such as BASCs, rather than AT2 cells, may account for the heterotypic colonies in our three-dimensional Matrigel cultures. However, proliferating cells coexpressing bright GFP and SPC without CCSP were detected even in tertiary alveolar colonies. Future studies to further fractionate GFP+ cells with additional surface markers, as well as analyses of more lung lineage genes, may reveal the existence of multiple stem/progenitor cell populations labeled with SPC H2B-GFP.
In conclusion, we have generated SPC H2B-GFP transgenic mice, implementing a reporter system that provides a new method for the identification, prospective enrichment, and functional analysis of AT2 cells and other SPC-expressing cells in vivo and in vitro. This murine model is most useful for the enrichment of AT2 cells in early postnatal lungs, because some discordance between SPC and GFP expression was observed with age. Nevertheless, our methods for isolating and expanding proliferative SPC-expressing cells using this line of transgenic mice have provided useful tools to identify stem/progenitor properties in lung epithelial cells. The future use of SPC H2B-GFP mice may allow for significant insights into the technical and scientific hurdles of stem cell–based therapeutics for lung diseases.
Supplementary Material
Acknowledgments
The authors thank Stuart Orkin and the Center of Molecular Developmental Hematopoiesis (2P01 HL032262) for transgene injections, Elaine Fuchs for the H2B-GFP cassette, and S. Nizza, A. Vo, E. Leder, and J. Wong for technical assistance. The authors further thank the Dana Farber Cancer Institute (DFCI) and Children's Hospital Boston (CHB) Hematology Oncology FACS Facilities and the Children's Hospital Boston Molecular Genetics Core Facility.
Footnotes
This work was supported by the Hope Fund for Cancer Research (J.-H.L.), National Institutes of Health grant RO1 HL090146 (B.R.S.), National Institutes of Health grant 1U01HL099997-01 under RFA-HL-09-004 (B.R.S. and C.F.K.), National Institutes of Health grants RO1 HL090136 and U01 HL100402 under RFA-HL-09-004, American Cancer Society Research Scholar Grant RSG-08-082-01-MGO, a Basil O’Conner March of Dimes Starter Award, and the Harvard Stem Cell Institute (C.F.K.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2011-0403OC on November 29, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008;5:328–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol 2004;286:L643–L649 [DOI] [PubMed] [Google Scholar]
- 3.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol 2004;164:577–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, Wang F, Hogan BL. The role of SCGB1A1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell 2009;4:525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds SD, Hong KU, Giangreco A, Mango GW, Guron C, Morimoto Y, Stripp BR. Conditional Clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256–L1263 [DOI] [PubMed] [Google Scholar]
- 6.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein–expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol 2001;24:671–681 [DOI] [PubMed] [Google Scholar]
- 7.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rock JR, Barkauskas CE, Cronce MJ, Xue Y, Harris JR, Liang J, Noble PW, Hogan BL. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci USA 2011;108:E1475–E1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Mitsialis SA, Kourembanas S, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 2012;302:L829–L837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823–835 [DOI] [PubMed] [Google Scholar]
- 11.Williams MC. Alveolar Type I cells: molecular phenotype and development. Annu Rev Physiol 2003;65:669–695 [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez RF, Allen L, Dobbs LG. Rat alveolar Type I cells proliferate, express OCT-4, and exhibit phenotypic plasticity in vitro. Am J Physiol Lung Cell Mol Physiol 2009;297:L1045–L1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fehrenbach H. Alveolar epithelial Type II cell: defender of the alveolus revisited. Respir Res 2001;2:33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adamson IY, Bowden DH. The Type 2 cell as progenitor of alveolar epithelial regeneration: a cytodynamic study in mice after exposure to oxygen. Lab Invest 1974;30:35–42 [PubMed] [Google Scholar]
- 15.Evans MJ, Cabral LJ, Stephens RJ, Freeman G. Transformation of alveolar Type 2 cells to Type 1 cells following exposure to NO2. Exp Mol Pathol 1975;22:142–150 [DOI] [PubMed] [Google Scholar]
- 16.Brody JS, Burki R, Kaplan N. Deoxyribonucleic acid synthesis in lung cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis 1978;117:307–316 [DOI] [PubMed] [Google Scholar]
- 17.Reddy R, Buckley S, Doerken M, Barsky L, Weinberg K, Anderson KD, Warburton D, Driscoll B. Isolation of a putative progenitor subpopulation of alveolar epithelial Type 2 cells. Am J Physiol Lung Cell Mol Physiol 2004;286:L658–L667 [DOI] [PubMed] [Google Scholar]
- 18.Adamson IY, Bowden DH. Derivation of Type 1 epithelium from Type 2 cells in the developing rat lung. Lab Invest 1975;32:736–745 [PubMed] [Google Scholar]
- 19.Reynolds SD, Giangreco A, Hong KU, McGrath KE, Ortiz LA, Stripp BR. Airway injury in lung disease pathophysiology: selective depletion of airway stem and progenitor cell pools potentiates lung inflammation and alveolar dysfunction. Am J Physiol Lung Cell Mol Physiol 2004;287:L1256–L1265 [DOI] [PubMed] [Google Scholar]
- 20.Londhe VA, Maisonet TM, Lopez B, Jeng JM, Li C, Minoo P. A subset of epithelial cells with CCSP promoter activity participates in alveolar development. Am J Respir Cell Mol Biol 2011;44:804–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y, Vu TH. Integrin alpha6beta4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest 2011;121:2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leary JF, Finkelstein JN, Notter RH, Shapiro DL. Isolation of Type II pneumocytes by laser flow cytometry. Am Rev Respir Dis 1982;125:326–330 [DOI] [PubMed] [Google Scholar]
- 23.Wilson JS, Steinkamp JA, Lehnert BE. Isolation of viable Type II alveolar epithelial cells by flow cytometry. Cytometry 1986;7:157–162 [DOI] [PubMed] [Google Scholar]
- 24.Funkhouser JD, Cheshire LB, Ferrara TB, Peterson RD. Monoclonal antibody identification of a Type II alveolar epithelial cell antigen and expression of the antigen during lung development. Dev Biol 1987;119:190–198 [DOI] [PubMed] [Google Scholar]
- 25.Harrison JH, Jr, Porretta CP, Leming K. Purification of murine pulmonary Type II cells for flow cytometric cell cycle analysis. Exp Lung Res 1995;21:407–421 [DOI] [PubMed] [Google Scholar]
- 26.Corti M, Brody AR, Harrison JH. Isolation and primary culture of murine alveolar Type II cells. Am J Respir Cell Mol Biol 1996;14:309–315 [DOI] [PubMed] [Google Scholar]
- 27.Roper JM, Staversky RJ, Finkelstein JN, Keng PC, O’Reilly MA. Identification and isolation of mouse Type II cells on the basis of intrinsic expression of enhanced green fluorescent protein. Am J Physiol Lung Cell Mol Physiol 2003;285:L691–L700 [DOI] [PubMed] [Google Scholar]
- 28.Lo B, Hansen S, Evans K, Heath JK, Wright JR. Alveolar epithelial Type II cells induce T cell tolerance to specific antigen. J Immunol 2008;180:881–888 [DOI] [PubMed] [Google Scholar]
- 29.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Potts E, Foster WM, Bertoncello I, Stripp BR. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol 2011;44:794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glasser SW, Burhans MS, Eszterhas SK, Bruno MD, Korfhagen TR. Human SP-C gene sequences that confer lung epithelium–specific expression in transgenic mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L933–L945 [DOI] [PubMed] [Google Scholar]
- 31.Glasser SW, Korfhagen TR, Wert SE, Bruno MD, McWilliams KM, Vorbroker DK, Whitsett JA. Genetic element from human surfactant protein SP-C gene confers bronchiolar–alveolar cell specificity in transgenic mice. Am J Physiol 1991;261:L349–L356 [DOI] [PubMed] [Google Scholar]
- 32.Glasser SW, Eszterhas SK, Detmer EA, Maxfield MD, Korfhagen TR. The murine SP-C promoter directs Type II cell–specific expression in transgenic mice. Am J Physiol Lung Cell Mol Physiol 2005;288:L625–L632 [DOI] [PubMed] [Google Scholar]
- 33.Leslie CC, McCormick-Shannon K, Robinson PC, Mason RJ. Stimulation of DNA synthesis in cultured rat alveolar Type II cells. Exp Lung Res 1985;8:53–66 [DOI] [PubMed] [Google Scholar]
- 34.Leslie CC, McCormick-Shannon K, Mason RJ, Shannon JM. Proliferation of rat alveolar epithelial cells in low density primary culture. Am J Respir Cell Mol Biol 1993;9:64–72 [DOI] [PubMed] [Google Scholar]
- 35.Uhal BD, Flowers KM, Rannels DE. Type II pneumocyte proliferation in vitro: problems and future directions. Am J Physiol 1991; 261(4, Suppl)110–117 [DOI] [PubMed] [Google Scholar]
- 36.Sugahara K, Mason RJ, Shannon JM. Effects of soluble factors and extracellular matrix on DNA synthesis and surfactant gene expression in primary cultures of rat alveolar Type II cells. Cell Tissue Res 1998;291:295–303 [DOI] [PubMed] [Google Scholar]
- 37.Shannon JM, Jennings SD, Nielsen LD. Modulation of alveolar Type II cell differentiated function in vitro. Am J Physiol 1992;262:L427–L436 [DOI] [PubMed] [Google Scholar]
- 38.Diglio CA, Kikkawa Y. The Type II epithelial cells of the lung: IV. Adaption and behavior of isolated Type II cells in culture. Lab Invest 1977;37:622–631 [PubMed] [Google Scholar]
- 39.Mason RJ, Dobbs LG. Synthesis of phosphatidylcholine and phosphatidylglycerol by alveolar Type II cells in primary culture. J Biol Chem 1980;255:5101–5107 [PubMed] [Google Scholar]
- 40.Dobbs LG, Williams MC, Brandt AE. Changes in biochemical characteristics and pattern of lectin binding of alveolar Type II cells with time in culture. Biochim Biophys Acta 1985;846:155–166 [DOI] [PubMed] [Google Scholar]
- 41.Mason RJ, Dobbs LG, Greenleaf RD, Williams MC. Alveolar Type II cells. Fed Proc 1977;36:2697–2702 [PubMed] [Google Scholar]
- 42.Copeland NG, Jenkins NA, Court DL. Recombineering: a powerful new tool for mouse functional genomics. Nat Rev Genet 2001;2:769–779 [DOI] [PubMed] [Google Scholar]
- 43.Kanda T, Sullivan KF, Wahl GM. Histone–GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Curr Biol 1998;8:377–385 [DOI] [PubMed] [Google Scholar]
- 44.McQualter JL, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proc Natl Acad Sci USA 2011;107:1414–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gonzalez R, Yang YH, Griffin C, Allen L, Tigue Z, Dobbs L. Freshly isolated rat alveolar Type I cells, Type II cells, and cultured Type II cells have distinct molecular phenotypes. Am J Physiol Lung Cell Mol Physiol 2005;288:L179–L189 [DOI] [PubMed] [Google Scholar]
- 46.Rawlins EL. Lung epithelial progenitor cells: lessons from development. Proc Am Thorac Soc 2008;5:675–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsh LM, Cakarova L, Kwapiszewska G, von Wulffen W, Herold S, Seeger W, Lohmeyer J. Surface expression of CD74 by Type II alveolar epithelial cells: a potential mechanism for macrophage migration inhibitory factor–induced epithelial repair. Am J Physiol Lung Cell Mol Physiol 2009;296:L442–L452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.