Abstract
In asthma, basic fibroblast growth factor (FGF-2) plays an important (patho)physiological role. This study examines the effects of FGF-2 on the transforming growth factor–β (TGF-β)–stimulated differentiation of airway smooth muscle (ASM) cells in vitro. The differentiation of human ASM cells after incubation with TGF-β (100 pM) and/or FGF-2 (300 pM) for 48 hours was assessed by increases in contractile protein expression, actin-cytoskeleton reorganization, enhancements in cell stiffness, and collagen remodeling. FGF-2 inhibited TGF-β–stimulated increases in transgelin (SM22) and calponin gene expression (n = 15, P < 0.01) in an extracellular signal-regulated kinase 1/2 (ERK1/2) signal transduction–dependent manner. The abundance of ordered α–smooth muscle actin (α-SMA) filaments formed in the presence of TGF-β were also reduced by FGF-2, as was the ratio of F-actin to G-actin (n = 8, P < 0.01). Furthermore, FGF-2 attenuated TGF-β–stimulated increases in ASM cell stiffness and the ASM-mediated contraction of lattices, composed of collagen fibrils (n = 5, P < 0.01). However, the TGF-β–stimulated production of IL-6 was not influenced by FGF-2 (n = 4, P > 0.05), suggesting that FGF-2 antagonism is selective for the regulation of ASM cell contractile protein expression, organization, and function. Another mitogen, thrombin (0.3 U ml−1), exerted no effect on TGF-β–regulated contractile protein expression (n = 8, P > 0.05), α-SMA organization, or the ratio of F-actin to G-actin (n = 4, P > 0.05), suggesting that the inhibitory effect of FGF-2 is dissociated from its mitogenic actions. The addition of FGF-2, 24 hours after TGF-β treatment, still reduced contractile protein expression, even when the TGF-β–receptor kinase inhibitor, SB431542 (10 μM), was added 1 hour before FGF-2. We conclude that the ASM cell differentiation promoted by TGF-β is antagonized by FGF-2. A better understanding of the mechanism of action for FGF-2 is necessary to develop a strategy for therapeutic exploitation in the treatment of asthma.
Keywords: airway wall remodeling, α–smooth muscle actin, asthma, cytoskeleton, transgelin
Clinical Relevance
Asthma comprises structural changes to the airway wall, including increased airway smooth muscle mass, which contributes to airway obstruction. Basic fibroblast growth factor (FGF-2) has been considered for therapeutic applications in regenerative medicine. Our study shows that FGF-2 antagonizes the pro-remodeling effects of transforming growth factor–β on airway smooth muscle cells, and thus supports the potential utility of FGF-2 as therapy in the treatment of asthma.
Airway wall remodeling (AWR) contributes to airway dysfunction in asthma. AWR comprises an array of persistent tissue structural changes that are thought to occur through a process of injury and dysregulated repair, linked to chronic airway inflammation. Features of AWR include the increased deposition of extracellular matrix (ECM), airway smooth muscle (ASM) hyperplasia and hypertrophy, mucous cell metaplasia, and angiogenesis (1). The deposition of collagens I and III in the subepithelial layer and within smooth muscle bundles by airway mesenchyma increases airway wall thickness and reduces airway wall distensibility (2, 3). An effective anti-remodeling treatment is expected to reduce airway reactivity and symptoms in asthma (4, 5).
Transforming growth factor–β (TGF-β) is an important mediator of AWR in asthma. TGF-β stimulates ASM cells to differentiate into a more contractile and hypertrophic phenotype (6, 7). TGF-β signaling is activated upon ligand binding to the heteromeric receptor that phosphorylates Smad proteins. Smads translocate into the nucleus and regulate gene transcription in association with other cofactors, including the serum-response factor (SRF) (8). The promoters of genes encoding contractile proteins, such as transgelin (SM22), calponin, and α–smooth muscle actin (α-SMA), as well as procollagen I and procollagen III, contain TGF-β and SRF response elements (9, 10). The inhibition of TGF-β signaling attenuates AWR in allergen-challenged mice (11, 12). However, the therapeutic potential of TGF-β inhibition in the treatment of chronic asthma is limited by the high likelihood of serious adverse effects.
Basic fibroblast growth factor (bFGF or FGF-2), a potent mitogen, binds to the FGF receptor (FGFR) family of receptor tyrosine kinases (RTKs). FGF-2 has potential relevance in asthma, because concentrations of FGF-2 are higher in the airway submucosa and bronchoalveolar lavage fluid of patients with asthma, and increase further after exposure to allergen (13, 14). In the airways, FGF-2 is predominantly expressed by epithelial cells, and is also detected in the ECM of the epithelial basement membrane, primarily bound to heparin, an important determinant of FGF-2 activity (14, 15). FGF-2 is a potent mitogen of ASM cells in vitro (16), and is thought to play a role in AWR by contributing to ASM-cell hyperplasia. However, the administration of recombinant FGF-2 to sensitized mice has been shown to inhibit airway hyperresponsiveness and aspects of AWR in an acute model of allergic airway inflammation (17). FGF-2 may prevent ASM cell differentiation into a more contractile phenotype (maturation), as has been reported for corneal fibroblasts (18) and microvascular pericytes (19). Acidic fibroblast growth factor (FGF-1) also inhibits the TGF-β–stimulated differentiation (or transdifferentiation) of lung epithelial cells and fibroblasts (20, 21). We present evidence of the antagonistic effects of FGF-2 on ASM cell differentiation into a more contractile phenotype in response to TGF-β, and show that this inhibitory effect is dissociated from the mitogenic actions of FGF-2.
Materials and Methods
Cell Culture
Human ASM cell cultures were established as described previously (22). Cells were maintained in serum-free Dulbecco’s Modified Eagle’s Medium for 24 hours before the addition of growth factors. Three hundred picomolar (pM) of FGF-2 (Promega, Madison, WI), 0.3 U/ml−1 Thrombin (Promega), or 5% v/v−1 FCS, in the absence or presence of 100 pM TGF-β (R&D Systems, Minneapolis, MN), was added to cells with 1% v/v−1 insulin–transferrin–selenium containing supplement (Monomed A; CSL, Parkville, Australia). See the online supplement for further details and for the use of pharmacological inhibitors.
RNA Extraction and Real-Time PCR
RNA extractions, reverse transcription, and real-time PCR were performed as previously described (23). For further details, see the online supplement.
Western Blot Analysis and Measurement of Interleukin-6 Concentrations
For details of the Western blotting procedure for SM22 in cell lysates, and for measuring IL-6 concentrations in the culture supernatants, see the online supplement.
Staining for α–SMA, F-Actin, and G-Actin
See the online supplement for details of α-SMA immunostaining and of F-actin and G-actin staining using phalloidin and DNase 1, respectively.
Preparation of Type I Collagen Gels for Floating Three-Dimensional Cell Culture
Floating three-dimensional (3D) type I fibrillar collagen gel cultures of ASM cells were established as previously described (24). See the online supplement for further details.
SM22 Promoter Reporter Assay
ASM cells maintained in 24-well plates were transfected with SM22 promoter–luciferase reporter plasmid and the pSEAP2 control plasmid (Clontech and BD Biosciences, San Jose, CA). Details of the reporter plasmids, transfection procedure, and measurement of luciferase and SEAP concentrations are provided in the online supplement.
Micropipette Aspiration
The micropipette aspiration technique is an established method for measuring the stiffness of single cells (25). A micropipette was positioned in contact with a dissociated cell at 37°C, using a micromanipulator and a controlled suction pressure applied to the cell’s surface via the micropipette. The pressure was finely regulated using an adjustable water reservoir. Images of the aspirated cell in the micropipette in real time were taken by a digital camera–fitted microscope (Leica DMI6000B; North Ryde, NSW, Australia). The applied pressure and corresponding cell elongation provided the relevant data for calculating the mechanical properties of the single cell (see the online supplement for further details).
Statistical Analysis
Data are presented as the mean ± SEM for n individual experiments. Each experiment was performed using cells from at least four different donors (n ≥ 4), except for the micropipette aspiration experiment, for which data were collected from individual cells (n ≥ 4) of the one donor. All data were statistically analyzed with one-way ANOVA and the Bonferroni post hoc test, using GraphPad Prism version 5.0 (GraphPad, San Diego, CA). P < 0.05 was considered statistically significant.
Results
FGF-2 Inhibits TGF-β–Stimulated Increases in Contractile Protein Expression
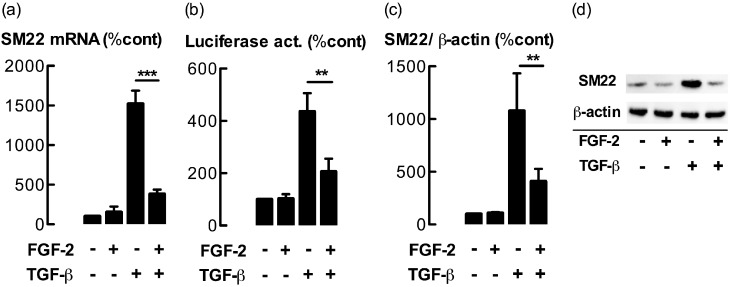
The effects of FGF-2 on the expression of the contractile apparatus–associated protein SM22 were examined. Cells incubated with TGF-β (100 pM) for 48 hours showed a large increase in concentrations of SM22 mRNA, which were greatly attenuated by coincubation with FGF-2 (300 pM, P < 0.01; Figure 1a). FGF-2 alone exerted little effect on the concentration of SM22 mRNA. The effects of FGF-2 at concentrations ranging from 10–1,000 pM in ASM cells, coincubated with either 100 or 1,000 pM TGF-β, are illustrated in Figure E1a in the online supplement. A similar inhibitory effect of FGF-2 on the mRNA abundance of calponin, another TGF-β–regulated contractile protein, was observed (Figure E1b). FGF-2 also attenuated TGF-β–stimulated increases in SM22 promoter activity in cells transfected with a reporter plasmid containing the intact SM22 promoter (P < 0.01; Figure 1b). Furthermore, TGF-β–stimulated increases in amounts of SM22 protein were significantly attenuated by coincubation with FGF-2 (P < 0.01; Figures 1c and 1d). However, TGF-β–stimulated increases in Smad 2/3 phosphorylation were not attenuated by coincubation with FGF-2 (P > 0.05; Figure E2).
Figure 1.
Basic fibroblast growth factor (FGF-2) attenuates transforming growth factor (TGF)–β–stimulated increases in transgelin (SM22) expression. (a) Concentrations of SM22 RNA. (b) SM22 promoter–reporter activity (Luciferase act.). (c) Concentrations of SM22 protein in airway smooth muscle (ASM) cells incubated with FGF-2 (300 pM) and/or TGF-β (100 pM) for 48, 24, and 48 hours, respectively. RNA and protein were analyzed by real-time PCR and Western blotting, respectively. (d) A representative Western blot. To measure the transcriptional activation of the SM22 promoter, cells were transfected with an SM22 promoter reporter plasmid. Values are expressed as a percentage of the control group (%cont). No significance (NS), P > 0.05. **P < 0.01. ***P < 0.001 (n = 6–15 different primary cultures established from separate donors).
Neither the Powerful Mitogens Thrombin nor FCS Influenced TGF-β–Regulated Contractile Protein Expression
To determine whether the effects of FGF-2 on TGF-β–regulated contractile protein expression were secondary to the mitogenic action of FGF-2, the effects of two other powerful ASM mitogens, thrombin and FCS, were examined. Under conditions known to be mitogenic (16), neither thrombin nor FCS attenuated TGF-β–stimulated increases in mRNA for SM22 (P > 0.05; Figure 2) or calponin (P > 0.05; Figure E3). However, thrombin stimulation increased the concentrations of SM22 and calponin mRNA. Furthermore, we have shown that the incubation of ASM cells with platelet-derived growth factor (PDGF) (25 ng/ml−1), another ASM cell mitogen, did not attenuate TGF-β–stimulated increases in either SM22 or calponin expression (P > 0.05; Figures E4a and E4b). Although epidermal growth factor (EGF) (300 pM) did attenuate TGF-β–stimulated SM22 gene expression, the effect was much weaker than that of FGF-2 (P < 0.01; Figure E4a). EGF exerted no effect on calponin gene expression (P > 0.05; Figure E4b).
Figure 2.
TGF-β–regulated SM22 gene expression is not modulated by thrombin or serum. Concentrations of SM22 mRNA in ASM cells incubated with FGF-2 (300 pM), thrombin (Thr, 0.3 U/ml−1) or FCS (5% v/v−1) in the presence or absence of TGF-β (100 pM) for 48 hours were analyzed by real-time PCR. NS, P > 0.05. **P < 0.01 (n = 8 different primary cultures established from separate donors).
FGF-2 Prevents the Formation of α-SMA Filaments by TGF-β
The effect of FGF-2 on the formation of α-SMA filaments, a characteristic of a contractile mesenchymal phenotype, was examined via immunocytochemistry. TGF-β induced an increase in the abundance of α-SMA filaments (Figures 3a and 3d). However, this elevation in α-SMA filaments was not observed in FGF-2–treated cells (Figures 3b and 3e). Thrombin alone exerted no effect on filament formation, nor did it reduce the abundance of filaments in the presence of TGF-β (Figures 3c and 3f).
Figure 3.
FGF-2, but not thrombin, interferes with TGF-β–stimulated α–smooth muscle actin (α-SMA) assembly into filaments. The immunocytochemical detection of α-SMA was undertaken in cells incubated with FGF-2 (300 pM) or thrombin (Thr, 0.3 U/ml−1) in the absence or presence of TGF-β (100 pM) for 48 hours. All images are ×200 magnification, and are representative of three experiments.
The TGF-β–Stimulated Increase in F-Actin Formation Is Diminished by FGF-2, but Not Thrombin
An increase in the formation of stress fibers by F-actin reorganization occurs in mesenchymal cells that differentiate into a more contractile phenotype. F-actin stress fibers that formed in ASM cells after exposure to TGF-β were visualized by phalloidin staining (Figures 4a and 4b). TGF-β–stimulated F-actin filament formation was abrogated by coincubation with FGF-2, but not by thrombin (Figures 4c and 4d). FGF-2 or thrombin alone exerted no effect (data not shown). The TGF-β–stimulated increase in the F-actin to G-actin ratio was reduced by FGF-2 incubation (P < 0.01), but not by thrombin (P > 0.05) (Figures 4e and 4f).
Figure 4.
FGF-2, but not thrombin, interferes with TGF-β–stimulated actin assembly into filaments. (a–d) Fluorescence staining of F-actin (green) and G-actin (red) was undertaken in ASM cells incubated with FGF-2 (300 pM) or thrombin (Thr, 0.3 U/mL−1) in the absence or presence of TGF-β (100 pM) for 48 hours. All images, ×400 magnification. (e and f) F-actin/G-actin ratios in cells treated with TGF-β and FGF-2 (e, n = 8) or thrombin (f, n = 4). NS, P > 0.05. **P < 0.01. ***P < 0.001.
FGF-2 Attenuates TGF-β–Stimulated Compaction of Collagen Gels Embedded with ASM Cells
To assess the impact of FGF-2 on a cell-contractile response, we analyzed the compaction of untensioned 3D collagen gels containing ASM cells. We found that collagen gels became more compacted in response to incubation with TGF-β, an effect that was blocked by coincubation with FGF-2 (Figure 5). However, TGF-β–stimulated gel compaction was not attenuated by thrombin, PDGF, or EGF (Figure E5).
Figure 5.
FGF-2 attenuates TGF-β–stimulated compaction of collagen gel plugs containing ASM cells. Cells were cultured in floating, Type I collagen gels in the presence of TGF-β (100 pM) and/or FGF-2 (300 pM) for 6 days. (a) Representative gels at t = 0 and t = 72 hours. (b) Percent gel compaction, as determined by change in gel area. *P < 0.05. **P < 0.01. ***P < 0.001 (n = 7 different primary cultures established from separate donors).
FGF-2 Reduces ASM Cell Stiffness
Changes in the organization of the actin cytoskeleton can influence the mechanical properties of ASM cells, including their stiffness (i.e., elastic modulus). We showed, using micropipette aspiration, that the stiffness of ASM cells was reduced by incubation with FGF-2 for 48 hours (P < 0.01; Figure 6). TGF-β–stimulated increases in cell stiffness were also attenuated by coincubation with FGF-2 (P < 0.001).
Figure 6.
FGF-2 reduces the stiffness of ASM cells. (a) Aspiration of isolated ASM cells into a micropipette by lowering the pipette pressure causes the cell to move along the lumen in a pressure-dependent manner. A series of decrements in luminal pressure generates a distance versus pressure curve, from which Young’s modulus (stiffness) is measured. (b) Young’s elastic (E) modulus of individual cells from the same donor culture treated with FGF-2 and/or TGF-β for 48 hours. **P < 0.01. ***P < 0.001.
FGF-2 Does Not Influence TGF-β–Stimulated Cytokine Production
To examine whether the inhibitory effects of FGF-2 are specific to contractile protein expression/organization, we measured the effects of FGF-2 on TGF-β–stimulated IL-6 release from ASM cells. The concentrations of IL-6 secreted in control cells or cells treated with FGF-2, TGF-β, or a combination of FGF-2 and TGF-β were 5,631 ± 1,009, 10,183 ± 2,107, 29,588 ± 2,247, and 27,660 ± 1,725 pg/ml−1, respectively (n = 4). Thus, TGF-β increased the concentrations of released IL-6 more than fivefold (P < 0.001), but coincubation with FGF-2 exerted no effect on the TGF-β–stimulated production of IL-6 (P > 0.05).
The FGF-2 Inhibitory Effect on Contractile Protein Expression Can Be Elicited in ASM Cells after Pretreatment with TGF-β
We examined whether FGF-2 stimulation was capable of decreasing the augmented SM22 expression observed in cells pretreated with TGF-β for 24 hours. We found that SM22 and calponin mRNA concentrations in cells incubated with TGF-β for 48 hours and with a delayed addition of FGF-2 were still lower than in control cells (P < 0.01) (Table 1). To determine whether the inhibitory effect of delayed FGF-2 stimulation was independent of TGF-β signaling, the TGF-β receptor kinase inhibitor SB431542 (10 μM) was added to TGF-β–pretreated ASM cells 1 hour before treatment with FGF-2. Even in the presence of SB431542, the delayed addition of FGF-2 still reduced SM22 mRNA concentrations in cells preincubated with TGF-β (P < 0.01) (Table 2). As a control, SB431542 (10 μM) was able to inhibit the TGF-β–stimulated release of IL-6 when added 1 hour before treatment with TGF-β (Figure E6), showing that this inhibitor was pharmacologically active under these conditions.
TABLE 1.
THE DELAYED ADDITION OF FGF-2 ATTENUATES TGF-β–STIMULATED INCREASES IN CONTRACTILE PROTEIN EXPRESSION
| Treatment | Control | TGF-β | FGF-2 (t = 0 h) + TGF-β | FGF-2 (t = 24 h) + TGF-β |
| SM22 | 100 | 2,198 ± 406 | 513 ± 212* | 910 ± 204* |
| Calponin | 100 | 2,075 ± 566 | 167 ± 19* | 354 ± 124* |
Definition of abbreviations: FGF-2, basic fibroblast growth factor; SM22, transgelin; TGF-β, transforming growth factor–β.
Relative levels (% control) of contractile protein mRNA (expressed as a percentage of control values) in ASM cells incubated with TGF-β (100 pM) for 48 hours. FGF-2 (300 pM) was either added at the same time as TGF-β (t = 0 h), or 24 hours after TGF-β (t = 24 h).
P < 0.01, versus TGF-β (n = 8).
TABLE 2.
THE DELAYED ADDITION OF FGF-2 STILL ATTENUATES TGF-STIMULATED RESPONSES IN THE PRESENCE OF THE TGF-β–RECEPTOR INHIBITOR, SB431542
| Treatment | Control | TGF-β | SB431542 (t = 23 h) + TGF-β | FGF-2 (t = 24 h) + SB431542 (t = 23 h) + TGF-β |
| SM22 | 9 ± 5 | 100 | 57 ± 8 | 36 ± 8* |
| Calponin | 16 ± 12 | 100 | 65 ± 11 | 14 ± 6* |
Definition of abbreviations: FGF-2, basic fibroblast growth factor; SM22, transgelin; TGF-β, transforming growth factor–β.
Relative levels of contractile protein mRNA (expressed as a percentage of TGF-β) in ASM cells incubated with TGF-β (100 pM) for 48 hours. SB431542 (10 μM) and FGF-2 (300 pM) were added 24 hours (t = 24 h) and 23 hours (t = 23 h) after TGF-β, respectively.
P < 0.05, versus SB431542 + TGF-β (n = 4).
Extracellular Signal–Regulated Kinase–Dependent Signaling Mediates the Inhibitory Effects of FGF-2
To analyze the signaling pathways involved in mediating the inhibitory effect of FGF-2 on TGF-β–stimulated differentiation, pharmacological inhibitors for the mitogen-activated protein kinase (MAPK) p38 and mitogen-activated protein kinase kinase (MEK) pathways, as well as the phosphoinositide 3–kinase (PI3K)/Akt pathway, were used. The MEK inhibitor PD98059 was the only inhibitor to significantly reduce the inhibitory effects of FGF-2 on TGF-β–stimulated increases in SM22 mRNA and protein abundance (Figures 7a and 7b), as well as SM22 promoter reporter activity (Figure E7). Similar results were observed for the concentrations of calponin mRNA (Table E1 in the online supplement). Another MEK inhibitor, UO126 (10 μM), also ameliorated the inhibitory effects of FGF-2 on TGF-β–stimulated SM22 and calponin gene expression (Table E2). The p38 MAPK and PI3K/Akt inhibitors, SB203580 and LY294002, respectively, exerted no detectable effect on TGF-β–elicited responses in the presence of FGF-2 (Figure 7).
Figure 7.
Extracellular signal-regulated kinase 1/2 signaling mediates the inhibitory effects of FGF-2 on TGF-stimulated SM22 expression. ASM cells were incubated with FGF-2 and/or TGF-β in the presence of the inhibitors PD98059 (PD, 10 μM), LY294002 (LY, 10 μM), or SB203580 (SB, 10 μM). (a) Concentrations of SM22 RNA and (b) SM22 protein in ASM cells after a 48-hour incubation. RNA and protein were analyzed by real-time PCR and Western blotting, respectively, with inset showing a representative blot. NS, P > 0.05. **P < 0.01 (n = 5–8 different primary cultures established from separate donors).
ETS1-like Transcription Factor-1 Knockdown Partly Reverses the Inhibitory Effect of FGF-2
Ets-like transcription factor-1 (Elk-1), a transcription factor phosphorylated downstream from extracellular signal-regulated kinase 1/2 (ERK 1/2) in the MEK pathway (26), binds to the SM22 promoter to regulate transcription (27). To investigate the role of Elk-1 in mediating the effects of FGF-2 on contractile protein expression, ASM cells were transfected with small interfering RNA (siRNA) targeting Elk-1, before incubation with TGF-β and FGF-2 for 48 hours. The transient transfection of Elk-1 siRNA reduced concentrations of Elk-1 mRNA by greater than 70%, and partly reversed the effect of FGF-2 on TGF-β–stimulated contractile protein expression (P < 0.05; Figure E8).
SRF–DNA Binding Activity Is Insufficient for the Inhibitory Function of FGF-2 on the Enhanced Response of TGF-β Stimulation
Previous work with ASM cells suggests that the stimulation of contractile protein gene expression requires the binding of SRF to the CArG box present in specific gene promoters (28). We show here that FGF-2 does not attenuate the TGF-β–stimulated increase of an artificial promoter that solely contains CArG sites and a minimal TATA, suggesting that other partners are needed in addition to SRF to elicit FGF-2 inhibitory functions (P > 0.05; Figure E9).
Discussion
The profibrotic cytokine TGF-β plays an important physiological (and pathophysiological) role in asthma (29), stimulating airway smooth muscle cells and fibroblasts to accumulate contractile proteins (7). Coincubation with the mitogen FGF-2 antagonized TGF-β responses in ASM cells, including the increased expression of the contractile apparatus proteins SM22 and calponin, the formation of ordered α-SMA filaments, and an increase in the ratio of F-actin to G-actin. These TGF-β responses are established markers of a contractile, hypertrophic mesenchymal phenotype (10, 30). The effect of FGF-2 on phenotype was associated with a reduction in cell stiffness, and with an inhibition of the compaction of untensioned three-dimensional collagen gels containing ASM cells, an assay that has been used to study the tractional remodeling of collagen fibrils by ASM cells (31). The effect of FGF-2 appears to be selective for TGF-β–regulated contractile protein expression and organization, because FGF-2 did not inhibit the TGF-β stimulation of IL-6 production, a response reported in previous studies (32).
FGF-2 inhibits corneal fibroblast differentiation into myofibroblasts (18). Similarly, acidic FGF (FGF-1) inhibits the differentiation (and transdifferentiation) of lung epithelial cells and fibroblasts into myofibroblasts (20, 21). Although FGF-2 is a potent mitogen of ASM cells (16), the lack of antagonism of TGF-β–stimulated contractile protein gene expression and organization by the powerful mitogens thrombin and FCS is not consistent with the idea that the antagonism of TGF-β’s effects is secondary to mitogenic signaling. This conclusion is further reinforced by observations that PDGF and EGF were much less effective than FGF-2 in inhibiting TGF–stimulated increases in contractile protein gene expression. Thus, although the inhibition of TGF-β–stimulated contractile protein expression in ASM is not an exclusive property of FGF-2, the response is much more pronounced than that for other growth factors of ASM. FGF-2 is also more effective than EGF in inhibiting the TGF-β–induced differentiation of multipotent dermal progenitor cells into myofibroblasts, whereas PDGF enhances this TGF-β response (33).
ASM cells in vivo are likely exposed to both FGF-2 and TGF-β (13–15). The combined effect of these molecules will depend on their abundance as well on spatial, temporal, and contextual factors. Similarly, the effect of FGF-2 on TGF-β–stimulated contractile protein expression in ASM cells may depend on culture conditions and the timing of FGF-2’s addition relative to TGF-β. Mutual influences of FGF-2 and TGF-β on gene expression may also contribute to the interactions between these two growth factors. For example, FGF-2 regulates the expression of TGF-β in airway macrophages (34). Conversely, the growth-like effect of TGF-β on renal fibroblasts is a consequence of its capacity to stimulate FGF-2 gene expression (35). Interactions between FGF-2 and TGF-β may also involve postreceptor signal transduction events. For example, the priming of ASM cells with TGF-β enhances the mitogenic effect of FGF-2, because both TGF-β and FGF-2 stimulate increases in the expression of the PDGF receptor and PDGF ligand, respectively (36). However, this type of coordinated response is unlikely to explain the antagonistic effects of FGF-2 on TGF-β–stimulated contractile expression reported here. In this study, in the majority of experiments, both FGF-2 and TGF-β were added to ASM cells simultaneously, and the inhibitory effect of FGF-2 was almost complete. This strongly suggests that the effect of FGF-2 is almost immediate, probably involving interactions between FGF-2 and the signaling pathways or the transcriptional apparatus that regulates contractile protein expression (and organization).
The simultaneous addition of FGF-2 and TGF-β has more relevance to a steady state in the airways than to the reversal of an asthma-like ASM phenotype, because the ASM cells have not yet differentiated into a hypercontractile phenotype. Therefore, we also examined the impact of the addition of FGF-2 24 hours after TGF-β, which is sufficient time for an increase in contractile protein gene expression. This model better represents the condition of ASM in established asthma. FGF-2 was able to reverse contractile protein gene expression in ASM cells incubated with TGF-β. This reversal does not appear to be dependent on an effect on TGF-β signaling, because FGF-2 was effective in the presence of delayed treatment with the TGF-β receptor kinase inhibitor, SB431542. Moreover, TGF-β–stimulated Smad2/3 phosphorylation was not attenuated by coincubation with FGF-2, regardless of the sequence of addition. Furthermore, TGF-β–stimulated increases in IL-6 concentrations, which are dependent on Smad2/3 signaling (37), were also not attenuated by FGF-2. In contrast to our observations in ASM cells, FGF-2 (or FGF-1) inhibits TGF-β–stimulated Smad 2/3 phosphorylation in aortic interstitial valvular cells (38) and in alveolar epithelial-like cell lines (20). However, TGF-β–stimulated Smad2/3 phosphorylation in nucleus pulposus cells is not attenuated by FGF-2 (39). These different functional interactions between FGF-2 and TGF-β are likely to be context-dependent and cell type–dependent.
Our findings beg the question of why FGF-2 does not cancel out the effects of TGF-β in vivo in the airways of patients with asthma. One plausible explanation states that the effective concentrations of FGF-2 and TGF-β within the microenvironment surrounding ASM create an imbalanced system in asthma, in which the concentrations of active FGF-2 are likely not sufficient to reduce contractile protein expression, especially in a profibrotic/inflamed environment. The threshold concentration for the effect of FGF-2 on TGF-β–stimulated contractile protein gene expression in our cell model was about 10–100 picomolar, depending on the gene and the concentration of TGF-β. Whether such concentrations of FGF-2 are physiologically (and pathophysiologically) relevant remains unknown, because concentrations of FGF-2 in asthmatic airways have been assessed primarily by immunohistochemistry (14). FGF-2 is largely associated with heparin as an inactive complex. Concentrations in the bronchoalveolar lavage fluid of patients with asthma (13) also provide little insight into the tissue concentrations of active FGF-2 near or within the ASM bundle in asthma.
The effects of FGF-2 occur primarily via binding to one of the four RTK isoforms (FGFR1–4), which activate the Ras/MAPK and PI3K/Akt signaling pathways (40, 41). In our study, inhibition of the MEK cascade abrogated the inhibitory effects of FGF-2 on TGF-β–stimulated SM22 and calponin expression. This effect occurred at a transcriptional level, as shown by the SM22 promoter reporter assay. It is feasible that Elk-1, a transcription factor phosphorylated downstream from the MEK (26), mediates the inhibitory effect of FGF-2 on contractile protein gene expression. Phosphorylated Elk-1 binds close to the distal CArG box in the SM22 promoter, facilitating complex formation between Elk-1 and SRF (27). This complex impedes myocardin binding to SRF, which is a required transcriptional cofactor for the SRF activation of TGF-β–responsive genes in mesenchymal cells. A cooperative effect of Elk-1 with SRF could explain why ERK pathway inhibition attenuated TGF-β–stimulated-SM22 promoter activity. One might have expected attenuation of the TGF-β response of the CArG box–dependent promoter by Elk-1 inhibition. However, unlike the natural SM22 promoter, the artificial CArG(x5) promoter does not contain an E twenty-six (ETS) domain binding site adjacent to the SRF binding site. Thus, Elk-1 could not influence CArG (SRF)–dependent promoter function. FGF-2 also does not inhibit the TGF-β–stimulated increase in SRF transcriptional activation in vascular smooth muscle cells when measured using a similar artificial CArG box reporter (42). The partial reversal of the inhibitory effect of FGF-2 on contractile protein expression by Elk-1 knockdown provides additional, albeit indirect, evidence of the role SRF suppression plays in mediating the antagonistic effects of FGF-2 in ASM cells.
Additional questions regard the mechanism by which FGF-2 mediates its antagonistic effects on ASM phenotype. For instance, why does thrombin, which also activates the MEK pathway in ASM cells (16), not inhibit TGF-β–stimulated SM22 and calponin gene expression? To propose one possibility, concomitant signaling pathways may also be required for FGF-2’s modulation of ASM phenotype. Indeed, FGF-2 activates noncanonical signaling cascades involving α(V)β(3) integrin and syndecans, which associate with focal adhesion complexes (43). Because these alternative receptors are highly abundant, their activation by FGF-2 can elicit diverse biological activity, even though the affinity of FGF-2 is lower than for the FGFRs. These alternative receptors may plausibly be involved in mediating the antagonistic effects of FGF-2 reported in this study. Supporting this possibility is the finding that α(V)β(3) integrin activation by vitronectin inhibits α-SMA production and the contractility of human lung fibroblasts (30).
The role of the actin cytoskeleton (CSK) of ASM cells is attracting interest for its possible contribution to airway obstruction in asthma, and particularly in muscle tension maintenance (44). Our findings suggest that the inhibition by FGF-2 of actin filament polymerization prevents cytoskeletal stiffness in ASM cells. The actin CSK is a deformable polymer network that plays a major role in maintaining cell shape (size), migration, and transmission, the distribution of mechanical forces within the cell, and signal transduction (45). The interactions of the actin CSK with the contractile apparatus and the ECM, involving integrins and focal adhesion complexes, are a key determinant of the mechanical and mechanosensing properties of ASM cells. The highly malleable actin CSK constantly rearranges, a process involving assembly and disassembly, in response to biophysical and chemical stimuli (46). Studies with dissociated cells showed that the modulation of ASM stiffness involves changes in the actin CSK organization (47). No actin-targeting drugs are in clinical use (48). Cytochalasins and latrunculins are cytotoxic, and they nonselectively disrupt actin filaments. Actin filaments may be targeted selectively to achieve a therapeutic effect by modulating the binding of specific actin-binding proteins. In this study, we found that FGF-2 selectively influences actin filaments to regulate ASM stiffness, hypertrophy, and the tractional remodeling of collagen in a noncytotoxic manner.
Asthma comprises structural changes to the AWR, including increased airway smooth muscle mass, which contributes to airway obstruction. No therapy specifically or effectively targets AWR. In experimental models of allergic asthma, the inhibition of TGF-β signaling attenuates AWR (11, 12). Our study shows that FGF-2 antagonizes and even reverses the pro-remodeling effects of TGF-β, thus supporting the therapeutic potential of exploiting this aspect of FGF-2’s action in the treatment of asthma. In an acute model of allergic asthma, the administration of recombinant FGF-2 attenuated airway hyperresponsiveness and goblet-cell metaplasia (17). FGF-2 has also been considered for other therapeutic applications in regenerative medicine. For instance, topical FGF-2 accelerates healing and lessens scarring in patients with second-degree burns (49). FGF-2 has been evaluated for “therapeutic angiogenesis” in patients with refractory angina (50), and shows no nontarget organ effects.
In conclusion, our study shows that FGF-2 inhibits the differentiation of ASM cells to a more contractile phenotype in response to TGF-β. This study provides evidence that FGF-2 anti-remodeling activity is mediated by multiple, possibly noncanonical, signaling mechanisms. The identification of these pathways may lead to the development of potential new therapies for AWR in asthma.
Supplementary Material
Acknowledgments
The authors thank the Department of Respiratory Medicine, the Department of Surgery, and the Department of Anatomical Pathology of Alfred Hospital, Prahran, Victoria, Australia, and Professor Catriona MacClean for assistance in obtaining human biopsies.
Footnotes
This work was supported by National Health and Medical Research Council (Australia) research grants 509001, 1045372 and by National Institutes of Health grant K01HL092588 (B.C.M.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0151OC on December 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Amin K, Ludviksdottir D, Janson C, Nettelbladt O, Bjornsson E, Roomans GM, Boman G, Seveus L, Venge P. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma: BHR Group. Am J Respir Crit Care Med 2000;162:2295–2301 [DOI] [PubMed] [Google Scholar]
- 2.Redington AE. Airway fibrosis in asthma: mechanisms, consequences, and potential for therapeutic intervention. Monaldi Arch Chest Dis 2000;55:317–323 [PubMed] [Google Scholar]
- 3.Wilson JW, Li X, Pain MC. The lack of distensibility of asthmatic airways. Am Rev Respir Dis 1993;148:806–809 [DOI] [PubMed] [Google Scholar]
- 4.Camoretti-Mercado B. Targeting the airway smooth muscle for asthma treatment. Transl Res 2009;154:165–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart AG, Tomlinson PR, Wilson J. Airway wall remodelling in asthma: a novel target for the development of anti-asthma drugs. Trends Pharmacol Sci 1993;14:275–279 [DOI] [PubMed] [Google Scholar]
- 6.Coutts A, Chen G, Stephens N, Hirst S, Douglas D, Eichholtz T, Khalil N. Release of biologically active TGF-beta from airway smooth muscle cells induces autocrine synthesis of collagen. Am J Physiol Lung Cell Mol Physiol 2001;280:L999–L1008 [DOI] [PubMed] [Google Scholar]
- 7.Goldsmith AM, Bentley JK, Zhou L, Jia Y, Bitar KN, Fingar DC, Hershenson MB. Transforming growth factor–beta induces airway smooth muscle hypertrophy. Am J Respir Cell Mol Biol 2006;34:247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camoretti-Mercado B, Solway J. Transforming growth factor–beta1 and disorders of the lung. Cell Biochem Biophys 2005;43:131–148 [DOI] [PubMed] [Google Scholar]
- 9.Bhogal RK, Stoica CM, McGaha TL, Bona CA. Molecular aspects of regulation of collagen gene expression in fibrosis. J Clin Immunol 2005;25:592–603 [DOI] [PubMed] [Google Scholar]
- 10.Solway J, Forsythe SM, Halayko AJ, Vieira JE, Hershenson MB, Camoretti-Mercado B. Transcriptional regulation of smooth muscle contractile apparatus expression. Am J Respir Crit Care Med 1998;158:S100–S108 [DOI] [PubMed] [Google Scholar]
- 11.Bottoms SE, Howell JE, Reinhardt AK, Evans IC, McAnulty RJ. TGF-beta isoform specific regulation of airway inflammation and remodelling in a murine model of asthma. PLoS ONE 2010;5:e9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMillan SJ, Xanthou G, Lloyd CM. Manipulation of allergen-induced airway remodeling by treatment with anti–TGF-beta antibody: effect on the Smad signaling pathway. J Immunol 2005;174:5774–5780 [DOI] [PubMed] [Google Scholar]
- 13.Redington AE, Roche WR, Madden J, Frew AJ, Djukanovic R, Holgate ST, Howarth PH. Basic fibroblast growth factor in asthma: measurement in bronchoalveolar lavage fluid basally and following allergen challenge. J Allergy Clin Immunol 2001;107:384–387 [DOI] [PubMed] [Google Scholar]
- 14.Shute JK, Solic N, Shimizu J, McConnell W, Redington AE, Howarth PH. Epithelial expression and release of FGF-2 from heparan sulphate binding sites in bronchial tissue in asthma. Thorax 2004;59:557–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans MJ, Van Winkle LS, Fanucchi MV, Baker GL, Murphy AE, Nishio SJ, Schelegle ES, Gershwin LJ, Sannes PL, Plopper CG. Fibroblast growth factor–2 in remodeling of the developing basement membrane zone in the trachea of infant rhesus monkeys sensitized and challenged with allergen. Lab Invest 2002;82:1747–1754 [DOI] [PubMed] [Google Scholar]
- 16.Ravenhall C, Guida E, Harris T, Koutsoubos V, Stewart A. The importance of ERK activity in the regulation of cyclin D1 levels and DNA synthesis in human cultured airway smooth muscle. Br J Pharmacol 2000;131:17–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeon SG, Lee CG, Oh MH, Chun EY, Gho YS, Cho SH, Kim JH, Min KU, Kim YY, Kim YK, et al. Recombinant basic fibroblast growth factor inhibits the airway hyperresponsiveness, mucus production, and lung inflammation induced by an allergen challenge. J Allergy Clin Immunol 2007;119:831–837 [DOI] [PubMed] [Google Scholar]
- 18.Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast growth factor reversal of the corneal myofibroblast phenotype. Invest Ophthalmol Vis Sci 2001;42:2490–2495 [PubMed] [Google Scholar]
- 19.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1–mediated induction of pericyte alpha–smooth muscle actin expression: a role for MYF-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci 2003;44:4994–5005 [DOI] [PubMed] [Google Scholar]
- 20.Ramos C, Becerril C, Montano M, Garcia-De-Alba C, Ramirez R, Checa M, Pardo A, Selman M. FGF-1 reverts epithelial–mesenchymal transition induced by TGF-{beta}1 through MAPK/ERK kinase pathway. Am J Physiol Lung Cell Mol Physiol 2010;299:L222–L231 [DOI] [PubMed] [Google Scholar]
- 21.Ramos C, Montano M, Becerril C, Cisneros-Lira J, Barrera L, Ruiz V, Pardo A, Selman M. Acidic fibroblast growth factor decreases alpha–smooth muscle actin expression and induces apoptosis in human normal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol 2006;291:L871–L879 [DOI] [PubMed] [Google Scholar]
- 22.Fernandes D, Guida E, Koutsoubos V, Harris T, Vadiveloo P, Wilson JW, Stewart AG. Glucocorticoids inhibit proliferation, cyclin D1 expression, and retinoblastoma protein phosphorylation, but not activity of the extracellular-regulated kinases in human cultured airway smooth muscle. Am J Respir Cell Mol Biol 1999;21:77–88 [DOI] [PubMed] [Google Scholar]
- 23.Schuliga MJ, See I, Ong SC, Soon L, Camoretti-Mercado B, Harris T, Stewart AG. Fibrillar collagen clamps lung mesenchymal cells in a nonproliferative and noncontractile phenotype. Am J Respir Cell Mol Biol 2009;41:731–741 [DOI] [PubMed] [Google Scholar]
- 24.Schuliga M, Harris T, Stewart AG. Plasminogen activation by airway smooth muscle is regulated by Type I collagen. Am J Respir Cell Mol Biol 2011;44:831–839 [DOI] [PubMed] [Google Scholar]
- 25.Hochmuth RM. Micropipette aspiration of living cells. J Biomech 2000;33:15–22 [DOI] [PubMed] [Google Scholar]
- 26.Buchwalter G, Gross C, Wasylyk B. ETS ternary complex transcription factors. Gene 2004;324:1–14 [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Wang DZ, Hockemeyer D, McAnally J, Nordheim A, Olson EN. Myocardin and ternary complex factors compete for SRF to control smooth muscle gene expression. Nature 2004;428:185–189 [DOI] [PubMed] [Google Scholar]
- 28.Camoretti-Mercado B, Liu HW, Halayko AJ, Forsythe SM, Kyle JW, Li B, Fu Y, McConville J, Kogut P, Vieira JE, et al. Physiological control of smooth muscle–specific gene expression through regulated nuclear translocation of serum response factor. J Biol Chem 2000;275:30387–30393 [DOI] [PubMed] [Google Scholar]
- 29.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-beta in airway remodeling in asthma. Immunol Cell Biol 2007;85:348–356 [DOI] [PubMed] [Google Scholar]
- 30.Scaffidi AK, Moodley YP, Weichselbaum M, Thompson PJ, Knight DA. Regulation of human lung fibroblast phenotype and function by vitronectin and vitronectin integrins. J Cell Sci 2001;114:3507–3516 [DOI] [PubMed] [Google Scholar]
- 31.Bourke JE, Li X, Foster SR, Wee E, Dagher H, Ziogas J, Harris T, Bonacci JV, Stewart AG. Collagen remodelling by airway smooth muscle is resistant to steroids and beta-agonists. Eur Respir J 2011;37:173–182 [DOI] [PubMed] [Google Scholar]
- 32.Elias JA, Wu Y, Zheng T, Panettieri R. Cytokine- and virus-stimulated airway smooth muscle cells produce IL-11 and other IL-6–type cytokines. Am J Physiol 1997;273:L648–L655 [DOI] [PubMed] [Google Scholar]
- 33.Tiede S, Ernst N, Bayat A, Paus R, Tronnier V, Zechel C. Basic fibroblast growth factor: a potential new therapeutic tool for the treatment of hypertrophic and keloid scars. Ann Anat 2009;191:33–44 [DOI] [PubMed] [Google Scholar]
- 34.Yum HY, Cho JY, Miller M, Broide DH. Allergen-induced coexpression of bFGF and TGF-beta1 by macrophages in a mouse model of airway remodeling: bFGF induces macrophage TGF-beta1 expression in vitro. Int Arch Allergy Immunol 2011;155:12–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, Muller G. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2). Kidney Int 2001;59:579–592 [DOI] [PubMed] [Google Scholar]
- 36.Bosse Y, Thompson C, Stankova J, Rola-Pleszczynski M. Fibroblast growth factor 2 and transforming growth factor beta1 synergism in human bronchial smooth muscle cell proliferation. Am J Respir Cell Mol Biol 2006;34:746–753 [DOI] [PubMed] [Google Scholar]
- 37.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2011;300:L295–L304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J 2008;22:1769–1777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsai TT, Guttapalli A, Oguz E, Chen LH, Vaccaro AR, Albert TJ, Shapiro IM, Risbud MV. doi: 10.1097/01.brs.0000257341.88880.f1. 2007. Fibroblast growth factor–2 maintains the differentiation potential of nucleus pulposus cells in vitro: implications for cell-based transplantation therapy. Spine (Phila) 1976;32:495–502. [DOI] [PubMed] [Google Scholar]
- 40.Choi JW, Kim S, Kim TM, Kim YM, Seo HW, Park TS, Jeong JW, Song G, Han JY. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS ONE 2010;5:e12968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kouhara H, Hadari YR, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored GRB2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 1997;89:693–702 [DOI] [PubMed] [Google Scholar]
- 42.Kawai-Kowase K, Sato H, Oyama Y, Kanai H, Sato M, Doi H, Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor–beta1–induced smooth muscle gene expression through extracellular signal–regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol 2004;24:1384–1390 [DOI] [PubMed] [Google Scholar]
- 43.Murakami M, Elfenbein A, Simons M. Non-canonical fibroblast growth factor signalling in angiogenesis. Cardiovasc Res 2008;78:223–231 [DOI] [PubMed] [Google Scholar]
- 44.Gunst SJ, Zhang W. Actin cytoskeletal dynamics in smooth muscle: a new paradigm for the regulation of smooth muscle contraction. Am J Physiol Cell Physiol 2008;295:C576–C587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gunst SJ, Tang DD. The contractile apparatus and mechanical properties of airway smooth muscle. Eur Respir J 2000;15:600–616 [DOI] [PubMed] [Google Scholar]
- 46.Deng L, Bosse Y, Brown N, Chin LY, Connolly SC, Fairbank NJ, King GG, Maksym GN, Pare PD, Seow CY, et al. Stress and strain in the contractile and cytoskeletal filaments of airway smooth muscle. Pulm Pharmacol Ther 2009;22:407–416 [DOI] [PubMed] [Google Scholar]
- 47.Smith PG, Roy C, Zhang YN, Chauduri S. Mechanical stress increases RhoA activation in airway smooth muscle cells. Am J Respir Cell Mol Biol 2003;28:436–442 [DOI] [PubMed] [Google Scholar]
- 48.Stehn JR, Schevzov G, O'Neill GM, Gunning PW. Specialisation of the tropomyosin composition of actin filaments provides new potential targets for chemotherapy. Curr Cancer Drug Targets 2006;6:245–256 [DOI] [PubMed] [Google Scholar]
- 49.Akita S, Akino K, Imaizumi T, Hirano A. Basic fibroblast growth factor accelerates and improves second-degree burn wound healing. Wound Repair Regen 2008;16:635–641 [DOI] [PubMed] [Google Scholar]
- 50.Aviles RJ, Annex BH, Lederman RJ. Testing clinical therapeutic angiogenesis using basic fibroblast growth factor (FGF-2). Br J Pharmacol 2003;140:637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.