Abstract
Mechanical ventilation with supraphysiological concentrations of oxygen (hyperoxia) is routinely used to treat patients with respiratory distress. However, a significant number of patients on ventilators exhibit enhanced susceptibility to infections and develop ventilator-associated pneumonia (VAP). Pseudomonas aeruginosa (PA) is one of the most common species of bacteria found in these patients. Previously, we demonstrated that prolonged exposure to hyperoxia can compromise the ability of alveolar macrophages (AMs), an essential part of the innate immunity, to phagocytose PA. This study sought to investigate the potential molecular mechanisms underlying hyperoxia-compromised innate immunity against bacterial infection in a murine model of PA pneumonia. Here, we show that exposure to hyperoxia (≥ 99% O2) led to a significant elevation in concentrations of airway high mobility group box–1 (HMGB1) and increased mortality in C57BL/6 mice infected with PA. Treatment of these mice with a neutralizing anti-HMGB1 monoclonal antibody (mAb) resulted in a reduction in bacterial counts, injury, and numbers of neutrophils in the lungs, and an increase in leukocyte phagocytic activity compared with mice receiving control mAb. This improved phagocytic function was associated with reduced concentrations of airway HMGB1. The correlation between phagocytic activity and concentrations of extracellular HMGB1 was also observed in cultured macrophages. These results indicate a pathogenic role for HMGB1 in hyperoxia-induced impairment with regard to a host’s ability to clear bacteria and inflammatory lung injury. Thus, HMGB1 may provide a novel molecular target for improving hyperoxia-compromised innate immunity in patients with VAP.
Keywords: hyperoxia, bacterial infection, macrophage function, HMGB1
Oxygen therapy using mechanical ventilation (MV) with supraphysiological concentrations of oxygen (i.e., hyperoxia) is a life-saving intervention for patients with respiratory distress. During an average day, MV is administered to at least 40% of the patients in an American intensive care unit (1). However, the application of MV is associated with many adverse effects on the respiratory system, including increased airway injury, alveolar damage, and susceptibility to microbial infections (2–4). Ventilator-associated pneumonia (VAP) has been defined as pneumonia occurring more than 48 hours after the initiation of endotracheal intubation and MV (5–7). VAP affects 8 to 28% of patients receiving MV (8, 9). According to the National Nosocomial Infection Survey, 86% of nosocomial pneumonia is associated with MV (10). VAP accounts for up to 60% of all deaths from healthcare-associated infections in United States (7), and continues to be a major cause of high morbidity and mortality for patients on ventilators (11–14).
Bacterial infections comprise one of the major contributors to VAP (8, 15). Both gram-negative and gram-positive strains of bacteria are frequently found in patients with VAP (8, 15). Pseudomonas aeruginosa (PA), a gram-negative aerobic bacterium, was reported to be associated with 21% of all nosocomial pneumonia cases (10). The overall prevalence of PA infections has been reported at approximately 0.4% in United States hospitals (http://www.cdc.gov/). Although antibiotics are routinely used, the management of PA infections in VAP remains difficult and complex because of their resistance to antibiotics (8, 15–17). Therefore, novel approaches are needed to enhance the efficacy of VAP treatment.
Corresponding to the poor clinical outcomes for patients with VAP, the mechanisms underlying the pathogenesis of VAP are not well elucidated. Invading microorganisms are cleared by host defenses, including innate immunity (18, 19). Both resident and recruited phagocytes are involved in the innate immunity to clear bacteria from the lungs and airways (20, 21). Alveolar macrophages (AMs) are professional phagocytes that reside in the airways (18). By engulfing and killing the invading pathogens, AMs form the first line of cell-mediated defense in the respiratory tract (19, 22, 23). We and others have previously shown that exposure to prolonged hyperoxia, which is routinely used during MV (24, 25), can compromise the ability of AMs to phagocytose PA (26, 27) and other bacteria, including Klebsiella pneumoniae (28, 29). Despite identifying the involvement of reactive oxygen species (ROS) (26), little is known about the downstream events that lead to the deleterious effects of prolonged hyperoxia on macrophage functions and the host defense system, and whether compromised macrophage function results in abridged survival in PA pneumonia.
We recently reported on the role of high mobility group box (HMGB)–1 in the phagocytic activity of AMs and host defense (30). HMGB1 belongs to the high mobility group family of nuclear proteins (31). In the nucleus, HMGB1 acts as a cotranscriptional factor and is implicated in stabilizing nucleosomes and regulating transcription and DNA repair (32–34). However, HMGB1 can be released into the extracellular milieu from immune cells in response to exogenous bacterial endotoxins or endogenous proinflammatory cytokines (35, 36). Once released, extracellular HMGB1 acts as an inflammatory cytokine, leading to lung injury and multiple organ failure (34, 35). In addition to its role as a proinflammatory cytokine, HMGB1 has been shown to play a role in bacterial pneumonia (30). Pronounced PA infection, a hallmark of cystic fibrosis (CF), occurs in the majority of adult patients (37, 38). We found that concentrations of airway HMGB1 were markedly increased in patients with CF, and elevated concentrations of airway HMGB1 can directly diminish the phagocytic activity of AMs (30). Using a murine model of PA pneumonia and cultured murine macrophages, we investigated in this study whether (1) exposure to hyperoxia induces an elevation in concentrations of airway HMGB1, and (2) HMGB1 plays a role in hyperoxia-compromised host defense in clearing bacteria and macrophage phagocytic function.
Materials and Methods
Special Reagents
Neutralizing anti-HMGB1 monoclonal and polyclonal antibodies were generated as described previously (35, 39). Briefly, recombinant HMGB1 with a purity of less than 0.1 pg/μg endotoxin was generated using rat HMGB1 cDNA, which was cloned onto a pCAL-n vector (Stratagene, La Jolla, CA) and expressed in Escherichia coli BL21(DE3)pLysS cells (35, 36, 40). Contaminating endotoxin was removed from HMGB1 preparations by Triton X-114 extraction (41). The extent of endotoxin contamination was assessed using the chromogenic Limulus amebocyte lysate assay (Endochrome; Charles River, Charleston, SC). Green fluorescent protein–PAO1, a nonmucoid strain of Pseudomonas aeruginosa, was cultured as described previously (42, 43).
Bronchoalveolar Lavage
Murine bronchoalveolar lavage (BAL) fluid was obtained as described previously (26, 44, 45). Briefly, mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (120 mg/kg). After a 1- to 2-cm incision was made on the neck, the trachea was dissected, and a 20-gauge × 1.25-inch intravenous catheter was inserted caudally into the lumen of the exposed trachea. The lungs were gently lavaged twice with 1 ml sterile, nonpyrogenic PBS solution (Mediatech, Inc., Hendon, VA). The BAL samples were centrifuged, and the resultant supernatants were stored in a freezer at −80°C for analyzing concentrations of HMGB1 and the total protein content, using Western blot analysis and a bicinchoninic acid assay.
Animal Studies
The experimental use of animals presented in this study was approved by the Institutional Animal Care and Use Committees of St. John’s University. The mice were housed in a specific pathogen–free environment. C57BL/6 mice obtained from the Jackson Laboratory (Bar Harbor, ME) were inoculated with 5 × 108 colony-forming units (CFUs) of PA (46) via intranasal aspiration after brief anesthesia with isoflurane. Mice were randomized to receive either neutralizing anti-HMGB1 (α-HMGB1) monoclonal antibody (mAb) or an isotypic control mAb, administrated by intraperitoneal injection 24 hours before bacterial inoculation, during hyperoxia. Twenty-four hours after bacterial inoculation, mice were anesthetized with intraperitoneal sodium pentobarbital (120 mg/kg) to obtain BAL and lung tissues, as depicted in Figure 1. In brief, mice were anesthetized and tracheas were dissected and cannulated, as already described. After lavage with PBS, the lungs were excised and immediately placed into 1 ml of cold PBS and homogenized.
Figure 1.
Antibody treatment protocol for mice with Pseudomonas aeruginosa (PA) infection. Male C57BL/6 mice were exposed to ≥ 99% O2 for 48 hours, followed by inoculation with PA (5 × 108 colony-forming units [CFUs]) via intranasal aspiration, and returned to 21% O2 after inoculation. These mice were randomized to receive either neutralizing anti-HMGB1 (αHMGB1) monoclonal antibody (mAb) or an isotypic control mAb intraperitoneally after 24 hours during hyperoxic exposure. Bronchoalveolar lavage (BAL) and lung tissues were harvested 24 hours after infection. The total protein content in BAL was measured using the bicinchoninic acid protein assay. The numbers of viable bacteria in the BAL and lungs were determined by plating serial dilutions of BAL and homogenized lung tissue. HMGB1, high mobility group box–1.
Exposure to Hyperoxia
Male C57BL/6 mice were placed in micro-isolator cages (Allentown Caging Equipment Co., Inc., Allentown, NJ), which were kept in a Plexiglas chamber (BioSpherix, Lacona, NY) and exposed to ≥ 99% O2 for up to 96 hours. The exposure of murine macrophage-like RAW 264.7 cells (American Type Culture Collection, Manassas, VA) was achieved in sealed, humidified Plexiglas chambers (Billups-Rothenberg, Inc., Del Mar, CA) flushed with 95% O2/5% CO2 at 37°C. An oxygen analyzer (MSA; Medical Products, Pittsburgh, PA) was used to monitor the O2 concentration in the chamber.
Phagocytosis Assay
The phagocytic activity of leukocytes was determined as described previously, with minor modifications (26). Male C57BL/6 mice were exposed to ≥ 99% O2 for 48 hours and treated with 50 μg/mouse of either anti-HMGB1 mAb (αHMGB1) or isotype control mAb, 24 hours before inoculation with PA and opsonized FITC-labeled latex beads (Polysciences, Warrington, PA). Isolated leukocytes were then treated with 0.04% trypan blue and washed with cold (4°C) PBS to quench the extracellular adherent particles. To visualize the uptake of PA and FITC-labeled latex beads, leukocytes were fixed with 2% paraformaldehyde, washed with PBS, and stained with Texas Red X–phalloidin (Molecular Probes, Eugene, OR) in 1% BSA. The slides were analyzed using an epifluorescence microscope (Nikon, Melville, NY). The uptake of beads by leukocytes was quantified by counting at least 100 leukocytes per slide.
Measurement of HMGB1
Concentrations of HMGB1 in BAL were determined using an immunoblotting analysis with anti-HMGB1 antibody, as described previously (35). In brief, samples were separated on SDS-PAGE. Proteins were electrotransferred to a polyvinyledene difluoride membrane, and then blocked with 5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20. The membrane was incubated with anti-HMGB1, and then with anti-rabbit horseradish peroxidase–coupled secondary antibodies (Bio-Rad, Hercules, CA). After washing, antibody binding was detected by enhanced chemiluminescence plus Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ). Western blots were scanned with a UVP Biospectrum 600 Imaging System (Vision Works LS, Upland, CA), and band intensities were quantified using Image acquisition and analysis software, version 6.8 (Vision Works LS, Upland, CA).
Quantitative Bacteriology
Viable bacterial counts in airways and lungs were determined using a CFU assay by plating serial dilutions of the BAL and lung homogenates, respectively, onto Pseudomonas Isolation Agar (Difco, Sparks, MD), and culturing at 37°C as already described.
Statistical Analysis
Results are presented as means ± SEMs from at least three independent experiments. The data were analyzed for statistical significance according to paired and unpaired t tests, ANOVA, or Kaplan-Meier analysis, using Microsoft Excel (Microsoft Corp., Seattle, WA). P ≤ 0.05 was considered statistically significant.
Results
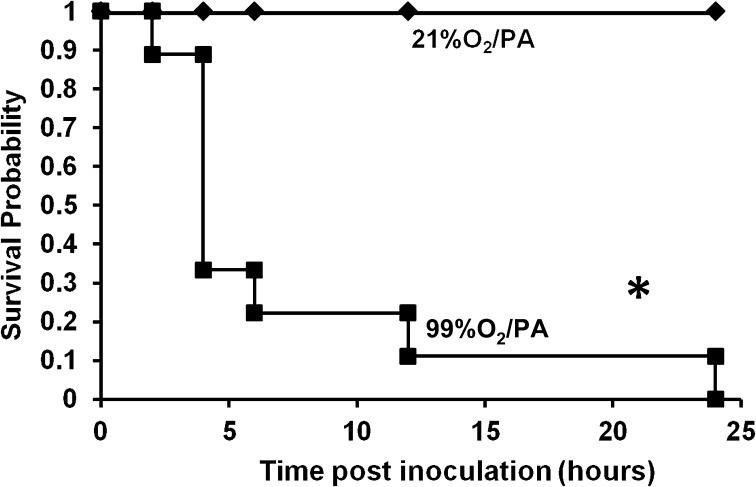
Exposure to Hyperoxia Increases the Mortality of Mice with PA Pneumonia
Previously, we have shown that exposure to hyperoxia reduces the phagocytic ability of AMs to engulf PA (26). To determine whether the hyperoxia-compromised phagocytic function of AMs affects an animal’s host defense against PA infection, C57BL/6 mice were exposed to ≥ 99% O2 (hyperoxia) for 72 hours, and then intranasally inoculated with PA. Mice exposed to hyperoxia exhibited clinical signs of severe illness, including lethargy and huddling together in the corners of their cages (data not shown). Seventy percent of the mice in this group died within the first 4 hours after inoculation, and none had survived by the end of 24 hours (Figure 2). In contrast, mice remaining at 21% O2 before inoculation with PA were relatively active, and all continued to survive 24 hours after infection (Figure 2). In addition, exposure to hyperoxia for up to 96 hours without PA infection did not induce any lethality in C57BL/6 mice (data not shown). These results indicate that exposure to hyperoxia not only reduces the ability of AM to phagocytose bacteria, but also compromises the survival of animals with PA infection.
Figure 2.
Exposure to hyperoxia increases the mortality of mice with bacterial pneumonia. Male C57BL/6 mice were exposed to ≥ 99% O2 for 72 hours (or 21% O2 in control mice). After the exposure, mice in both groups were intranasally inoculated with PA (5 × 108 CFUs/mouse), returned to 21% O2, and observed for survival up to 24 hours. Data were analyzed using Kaplan-Meier analysis (n = 9 mice per group). *P < 0.05, compared with 21% O2/PA mice.
Exposure to Hyperoxia Reduces Bacterial Clearance from the Lung in PA Pneumonia
To determine the mechanism underlying hyperoxia-increased mortality in PA pneumonia, bacterial loads in the lungs were assessed in mice exposed to ≥ 99% O2 (48 hours) and inoculated with PA. The bacterial load in lung lavage fluids was 6-fold greater (149.33 ± 37.92 × 104 CFUs/ml versus 24.91 ± 6.92 × 104 CFUs/ml of BAL, P < 0.01; Figure 3A) in mice exposed to hyperoxia than in mice remaining at 21% O2. Similar results were observed in the lung tissue of these mice (61.77 ± 9.51 × 104 CFUs/ml versus 8.78 ± 2.16 × 104 CFUs/ml, P < 0.01; Figure 3B). These results indicate that mice exposed to hyperoxia have a compromised ability to clear PA from the lung and airways, suggesting that higher bacterial loads in the lung contribute to the increased mortality of these animals.
Figure 3.
Exposure to hyperoxia reduces bacterial clearance in the lung. Male C57BL/6 mice were exposed to ≥ 99% O2 for 48 hours (or 21% O2 in control mice), and then inoculated with PA (5 × 108 CFUs/mouse). Mice were killed 24 hours after inoculation, and the BAL and lungs were harvested. Viable bacteria in airways and lungs were quantified by plating serial dilutions of BAL (A) and homogenized lung (B), and are expressed as CFUs/ml of BAL and CFUs/lung, respectively. Data represent means ± SEMs from three independent experiments (n = 9 mice per group). **P < 0.01, compared with 21% O2/PA mice.
Exposure to Hyperoxia Increases Concentrations of Airway HMGB1
Recent studies in our laboratory revealed a novel role for extracellular HMGB1 in impairing the function of AMs in phagocytosis (30). To investigate whether extracellular HMGB1 is responsible for hyperoxia-suppressed bacterial clearance in PA pneumonia, concentrations of airway HMGB1 were determined in lung lavage fluids isolated from mice exposed to hyperoxia. No concentration of HMGB1 was detectable in mice that remained at 21% O2. However, exposure to hyperoxia resulted in an incremental elevation in concentrations of airway HMGB1 in the BAL of mice exposed to ≥ 99% O2 for up to 96 hours (162% ± 8%, 262% ± 107%, 347% ± 103%, and 437% ± 73% at 24 hours, 48 hours, 72 hours, and 96 hours, respectively; Figure 4).
Figure 4.
Exposure to hyperoxia increases concentrations of airway HMGB1. Male C57BL/6 mice were exposed to ≥ 99% O2 for different periods of time (24, 48, 72, and 96 hours). Mice were killed at the end of exposure to hyperoxia, and BAL fluids and lungs were harvested. HMGB1 concentrations in the BAL were measured by Western blot analysis. Data represent the means ± SEMs from three independent experiments (n = 4–6 mice per group). *P < 0.05, compared with mice remaining at 21% O2.
Pretreatment with Neutralizing Anti-HMGB1 mAb Reduces Bacterial Load in the Lung and Ameliorates Acute Lung Injury
To determine whether elevated concentrations of airway HMGB1 play a causal role in hyperoxia-compromised bacterial clearance, neutralizing anti-HMGB1 mAb was administered to mice. During exposure to ≥ 99% O2, C57BL/6 male mice were treated with either neutralizing anti-HMGB1 mAb or isotype control mAb (control group) at 24 hours (Figure 1). Treatment with anti-HMGB1 mAb significantly reduced bacterial counts in the airways (0.21 ± 0.04 × 106 CFUs/ml versus 15.07 ± 0.23 × 106 CFUs/ml of BAL, P < 0.01; Figure 5A) and in the lungs (0.41 ± 0.04 × 106 CFUs/ml versus 40.20 ± 2.82 × 106 CFUs/ml, P < 0.01; Figure 5B). In addition, anti-HMGB1 mAb also significantly lowered the total protein content (a marker of lung injury) in the airways, compared with the total protein content of mice treated with control mAb (6.46 ± 1.7 × 102 μg/ml versus 38.66 ± 5.8 × 102 μg/ml, P < 0.01; Figure 6A). These data suggest that HMGB1 plays a critical role in hyperoxia-impaired bacterial clearance in PA pneumonia and bacteria-elicited lung injury.
Figure 5.
Anti-HMGB1 mAb reduces the bacterial load in lungs during PA pneumonia. The experiment with antibody treatment was performed as described in Figure 1. Viable bacteria in the airways and lungs were quantified by plating serial dilutions of BAL (A) and homogenized lung (B), and are expressed as CFUs/ml of BAL and CFUs/lung, respectively. Data represent the means ± SEMs from three independent experiments (n = 6 mice per group). **P < 0.01, compared with mice receiving control mAb.
Figure 6.
Anti-HMGB1 mAb reduces lung injury and leukocyte infiltration into the lung. The experiment with antibody treatment was performed as described in Figure 1. The total protein content in the BAL (A) was measured as a marker of lung injury. Total leukocyte counts in the BAL (C) and in lung sections with hematoxylin and eosin staining (B) were analyzed for leukocyte infiltration. Arrows indicate leukocytes. Data represent the means ± SEMs from three independent experiments (n = 4–9 mice per group). Ab, antibody; PMNs, polymorphonuclear leukocytes. #P < 0.05, compared with 21% O2/PA mice. **P < 0.01, compared with mice receiving control mAb.
Pretreatment with Neutralizing Anti-HMGB1 mAb Reduces Numbers of Leukocytes in the Lung
Leukocyte recruitment is important in clearing bacterial infections from the lung (43, 47–49). To determine the cellular mechanisms underlying the HMGB1-mediated impairment of host defense in PA pneumonia, numbers of leukocytes in the lung were assessed. Histological examination showed fewer leukocytes in the lungs of mice treated with anti-HMGB1 mAb (Figure 6B). In addition, the numbers of leukocytes in lung lavage fluids were significantly reduced in mice treated with anti-HMGB1 mAb compared with control mice (850.48 ± 117.1 × 104 versus 107.69 ± 36.75 × 104, P < 0.01; Figure 6C). Furthermore, numbers of neutrophils were markedly lower in airways of mice that received anti-HMGB1 mAb (347.07 ± 80.32 × 104 versus 35.51 ± 15.97 × 104, P < 0.01; Figure 6C). Therefore, the improved host defense against bacterial infection in mice treated with anti-HMGB1 mAb is not associated with increased numbers of leukocytes, especially neutrophils, in the lungs.
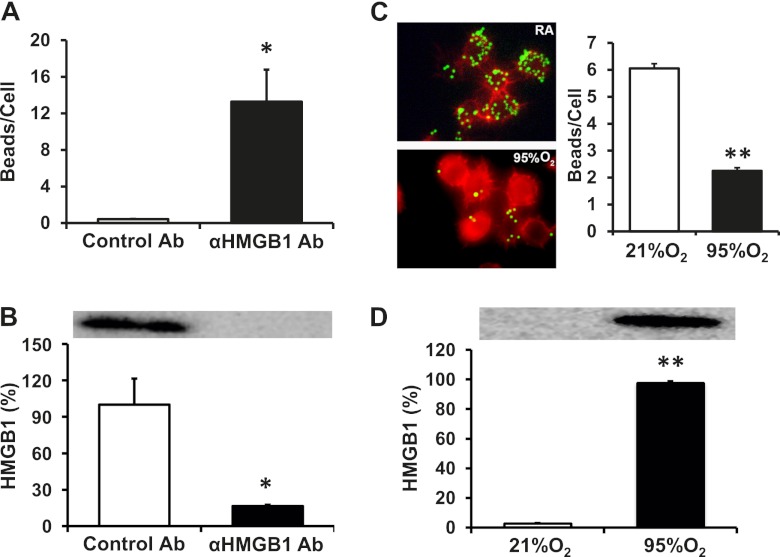
Anti-HMGB1 mAb Attenuates Hyperoxia-Compromised Leukocyte Phagocytic Activity and Reducesthe Concentrations of Airway HMGB1
To investigate further the potential cellular mechanisms underlying the improved host defense against PA infection with reduced numbers of neutrophils in the lungs of mice receiving anti-HMGB1 mAb, the phagocytic activity of lung leukocytes was assessed. The phagocytic activity of leukocytes in lung lavage fluids was significantly elevated in mice treated with anti-HMGB1 mAb, compared with control mice (0.42 ± 0.03 versus 13.28 ± 3.5 beads/cell, P < 0.05; Figure 7A). These data suggest that the HMGB1-mediated impairment of host defense is triggered by altering leukocyte function in bacterial clearance. Interestingly, the increased phagocytic activity of leukocytes in mice treated with anti-HMGB1 mAb was associated with a decreased accumulation of airway HMGB1 (80% less than that of control mice, P < 0.05; Figure 7B). Previously, we demonstrated that AMs isolated from mice exposed to hyperoxia exhibit significantly reduced phagocytic activity (26). To determine whether elevated concentrations of extracellular HMGB1 contribute to the molecular mechanisms underlying hyperoxia-compromised macrophage function, the phagocytic activity and concentrations of extracellular HMGB1 were analyzed in cultured macrophages. The phagocytic activity of RAW 264.7 cells, after exposure to 95% O2 for 24 hours, was reduced by approximately 3-fold (6.08 ± 0.21 versus 2.25 ± 0.11, P < 0.01; Figure 7C), whereas concentrations of extracellular HMGB1 in the culture media were 35-fold higher compared with those of cells remaining at 21% O2. These results suggest that HMGB1 mediates hyperoxia-impaired macrophage function and host defense in bacterial clearance during PA pneumonia.
Figure 7.
Extracellular HMGB1 compromises the leukocyte function of phagocytosis. The experiment with antibody treatment was performed as described in Figure 1. Airway leukocytes were isolated from BAL samples. RAW 264.7 cells were exposed to 95% O2 and incubated with fluorescent latex beads. Phagocytosis by leukocytes (ex vivo) (A) and phagocytosis by RAW cells (in vitro) (C) were expressed as beads/cell. At least 100 cells/slide were counted. HMGB1 concentrations in the BAL (B) and cell culture media (D) were measured by Western blot analysis. Data represent the means ± SEMs from three independent experiments (n = 4–6 mice per group). *P < 0.05, compared with mice receiving control mAb. **P < 0.01, compared with cells remaining at 21% O2 control level.
Discussion
We have previously shown that prolonged exposure to hyperoxia can compromise the function of macrophages to phagocytose PA. In this study, we further investigated the effects of hyperoxia on animal survival in PA pneumonia, and explored the potential underlying cellular and molecular mechanisms. We demonstrate that prolonged exposure to hyperoxia results in both a marked increase in animal mortality from PA pneumonia and an impaired host defense to effectively clear PA from the lung. Hyperoxia-compromised host defense was associated with a substantial elevation in concentrations of airway HMGB1. Interestingly, not only did treatment of mice with anti-HMGB1 mAb effectively reduce both leukocyte counts in the lung and the extent of inflammatory lung injury, but it also significantly enhanced bacterial clearance in the lung. The increased bacterial clearance in mice treated with anti-HMGB1 mAb was associated with both enhanced phagocytic activities in airway leukocytes and reduced concentrations of airway HMGB1. The critical role of extracellular HMGB1 in hyperoxia-compromised phagocytic activity was also confirmed in cultured macrophages. Thus, the experiments in this report indicate that HMGB1 mediates hyperoxia-impaired host defense against bacterial infection, at least in part, by inhibiting phagocyte functions.
Patients on MV are known to be more susceptible to bacterial infections and prone to develop VAP (6). Either the mechanical stretching caused by MV or exposure to the hyperoxia applied during MV may independently predispose subjects to VAP (28, 50). In this study, we examined the effects of pre-exposure to hyperoxia alone on animal survival in a murine model of PA pneumonia. The results presented in Figure 2 demonstrate that prolonged exposure to hyperoxia (≥ 99% O2) can cause a marked increase in lethality of mice with PA pneumonia (100% versus 0% without hyperoxic exposure). Similarly, the exposure of animals with Klebsiella infection to hyperoxia was also reported to increase animal lethality significantly (28). Thus, prolonged exposure to hyperoxia alone can lead to fatal outcomes in bacterial pneumonia. In addition, Kikuchi and colleagues reported that the PA infection of mice before exposure to 90% O2 increased bacterial dissemination to organs other than the lung, such as the liver (51). Baleeiro and colleagues demonstrated that mice infected with Klebsiella pneumoniae during hyperoxic exposure (> 95% O2) manifested an increased bacterial burden in both the lung and liver (28). Together, these studies suggest that hyperoxia directly contributes to adverse clinical outcomes, leading to the high mortality and morbidity observed in patients with VAP (5).
To determine the mechanisms underlying hyperoxia-increased mortality in PA pneumonia, we further investigated whether animals pre-exposed to hyperoxia can effectively clear PA from their lungs. The results presented in Figure 3 show that exposure to hyperoxia increased the bacterial burden in airways and lungs by more than 6-fold. The increased bacterial burden in lung lavage fluids of hyperoxia-exposed mice was accompanied by severe lung injury (Figure 6A). Hyperoxia-compromised bacterial clearance was also observed by others in several murine models of bacterial pneumonia (28, 52). Baleeiro and colleagues investigated the effects of exposure to 95% O2 on innate immunity in animals with K. pneumoniae infection. In their study, mice were inoculated with K. pneumoniae on the third day of hyperoxic exposure, and the exposure was continued for another 24 hours after the inoculation (28). The authors observed that hyperoxia-exposed mice exhibited increased mortality, with an increased bacterial burden in the lungs, suggesting that hyperoxia was responsible for the reduced bacterial clearance. In another study, Dunn and Smith exposed mice to 100% O2 for 48 hours and inoculated them with PA. They found that these mice manifested an elevated bacterial burden in their lungs, 4 hours after inoculation (52). Thus, the hyperoxia-increased mortality in mice with PA pneumonia is caused, at least in part, by impaired host defense to clear bacteria from both the airways and lungs.
Few published studies have sought to elucidate the molecular mechanisms underlying the observed deleterious effects of hyperoxia on bacterial pneumonia. The role of inflammatory cytokine TNF-α in host defense against bacterial infection has been examined under hyperoxic conditions (28, 53). The production of TNF-α was found to be profoundly suppressed in AMs isolated from mice exposed to hyperoxia and then subjected to Klebsiella or Legionella infection, suggesting that TNF-α contributes to bacterial clearance under hyperoxic conditions (28, 53). However, the administration of TNF-α did not improve bacterial clearance in hyperoxia-exposed animals (53). Thus, the role of proinflammatory cytokines in bacterial clearance among animals exposed to hyperoxia requires further investigation.
Here, we provide compelling evidence that the proinflammatory cytokine HMGB1 plays a critical role in hyperoxia-compromised host defense during PA pneumonia. The results shown in Figure 4 demonstrate that hyperoxia induces an accumulation of high concentrations of HMGB1 in murine airways. Interestingly, neutralizing HMGB1 in hyperoxia-exposed mice with an anti-HMGB1 mAb significantly increased bacterial clearance in both the airways and lungs by 75-fold and 100-fold, respectively (Figures 5A and 5B). The increased bacterial clearance was associated with substantially reduced concentrations of airway HMGB1 (80%; Figure 7B) in these mice. Thus, hyperoxia-induced high concentrations of airway HMGB1 contribute to the molecular mechanisms underlying hyperoxia-compromised bacterial clearance. In addition, PA infection without previous exposure to hyperoxia also increased concentrations of airway HMGB1, although it is difficult to quantify which treatment alone induces higher concentrations of HMGB1 release, which is dependent on the dose and length of exposure (unpublished observations). Regardless, the combination of hyperoxia and PA induces the release of more HMGB1 than either treatment alone (unpublished observations). Interestingly, it takes more than 48 hours for patients on MV to develop VAP. This time window coincides with the significant increase in concentrations of airway HMGB1 among animals exposed to hyperoxia (Figure 4). HMGB1 is ubiquitously expressed and highly conserved throughout the animal kingdom, and has been shown to play essential roles in a number of animal models of inflammatory diseases as well as in human subjects (54). Recently, we demonstrated that high concentrations of airway HMGB1 in cystic fibrosis transmembrane conductance regulator (CFTR)−/− mice compromise the functions of alveolar macrophages against PA infection. Importantly, highly elevated concentrations of airway HMGB1 in lung lavage samples from patients with CF were also found to suppress macrophage functions (30). Thus, these findings suggest that the critical roles of airway HMGB1 in pathogenesis of PA infection in murine models may be applicable to human subjects. Taken together with the clinical observation of an increased incidence of bacterial infection in patients undergoing hyperoxia, our findings on the role of airway HMGB1 in host defense against PA infection warrants the recommendation to wean patients off of the relatively high concentrations of oxygen as quickly as possible.
We further explored the mechanisms underlying HMGB1-impaired host defense in PA infection under hyperoxic conditions. AMs play important roles as the first line of defense against invading pathogens in the lung (55, 56). However, AMs that were isolated from mice exposed to hyperoxia exhibited significantly decreased phagocytic activities (26, 28). Previous experiments performed in our laboratory, using antioxidants, demonstrated the essential role of ROS in hyperoxia-compromised phagocyte function (26). In that earlier study, experiments were designed to further explore downstream events leading to the macrophage dysfunction induced by oxygen toxicity. The results presented in Figure 4 demonstrate that concentrations of HMGB1 in airways of mice exposed to prolonged hyperoxia (48 h) were much higher than those of the mice remaining at 21% O2. Similarly, the concentrations of extracellular HMGB1 were substantially increased (35-fold) in cultured macrophages exposed to hyperoxia (Figure 7D). We have recently shown that HMGB1 can directly impair the ability of macrophages to phagocytose PA (30). The association between the elevated concentrations of extracellular HMGB1 and the hyperoxia-compromised phagocytic ability of macrophages in both a murine model of PA infection and cultured macrophages (Figures 7C and 7D) implicates the role of HMGB1 in impairing phagocyte functions under hyperoxic conditions. The improved phagocytic function of leukocytes, including macrophages, in hyperoxic animals treated with anti-HMGB1 mAb helps to establish a causal role for HMGB1 in hyperoxia-induced phagocytic dysfunction. Indeed, enhanced phagocyte activities were associated with attenuated accumulations of HMGB1 in the airways of anti-HMGB1 mAb–treated mice (Figure 7B). These results demonstrate that hyperoxia-induced elevations in concentrations of airway HMGB1 significantly compromise phagocytic function, resulting in suppressed host defense, which can lead to increased pulmonary bacterial burden and mortality in animals with PA infection.
The inhibition of airway HMGB1 may also improve clinical outcomes by modulating neutrophilic inflammation with PA infection under hyperoxic conditions. Although the inflammatory response with leukocytic recruitment is important for the effective clearance of invading microorganisms (57–59), a prolonged presence of neutrophils in the lung may cause tissue injury by releasing excessive ROS and hydrolytic enzymes (43, 47–49). The roles of excessive neutrophilic accumulation in mediating hyperoxia-induced lung injury have been examined in both neonatal and adult animal models (60–62). The infiltration of neutrophils into the lung is directed by cytokines such as IL-8, one of the most studied cytokines (63). However, the upstream events leading to hyperoxia-mediated neutrophil recruitment are not well understood. HMGB1 is a potent chemoattractant for neutrophils (64). The intratracheal instillation of recombinant HMGB1 has been shown to markedly increase the secretion of IL-8 and cause lung injury with pronounced neutrophil infiltration (64). Blocking the α-chemokine receptor, one of the receptors for IL-8 (65), significantly attenuated HMGB1-mediated inflammatory lung injury by inhibiting neutrophil infiltration (45).
In addition, HMGB1 can hamper the removal of neutrophils by impairing the clearance of apoptotic neutrophils by macrophages (66). The accumulated apoptotic neutrophils can release more HMGB1 into the airways to attract more neutrophils to the lungs, establishing a vicious cycle that results in severe lung injury. In this study, we show that the exposure of mice to hyperoxia markedly induced concentrations of airway HMGB1 (Figure 4). The elevated concentrations of airway HMGB1 in hyperoxic animals may result in a significant accumulation of apoptotic neutrophils, further aggravating PA-elicited lung injury. Thus, inhibiting airway HMGB1 in these animals with neutralizing anti-HMGB1 mAb was anticipated to reduce neutrophil counts by inhibiting their recruitment and enhancing the clearance of apoptotic neutrophils. Indeed, inhibiting HMGB1 by the anti-HMGB1 mAb significantly reduced neutrophil counts and tissue injury in the lung (Figure 6). Therefore, these results suggest that HMGB1 plays an important role in mediating inflammatory lung injury in hyperoxic animals with PA infection.
In conclusion, the experiments in this report demonstrate that the elevation in concentrations of airway HMGB1 induced by prolonged exposure to hyperoxia is critical in both compromising host immunity against PA infection and mediating inflammatory lung injury. In addition to improving phagocytic function against bacterial infection, the inhibition of HMGB1 breaks the vicious cycle, wherein HMGB1 induces both neutrophil recruitment and the accumulation of apoptotic neutrophils that subsequently result in the further accumulation of HMGB1. Therefore, HMGB1 provides a novel target in modulating hyperoxia-compromised innate immunity, and understanding its regulation may help to devise therapeutic interventions for patients with VAP.
Supplementary Material
Acknowledgments
The authors thank Dr. Louis Trombetta, Dr. Charles Ashby, and Dr. Matthew Wargo for insightful discussions and generous support.
Footnotes
This work was supported by National Heart, Lung, and Blood Institute grant HL093708 (L.L.M.) from the National Institutes of Health and by St. John’s University.
Originally Published in Press as DOI: 10.1165/rcmb.2012-0279OC on October 18, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Esteban A, Anzueto A, Alia I, Gordo F, Apezteguia C, Palizas F, Cide D, Goldwaser R, Soto L, Bugedo G, et al. How is mechanical ventilation employed in the intensive care unit? An international utilization review. Am J Respir Crit Care Med 2000;161:1450–1458 [DOI] [PubMed] [Google Scholar]
- 2.Huber GL, Porter SL, Burley SW, La Force FM, Mason RJ. The effect of oxygen toxicity on the inactivation of bacteria by the lung. Chest 1972;61:66S. [DOI] [PubMed] [Google Scholar]
- 3.Coalson JJ, King RJ, Winter VT, Prihoda TJ, Anzueto AR, Peters JI, Johanson WG., Jr O2- and pneumonia-induced lung injury: I. Pathological and morphometric studies. J Appl Physiol 1989;67:346–356 [DOI] [PubMed] [Google Scholar]
- 4.Sinclair SE, Altemeier WA, Matute-Bello G, Chi EY. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med 2004;32:2496–2501 [DOI] [PubMed] [Google Scholar]
- 5.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388–416 [DOI] [PubMed] [Google Scholar]
- 6.Craven DE, Kunches LM, Kilinsky V, Lichtenberg DA, Make BJ, McCabe WR. Risk factors for pneumonia and fatality in patients receiving continuous mechanical ventilation. Am Rev Respir Dis 1986;133:792–796 [PubMed] [Google Scholar]
- 7.Tablan OC, Anderson LJ, Besser R, Bridges C, Hajjeh R, Centers for Disease Control, Healthcare Infection Control Practices Advisory Committee Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep 2004;53:1–36 [PubMed] [Google Scholar]
- 8.Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867–903 [DOI] [PubMed] [Google Scholar]
- 9.Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, Jaeschke RZ, Brun-Buisson C. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med 1998;129:433–440 [DOI] [PubMed] [Google Scholar]
- 10.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in medical intensive care units in the United States: National Nosocomial Infections Surveillance System. Crit Care Med 1999;27:887–892 [DOI] [PubMed] [Google Scholar]
- 11.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH, VAP Outcomes Scientific Advisory Group Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest 2002;122:2115–2121 [DOI] [PubMed] [Google Scholar]
- 12.Ramirez Barba EJ, Rosenthal VD, Higuera F, Oropeza MS, Hernandez HT, Lopez MS, Lona EL, Duarte P, Ruiz J, Hernandez RR, et al. Device-associated nosocomial infection rates in intensive care units in four Mexican public hospitals. Am J Infect Control 2006;34:244–247 [DOI] [PubMed] [Google Scholar]
- 13.Davis KA. Ventilator-associated pneumonia: a review. J Intensive Care Med 2006;21:211–226 [DOI] [PubMed] [Google Scholar]
- 14.Leblebicioglu H, Rosenthal VD, Arikan OA, Ozgultekin A, Yalcin AN, Koksal I, Usluer G, Sardan YC, Ulusoy S. Turkish branch of INICC: device-associated hospital-acquired infection rates in Turkish intensive care units: findings of the International Nosocomial Infection Control Consortium (INICC). J Hosp Infect 2007;65:251–257 [DOI] [PubMed] [Google Scholar]
- 15.Joseph NM, Sistla S, Dutta TK, Badhe AS, Rasitha D, Parija SC. Ventilator-associated pneumonia in a tertiary care hospital in India: role of multi-drug resistant pathogens. J Infect Dev Ctries. 2010;4:218–225 [DOI] [PubMed] [Google Scholar]
- 16.Crouch Brewer S, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated pneumonia due to Pseudomonas aeruginosa. Chest 1996;109:1019–1029 [DOI] [PubMed] [Google Scholar]
- 17.Bergogne-Berezin E. Treatment and prevention of nosocomial pneumonia. Chest 1995; 108(2, Suppl)26S–34S [DOI] [PubMed] [Google Scholar]
- 18.Berg JT, Lee ST, Thepen T, Lee CY, Tsan MF. Depletion of alveolar macrophages by liposome-encapsulated dichloromethylene diphosphonate. J Appl Physiol 1993;74:2812–2819 [DOI] [PubMed] [Google Scholar]
- 19.Kooguchi K, Hashimoto S, Kobayashi A, Kitamura Y, Kudoh I, Wiener-Kronish J, Sawa T. Role of alveolar macrophages in initiation and regulation of inflammation in Pseudomonas aeruginosa pneumonia. Infect Immun 1998;66:3164–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadikot RT, Blackwell TS, Christman JW, Prince AS. Pathogen–host interactions in Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 2005;171:1209–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wesselkamper SC, Eppert BL, Motz GT, Lau GW, Hassett DJ, Borchers MT. NKG2D is critical for NK cell activation in host defense against Pseudomonas aeruginosa respiratory infection. J Immunol 2008;181:5481–5489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Brien AD, Standiford TJ, Bucknell KA, Wilcoxen SE, Paine R., III Role of alveolar epithelial cell intercellular adhesion molecule–1 in host defense against Klebsiella pneumoniae. Am J Physiol 1999;276:L961–L970 [DOI] [PubMed] [Google Scholar]
- 23.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature 2000;406:782–787 [DOI] [PubMed] [Google Scholar]
- 24.Dos Santos CC. Hyperoxic acute lung injury and ventilator-induced/associated lung injury: new insights into intracellular signaling pathways. Crit Care 2007;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gore A, Muralidhar M, Espey MG, Degenhardt K, Mantell LL. Hyperoxia sensing: from molecular mechanisms to significance in disease. J Immunotoxicol 2010;7:239–254 [DOI] [PubMed] [Google Scholar]
- 26.Morrow DM, Entezari-Zaher T, Romashko J, III, Azghani AO, Javdan M, Ulloa L, Miller EJ, Mantell LL. Antioxidants preserve macrophage phagocytosis of Pseudomonas aeruginosa during hyperoxia. Free Radic Biol Med 2007;42:1338–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arita Y, Kazzaz JA, Joseph A, Koo H, Li Y, Davis JM. Antioxidants improve antibacterial function in hyperoxia-exposed macrophages. Free Radic Biol Med 2007;42:1517–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baleeiro CE, Wilcoxen SE, Morris SB, Standiford TJ, Paine R., III Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol 2003;171:955–963 [DOI] [PubMed] [Google Scholar]
- 29.O’Reilly PJ, Hickman-Davis JM, Davis IC, Matalon S. Hyperoxia impairs antibacterial function of macrophages through effects on actin. Am J Respir Cell Mol Biol 2003;28:443–450 [DOI] [PubMed] [Google Scholar]
- 30.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med 2012;18:477–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodwin GH, Sanders C, Johns EW. A new group of chromatin-associated proteins with a high content of acidic and basic amino acids. Eur J Biochem 1973;38:14–19 [DOI] [PubMed] [Google Scholar]
- 32.Bustin M. Regulation of DNA-dependent activities by the functional motifs of the high-mobility–group chromosomal proteins. Mol Cell Biol 1999;19:5237–5246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Degryse B, de Virgilio M. The nuclear protein HMGB1: a new kind of chemokine? FEBS Lett 2003;553:11–17 [DOI] [PubMed] [Google Scholar]
- 34.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 2004;255:320–331 [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251 [DOI] [PubMed] [Google Scholar]
- 36.Andersson U, Wang H, Palmblad K, Aveberger AC, Bloom O, Erlandsson-Harris H, Janson A, Kokkola R, Zhang M, Yang H, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med 2000;192:565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoiby N, Koch C. Cystic fibrosis: 1. Pseudomonas aeruginosa infection in cystic fibrosis and its management. Thorax 1990;45:881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brennan AL, Geddes DM. Cystic fibrosis. Curr Opin Infect Dis 2002;15:175–182 [DOI] [PubMed] [Google Scholar]
- 39.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, et al. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med 2006;203:1637–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Wang H, Mason JM, Levine J, Yu M, Ulloa L, Czura CJ, Tracey KJ, Yang H. Recombinant HMGB1 with cytokine-stimulating activity. J Immunol Methods 2004;289:211–223 [DOI] [PubMed] [Google Scholar]
- 41.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods 1990;132:191–195 [DOI] [PubMed] [Google Scholar]
- 42.Allard JB, Poynter ME, Marr KA, Cohn L, Rincon M, Whittaker LA. Aspergillus fumigatus generates an enhanced Th2-biased immune response in mice with defective cystic fibrosis transmembrane conductance regulator. J Immunol 2006;177:5186–5194 [DOI] [PubMed] [Google Scholar]
- 43.Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol 1994;56:672–686 [DOI] [PubMed] [Google Scholar]
- 44.Mantell LL, Kazzaz JA, Xu J, Palaia TA, Piedboeuf B, Hall S, Rhodes GC, Niu G, Fein AF, Horowitz S. Unscheduled apoptosis during acute inflammatory lung injury. Cell Death Differ 1997;4:600–607 [DOI] [PubMed] [Google Scholar]
- 45.Lin X, Yang H, Sakuragi T, Hu M, Mantell LL, Hayashi S, Al-Abed Y, Tracey KJ, Ulloa L, Miller EJ. Alpha-chemokine receptor blockade reduces high mobility group box 1 protein–induced lung inflammation and injury and improves survival in sepsis. Am J Physiol Lung Cell Mol Physiol 2005;289:L583–L590 [DOI] [PubMed] [Google Scholar]
- 46.Stover CK, Pham XQ, Erwin AL, Mizoguchi SD, Warrener P, Hickey MJ, Brinkman FS, Hufnagle WO, Kowalik DJ, Lagrou M, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 2000;406:959–964 [DOI] [PubMed] [Google Scholar]
- 47.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med 1989;320:365–376 [DOI] [PubMed] [Google Scholar]
- 48.Ras GJ, Theron AJ, Anderson R, Taylor GW, Wilson R, Cole PJ, van der Merwe CA. Enhanced release of elastase and oxidative inactivation of alpha–1-protease inhibitor by stimulated human neutrophils exposed to Pseudomonas aeruginosa pigment 1-hydroxyphenazine. J Infect Dis 1992;166:568–573 [DOI] [PubMed] [Google Scholar]
- 49.Seitz DH, Perl M, Mangold S, Neddermann A, Braumuller ST, Zhou S, Bachem MG, Huber-Lang MS, Knoferl MW. Pulmonary contusion induces alveolar Type 2 epithelial cell apoptosis: role of alveolar macrophages and neutrophils. Shock 2008;30:537–544 [DOI] [PubMed] [Google Scholar]
- 50.Charles PE, Tissieres P, Barbar SD, Croisier D, Dufour J, Dunn-Siegrist I, Chavanet P, Pugin J. Mild-stretch mechanical ventilation upregulates Toll-like receptor 2 and sensitizes the lung to bacterial lipopeptide. Crit Care 2011;15:R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kikuchi Y, Tateda K, Fuse ET, Matsumoto T, Gotoh N, Fukushima J, Takizawa H, Nagase T, Standiford TJ, Yamaguchi K. Hyperoxia exaggerates bacterial dissemination and lethality in Pseudomonas aeruginosa pneumonia. Pulm Pharmacol Ther 2009;22:333–339 [DOI] [PubMed] [Google Scholar]
- 52.Dunn MM, Smith LJ. The effects of hyperoxia on pulmonary clearance of Pseudomonas aeruginosa. J Infect Dis 1986;153:676–681 [DOI] [PubMed] [Google Scholar]
- 53.Nara C, Tateda K, Matsumoto T, Ohara A, Miyazaki S, Standiford TJ, Yamaguchi K. Legionella-induced acute lung injury in the setting of hyperoxia: protective role of tumour necrosis factor–alpha. J Med Microbiol 2004;53:727–733 [DOI] [PubMed] [Google Scholar]
- 54.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331–342 [DOI] [PubMed] [Google Scholar]
- 55.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 1990;141:471–501 [DOI] [PubMed] [Google Scholar]
- 56.Franke-Ullmann G, Pfortner C, Walter P, Steinmuller C, Lohmann-Matthes ML, Kobzik L. Characterization of murine lung interstitial macrophages in comparison with alveolar macrophages in vitro. J Immunol 1996;157:3097–3104 [PubMed] [Google Scholar]
- 57.Lovchik JA, Lipscomb MF. Role for C5 and neutrophils in the pulmonary intravascular clearance of circulating Cryptococcus neoformans. Am J Respir Cell Mol Biol 1993;9:617–627 [DOI] [PubMed] [Google Scholar]
- 58.Buisman AM, Langermans JA, van Furth R. Effect of granulocyte colony–stimulating factor on the course of infection with Gram-positive bacteria in mice during granulocytopenia induced by sublethal irradiation or cyclophosphamide. J Infect Dis 1996;174:417–421 [DOI] [PubMed] [Google Scholar]
- 59.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol 2010;185:6930–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keeney SE, Mathews MJ, Haque AK, Schmalstieg FC. Comparison of pulmonary neutrophils in the adult and neonatal rat after hyperoxia. Pediatr Res 1995;38:857–863 [DOI] [PubMed] [Google Scholar]
- 61.Auten RL, Whorton MH, Nicholas Mason S. Blocking neutrophil influx reduces DNA damage in hyperoxia-exposed newborn rat lung. Am J Respir Cell Mol Biol 2002;26:391–397 [DOI] [PubMed] [Google Scholar]
- 62.Sue RD, Belperio JA, Burdick MD, Murray LA, Xue YY, Dy MC, Kwon JJ, Keane MP, Strieter RM. CXCR2 is critical to hyperoxia-induced lung injury. J Immunol 2004;172:3860–3868 [DOI] [PubMed] [Google Scholar]
- 63.Witko-Sarsat V, Rieu P, Descamps-Latscha B, Lesavre P, Halbwachs-Mecarelli L. Neutrophils: molecules, functions and pathophysiological aspects. Lab Invest 2000;80:617–653 [DOI] [PubMed] [Google Scholar]
- 64.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 2000;165:2950–2954 [DOI] [PubMed] [Google Scholar]
- 65.Hay DW, Sarau HM. Interleukin-8 receptor antagonists in pulmonary diseases. Curr Opin Pharmacol 2001;1:242–247 [DOI] [PubMed] [Google Scholar]
- 66.Liu G, Wang J, Park YJ, Tsuruta Y, Lorne EF, Zhao X, Abraham E. High mobility group protein–1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J Immunol 2008;181:4240–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.