Abstract
The respiratory epithelium plays a critical role in innate defenses against airborne pathogens and pollutants, and alterations in epithelial homeostasis and repair mechanisms are thought to contribute to chronic lung diseases associated with airway remodeling. Previous studies implicated the nicotinamide adenine dinucleotide phosphate–reduced oxidase dual oxidase–1 (DUOX1) in redox signaling pathways involved in in vitro epithelial wound responses to infection and injury. However, the importance of epithelial DUOX1 in in vivo epithelial repair pathways has not been established. Using small interfering (si)RNA silencing of DUOX1 expression, we show the critical importance of DUOX1 in wound responses in murine tracheal epithelial (MTE) cells in vitro, as well as its contribution to epithelial regeneration in vivo in a murine model of epithelial injury induced by naphthalene, a selective toxicant of nonciliated respiratory epithelial cells (club cells [Clara]). Whereas naphthalene-induced club-cell injury is normally followed by epithelial regeneration after 7 and 14 days, such airway reepithelialization was significantly delayed after the silencing of airway DUOX1 by oropharyngeal administration of DUOX1-targeted siRNA. Wound closure in MTE cells was related to DUOX1-dependent activation of the epidermal growth factor receptor (EGFR) and the transcription factor signal transducer and activator of transcription–3 (STAT3), known mediators of epithelial cell migration and wound responses. Moreover, in vivo DUOX1 silencing significantly suppressed naphthalene-induced activation of STAT3 and EGFR during early stages of epithelial repair. In conclusion, these experiments demonstrate for the first time an important function for epithelial DUOX1 in lung epithelial regeneration in vivo, by promoting EGFR–STAT3 signaling and cell migration as critical events in initial repair.
Keywords: airway injury, redox signaling, EGFR, STAT3, naphthalene
Clinical Relevance
The results presented in this study demonstrate that the nicotinamide adenine dinucleotide phosphate–reduced oxidase dual oxidase–1 (DUOX1) plays a critical role in airway epithelial regeneration after injury by the redox-dependent activation of Src, epidermal growth factor receptor, and signal transducer and activator of transcription–3 signaling pathways. Abnormal expression or activation of DUOX1 may result in ineffective or dysregulated epithelial repair processes, and could thereby contribute to the pathology of chronic lung disease.
The respiratory epithelium forms an interface between the airways and the external environment, and represents the first line of defense against inhaled pollutants, microorganisms, and allergens. Critical in the proper functioning of the airway epithelium is the maintenance of a carefully regulated physical epithelial barrier that minimizes microbial invasion and allows for optimal surface hydration and mucociliary clearance in the upper airways, and for efficient gas exchange in the alveolar regions. Epithelial cells must therefore be capable of evoking rapid responses to injurious stimuli to minimize the loss of epithelial integrity, and disorderly epithelial repair mechanisms are thought to contribute to structural and functional alterations as important features of chronic respiratory diseases such as asthma, emphysema, pulmonary fibrosis, and lung cancer (1, 2). Epithelial defense mechanisms in response to infectious or injurious challenges rely on the presence of a wide range of pattern recognition receptors at the epithelial surface that rapidly detect and respond to a variety of pathogen-associated molecular patterns and damage-associated molecular patterns (DAMPs) that are released during injury, such as ATP, uric acid, lipids, and nuclear proteins.
One recently recognized aspect of such innate epithelial defense is the regulated production of hydrogen peroxide (H2O2) within the apical lumen in response to various stimuli, mediated by the activation of recently identified homologues of nicotinamide adenine dinucleotide phosphate–reduced (NADPH) oxidase, the dual oxidases 1 and 2 (DUOX1/2). Epithelial H2O2 production, presumably by DUOX2, is thought to aid in direct oxidative microbial killing in association with secreted lactoperoxidase (3, 4), and the prominent induction of DUOX2 in response to microbial and viral stimuli further supports its role in host defense (5, 6). Studies of Drosophila and zebrafish have established the importance of their single DUOX gene in the maintenance of gut immunity (7–9), and analogous host-defense properties have been demonstrated for DUOX2 in the mammalian gastrointestinal tract (10, 11). In contrast to the well-appreciated functional properties of intestinal and airway DUOX2 in antimicrobial defense, the functional properties of the more prominently expressed DUOX1 within the airway epithelium are less well understood. Most specific insights into the functional properties of airway epithelial DUOX1 have come from in vitro studies in airway or bronchial epithelial cell lines, which have demonstrated a contribution of DUOX1 to the enhanced production of epithelial mediators such as MUC5AC, IL-8, or matrix metalloproteinase (MMP)–9, in response to environmental and microbial stimuli, mediated by the activation of epidermal growth factor receptor (EGFR)/extracellular signal–regulated kinase (ERK)/NF-κB signaling pathways (12–15), indicating a more indirect role for DUOX1 in innate host defense and epithelial wound responses. Epithelial DUOX1 was also found to play an important role in epithelial cell migration as a critical event in epithelial wound healing (15, 16), suggesting a central role for epithelial DUOX1 in airway epithelial homeostasis by mediating common wound responses. Moreover, these actions of DUOX1 are in many cases initiated by ATP, which functions as a DAMP and promotes Ca2+-dependent DUOX1 activation by purinergic P2Y receptor stimulation (14, 15, 17). Elegant studies of zebrafish (18, 19) or Drosophila (20) have demonstrated the importance of DUOX in in vivo injury responses, but a definitive role for DUOX1 in epithelial regeneration after in vivo injury, especially in mammalian systems, has not been established.
In the present study, we established the involvement of DUOX1-mediated epithelial signaling in epithelial regeneration in a chemical model of airway epithelial injury. Our results show that DUOX1 promotes epithelial wound responses in in vitro studies with primary murine tracheal epithelial (MTE) cells and promotes epithelial regeneration in an in vivo murine model of naphthalene-induced epithelial injury, in part through the activation of EGFR and signal transducer and activator of transcription–3 (STAT3) signaling as critical events in promoting epithelial cell migration (21–23) and effective epithelial regeneration after injury (24, 25).
Materials and Methods
Cell Culture and Treatments
Primary MTE cells were isolated from C57BL/6 mice (Jackson Laboratories, Bar Harbor, ME), and cultured as described previously (26, 27). For experimentation, MTE cells at passages 2–4 were seeded at 1 × 105 cells/cm2 in 24-well plates (BD Labware, Bedford, MA) and subjected to scratch wound injury or cell stimulation with ATP (100 μM; Sigma Chemical Co., St. Louis, MO) or epidermal growth factor (EGF, 100 ng/ml; Calbiochem, San Diego, CA).
Epithelial Wound Closure and Cell Migration
Confluent MTE cell monolayers were scratched using a sterile pipette tip, and the closure of wound areas was monitored for 24 hours using Image J software (National Institutes of Health, Bethesda, MD). Quantitative cell migration was evaluated by seeding MTE cells (1 × 105 cells/well) on fibronectin-coated, 8-μm polycarbonate inserts, and analysis of migrated cells was performed after 24 hours (28).
Naphthalene-Induced Airway Epithelial Injury
Airway epithelial injury was induced in C57BL/6 mice by an intraperitoneal injection of 200 mg/kg naphthalene (Sigma Chemical Co.) in corn oil, as described previously (29, 30). At 2, 7, and 14 days after naphthalene treatment, lungs were collected for immunohistochemical analyses, or stored in RNAlater (Invitrogen, Grand Island, NYY) or snap-frozen for biochemical analyses. All animal studies were approved by the Institutional Animal Care and Use Committee at the University of Vermont.
Silencing of DUOX1 by RNA Interference
MTE cells were transfected at 60–70% confluence with two DUOX1 small interfering (si)RNA targets (100 nM each; Table 1) or nontarget (NS) siRNA (200 nM; Ambion, Austin, TX), using the DharmaFECT transfection reagent (Dharmacon, Lafayette, CO) for experimentation after 72 hours. For the in vivo siRNA silencing of DUOX1, two DUOX1 siRNA targets in sterile PBS (35 μg/target sequence/mouse) or NS-siRNA (70 μg/mouse) were instilled oropharyngeally in mice under brief isofluorane anesthesia, before naphthalene injection.
TABLE 1.
SIRNA TARGET SEQUENCES
| DUOX1 siRNA |
||
| Target 1 | Sense: | GAGUUCCUCAACAUUUACATT |
| Antisense: | UGUAAAUGUUGAGGAACUCTG | |
| Target 2 | Sense: | GGACAUGCAAGAUUUCUGGTT |
| Antisense: | CCAGAAAUCUUGCAUGUCCTC | |
| NS-siRNA | Sense: | AGUACUGCUUACGAUACGGTT |
| Antisense: | CCGUAUCGUAAGCAGUACUTT | |
Definition of abbreviations: DUOX1, dual oxidase–1; NS, nontarget; siRNA, small interfering RNA.
Western Blot Analysis
MTE cell lysates or lung-tissue homogenates were separated by SDS-PAGE for Western blotting, using primary antibodies against phospho-EGFR (Y845), phospho-EGFR (Y1068), EGFR, phospho-ERK1/2, ERK1/2, phospho-Src (Tyr416), Src, phospho-STAT3 (Tyr705), STAT3 (Cell Signaling, Danvers, MA), β-actin (Sigma Chemical Co.), or a rabbit polyclonal antibody against the Arg618–His1044 fragment of DUOX1, kindly provided by F. Miot (Free University of Brussels, Belgium) (31). Antibodies were visualized by enhanced chemiluminescence, and band densities were quantified using ImageJ software.
RT-PCR
RNA was extracted using TRIzol (Invitrogen) and the RNeasy Mini Kit (Qiagen, Germantown, MD) and reverse-transcribed, and PCR reactions were performed using Platinum Taq DNA Polymerase (Invitrogen) and appropriate primer sets (Table E2) for analysis on ethidium bromide–stained agarose gels. Alternatively, quantitative PCR was performed using the SYBR Green PCR Supermix (Bio-Rad, Hercules, CA) with appropriate primers (Table E2), normalized to glyceraldehyde 3–phosphate dehydrogenase, using the ΔΔCT method.
Immunohistochemistry
Lung-tissue sections were analyzed using rabbit α-DUOX1 (31), rabbit α-CCSP (1:2,000; Millipore, Billerica, MA), mouse anti–β-tubulin IV (1:200; BioGenex, Fremont, CA), or rabbit anti–phospho-STAT3 (Y705), which were visualized using Alexa Fluor 555 goat anti-rabbit (1:500; Invitrogen) or the Vector M.O.M. Immunodetection kit (Vector Laboratories, Burlingame, CA) and streptavidin Alexa Fluor 647 conjugate (1:500; Invitrogen). Sections were counterstained with 4,6-diamidino-2-phenylindole (DAPI), and evaluated by confocal microscopy and analyzed using Metamorph imaging software (Molecular Devices, Sunnyvale, CA).
Statistical Analysis
Quantitative data are presented as mean ± SE, and statistical differences were analyzed using the Student t test. Differences were considered significant at P < 0.05.
More detailed information is provided in the online supplement.
Results
Activation of DUOX1 Contributes to Epithelial Cell Migration In Vitro
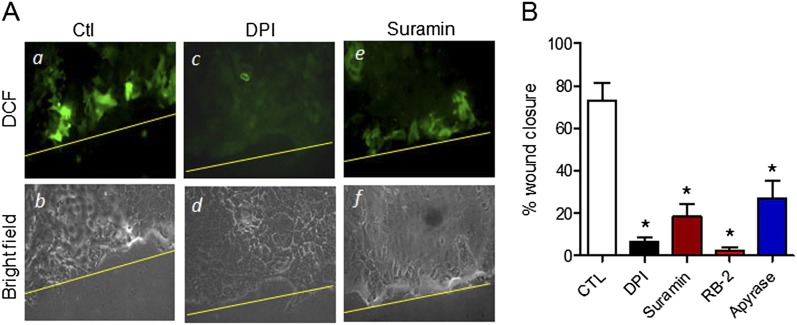
We first evaluated the involvement of ATP-dependent purinergic signaling and the activation of NADPH oxidase–dependent production of reactive oxygen species (ROS) in wound responses of MTE cells, based on previous findings with human airway epithelial cells (15). As expected, the scratch wounding of MTE cell monolayers was associated with increased ROS production near the wound margin, as illustrated by enhanced 2',7'-dichlorodihydrofluorescein (DCF) fluorescence, and this increase in ROS production was largely prevented in the presence of the NADPH oxidase inhibitor DPI (Sigma) or the P2YR antagonist suramin (Figure 1A). Furthermore, as shown in Figure 1B, epithelial wound closure was significantly attenuated in the presence of DPI and by two distinct P2YR antagonists (suramin and RB-2; Sigma) as well as the ATP hydrolyzing enzyme Apyrase (Sigma), confirming the critical importance of P2YR-dependent NADPH oxidase activation in wound responses of MTE cells, similar to previous observations in other cell types (15, 32, 33).
Figure 1.
Nicotinamide adenine dinucleotide phosphate–reduced oxidase (NADPH) oxidase activation contributes to wound responses in murine tracheal epithelial (MTE) cells. (A) Confluent MTE cell monolayers on chamber slides were loaded with H2 DCF for 30 minutes before scratch wounding with a pipette tip in the absence (a and b) or presence of inhibitors of NADPH oxidase (diphenylene iodonium; 1 μM; c and d) or purinergic P2Y receptors (suramin, 100 μM; e and f). DCF fluorescence was measured 10 minutes after wounding. Ctl, control. (B) Wound closure after scratch injury of MTE cell monolayers was followed for 24 hours in the absence or presence of DPI (1 μM), the P2YR antagonists suramin (100 μM) or reactive blue–2 (RB-2; 100 μM), or the ATPase Apyrase (10 U/ml). CTL, control. Data represent the mean ± SE of four replicates from two separate experiments. *P < 0.05, compared with untreated control samples.
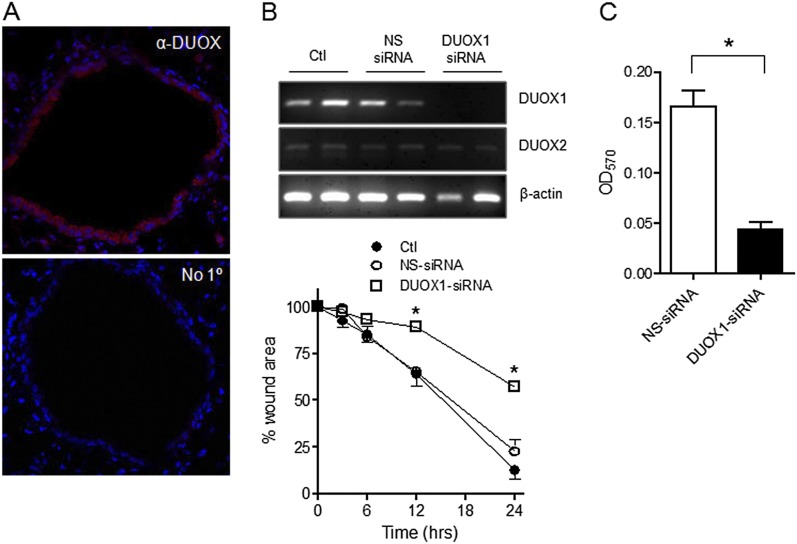
Although previous studies established a role for DUOX1 in wound responses in human airway epithelia (15, 16), whether DUOX1 plays a similar role in murine airway epithelia remains unclear, and its presence in murine airway epithelia has been questioned (4). However, the immunohistochemical analysis of murine lung tissue indicated the epithelial expression of DUOX protein (Figure 2A), and RT-PCR analysis confirmed the expression of DUOX1 and to a lesser extent DUOX2 in MTE cells (Figure 2B). Notably, the antibody used in the present study differs from those used previously in related studies (4), and recognizes the Arg618–His1044 fragment within DUOX1 that represents its intracellular EF-hand binding domain, and is 92% identical between mouse and human. Furthermore, analogous to earlier findings with human epithelial cells (15), the siRNA-mediated suppression of DUOX1 was found to attenuate wound closure rates markedly in injured MTE monolayers (Figure 2B), indicating the critical contribution of DUOX1 to MTE cell migration. This was further confirmed in an alternative migration assay, which showed a significant inhibition of MTE cell haptotaxis after the siRNA silencing of DUOX1 (Figure 2C).
Figure 2.
Wound responses in MTE cells are mediated by dual oxidase–1 (DUOX1). (A) Immunofluorescence analysis of DUOX protein in lung tissue from C57BL6 mice. Top: Immunofluorescence detection of α-DUOX (red) and nuclear counterstaining with 4,6-diamidino-2-phenylindole (DAPI) (blue). Bottom: Similar analysis without primary (1°) antibody. (B) DUOX1 expression in MTE was silenced by small interfering (si)RNA, as confirmed by RT-PCR (above). The effects of DUOX1 silencing on wound closure were evaluated in a scratch wound assay (below). Mean values ± SEs of 4–6 replicates from 2–3 experiments are presented. *P < 0.05, compared with nontarget (NS)–siRNA. (C) MTE cells transfected with DUOX1 siRNA or NS-siRNA were seeded on fibronectin-coated inserts for analyses of cell migration, as measured by the absorbance (optical density; OD) at 570 nm of crystal violet–stained migrated cells (n = 5). *P < 0.05, compared with NS-siRNA.
Epithelial DUOX1 generates H2O2 as its primary product (34), and we therefore evaluated the importance of H2O2 in epithelial wound responses in several ways. First, the addition of extracellular catalase after MTE cell wounding failed to attenuate wound closure (Figure E1A in the online supplement), ruling out the involvement of paracrine signaling by extracellular H2O2 production. Although H2O2 is capable of directly stimulating migration in several cell types (35), attempts to mimic the epithelial wound response in MTE cells with exogenous H2O2 showed minimal effects of H2O2 at low micromolar concentrations (Figure E1A). Higher concentrations tended to inhibit wound closure, most likely because of global oxidative stress under these conditions (15). These findings do not necessarily argue against a role for localized intracellular H2O2 production by DUOX1 activation, and so to address a potential endogenous role for H2O2, we evaluated wound closure in the presence of two cell-permeable and structurally unrelated catalysts of H2O2 decomposition, ebselen (10 μM; Sigma) and EUK (50 μM; Cayman Chemical Co., Ann Arbor, MI), which were both found to attenuate wound closure dramatically (Figure E1B). Neither compound significantly affected cell viability at these concentrations. As a secondary approach, we compared wound responses in MTE cells from wild-type (WT) C57BL/6 mice and from Tg (CAT)+/+ mice, in which the expression and activity of catalase were increased 2- to 3-fold (Figure E2). Although wound closure in MTE cells from Tg (CAT)+/+ mice tended to be somewhat delayed compared with MTE cells from WT mice, this result was not statistically significant. The relative lack of effect for catalase overexpression on wound closure is most likely attributable to the relatively modest increase in catalase activity in Tg (CAT)+/+ mice (2- to 3-fold), and to the fact that endogenous catalase is unlikely to control localized H2O2-dependent signaling events effectively near the plasma membrane because of its cellular location (36). These collective findings support the involvement of localized intracellular H2O2 production by DUOX1 activation in wound responses in MTE cells, analogous to previous findings in human epithelial cells (15).
DUOX1 Contributes to Airway Epithelial Regeneration after Naphthalene-Induced Injury In Vivo
To address the importance of DUOX1 in epithelial repair processes in vivo, we used the naphthalene model of airway injury, which is characterized by selective injury to nonciliated secretory bronchiolar epithelial cells (club cells [Clara]) and subsequent epithelial regeneration by the migration, transdifferentiation, and proliferation of remaining cell types (30, 37–39). The evaluation of naphthalene-induced lung injury by hematoxylin-and-eosin staining revealed the presence of exfoliated epithelial cells in the airway lumen 2 days after an intraperitoneal naphthalene injection (2 DPN), followed by gradual epithelial regeneration at 7 and 14 DPN (Figure S3A). More specific analysis of ciliated cells and secretory (club) cells, using the immunofluorescence analysis of specific marker proteins, confirmed the selective desquamation of CCSP+ club cells at 2 DPN, with remaining β-tubulin IV+ ciliated cells becoming flattened and extending beneath the injured club cells, followed by reepithelialization with club cells after 7 and 14 DPN (Figure E3B).
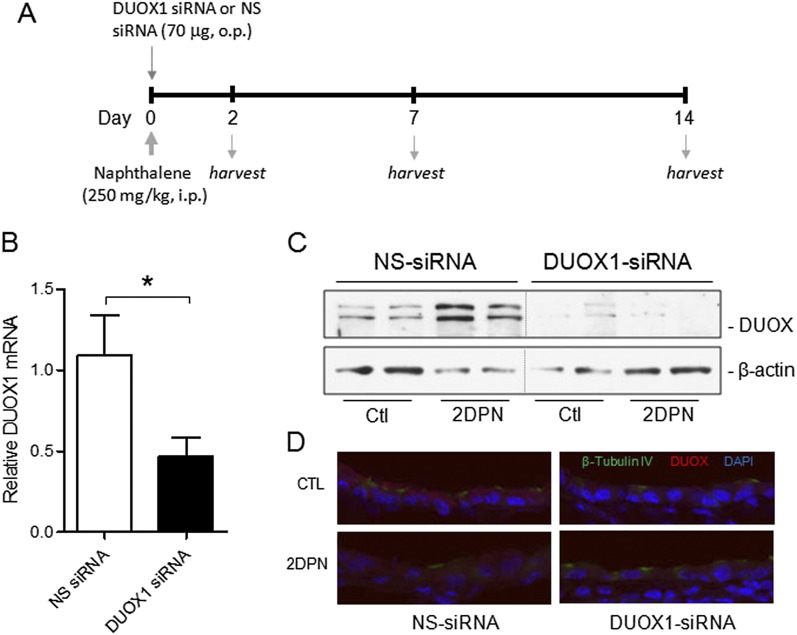
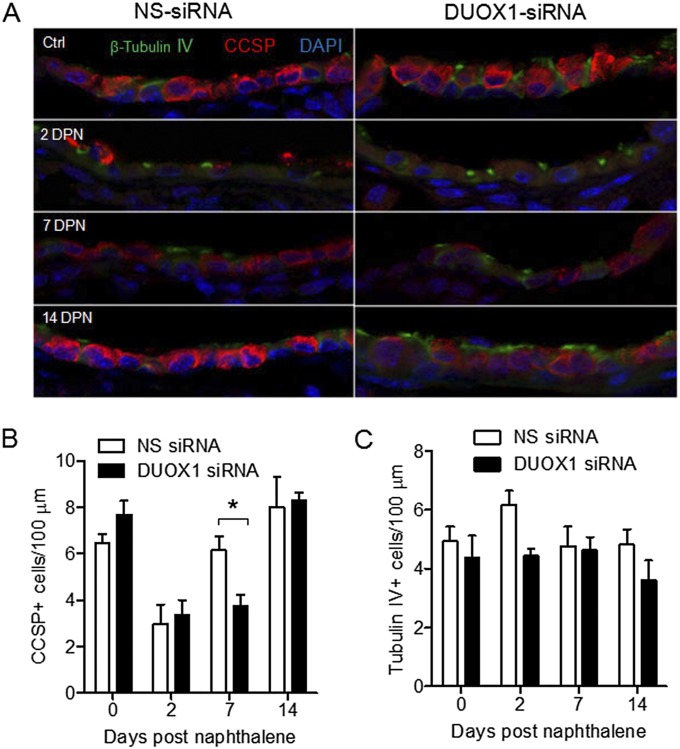
To determine the importance of DUOX1 in epithelial regeneration after naphthalene-induced injury, we oropharyngeally instilled DUOX1-targeted siRNA into the airways 30 minutes before naphthalene injection (Figure 3A) to suppress DUOX1 expression during the early stages of epithelial regeneration, which involve the spreading and migration of surviving ciliated cells (30, 37). An analysis of DUOX1 mRNA showed significant suppression at 2 days after siRNA instillation (Figure 3B), consistent with the reduced presence of DUOX protein shown by Western blotting (Figure 3C) or immunofluorescence analysis (Figure 3D). DUOX protein was largely associated with ciliated cell populations, and its overall concentration was surprisingly somewhat enhanced 2 days after naphthalene, compared with time-matched control samples (Figure 3C). Analyses of lung sections for β-tubulin IV+ ciliated cells and CCSP+ club cells at various time points indicated that CCSP+ club cells were lost at 2 DPN, but were restored to normal concentrations at 7 and 14 DPN, consistent with earlier reports (30, 37). In vivo DUOX1 silencing did not prevent naphthalene-induced club cell injury at 2 DPN, but resulted in delayed reepithelialization with club cells at 7 DPN, which appeared to be normalized at 14 DPN (Figure 4). As expected (39), numbers of tubulin IV+ ciliated cells did not change significantly at any time point after naphthalene-induced injury or after DUOX1 siRNA instillation. These findings are consistent with a role for DUOX1 in early stages of epithelial repair, most likely by contributing to the spreading and migration of remaining ciliated cell types. Although DUOX was recently implicated in wound responses in vivo in zebrafish (18) and Drosophila (20), to our knowledge, this is the first direct demonstration of a role for DUOX1 in epithelial regeneration in vivo in a mammalian organism.
Figure 3.
In vivo siRNA silencing of airway DUOX1 in mice. (A) Study design illustrates timing of oropharyngeal (o.p.) siRNA instillation, naphthalene injection, and harvesting of lung tissues for analysis. (B) Analysis of DUOX1 mRNA expression in lung tissues at 2 days after siRNA instillation. *P < 0.05, compared with NS-siRNA (n = 6). Lung tissue was harvested 2 days after siRNA instillation and naphthalene injection (2 DPN) or control injection with corn oil (Ctl) for the analysis of DUOX protein expression according to Western blot analysis of whole-lung lysates (C) or by immunofluorescence analysis of lung-tissue sections (D), using an α-DUOX antibody (provided by Dr. F. Miot). i.p., intraperitoneal.
Figure 4.
In vivo DUOX1 silencing attenuates epithelial regeneration after naphthalene-induced injury. (A) Representative immunofluorescence images of lung sections obtained after 0, 2, 7, and 14 days after naphthalene injection (DPN), after staining for β-tubulin IV+ ciliated cells (green) and club cell secretory protein–positive (CCSP+) club cells (red). (B) Quantification of numbers of CCSP+ (club) cells per 100 μm using Metamorph software (Molecular Devices, Sunnyvale, CA), based on at least five airway sections per mouse. (C) Similar quantification of tubulin IV+ (ciliated) cells. Data represent the means ± SEs from 4–5 mice. *P < 0.05.
DUOX1 Mediates Epithelial Repair by the Activation of Src–EGFR–STAT3 Signaling
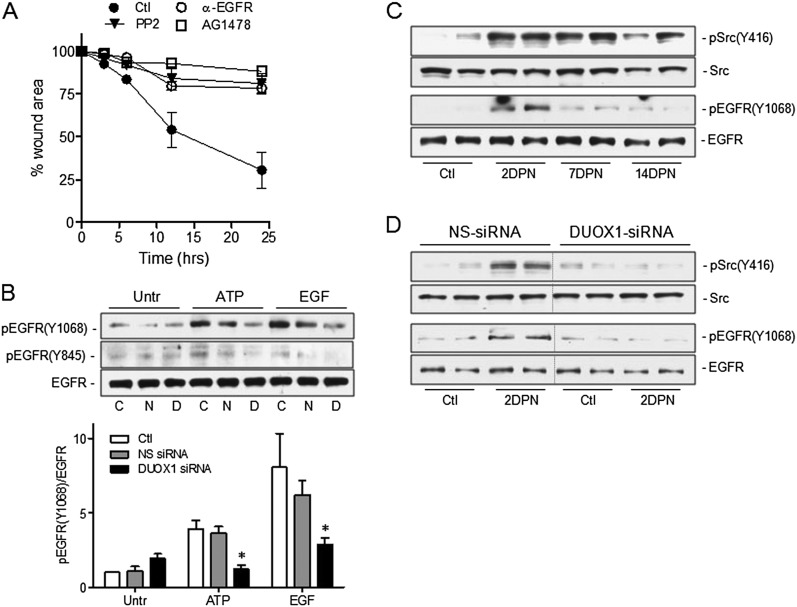
With respect to the potential mechanisms by which DUOX1 activation promotes epithelial cell migration, previous studies with airway epithelial cells indicated a critical role for DUOX1 in the oxidant-dependent activation of EGFR signaling (12, 14), and the activations of EGFR and its ligands comprise critical events in epithelial repair pathways (40, 41), and contribute to epithelial regeneration after naphthalene injury (24, 29). Moreover, the H2O2-dependent activation of EGFR has also been demonstrated to involve an initial activation of the nonreceptor tyrosine kinase Src (42, 43), which is known to play critical roles in epithelial motility and migration (32, 33). Using selective inhibitors of either Src (PP2; Calbiochem) or EGFR (AG1478; Sigma; or an EGFR-blocking antibody, mAb 225; Calbiochem), we confirmed their importance in wound closure in injured MTE cells (Figure 5A). Moreover, EGFR activation in MTE cells in response to either exogenous ATP or EGF, evaluated by tyrosine phosphorylation at Tyr1068 or Tyr845 (a known target for Src), was significantly attenuated after the siRNA silencing of DUOX1 (Figure 5B). The ability of DUOX1 to mediate ligand-mediated EGFR autophosphorylation (at Tyr1068) is most likely related to the DUOX1-dependent activation of Src (illustrated by enhanced EGFR phosphorylation at Tyr845), which is known to promote EGFR autophosphorylation and activation.
Figure 5.
Role of Src–epidermal growth factor receptor (EGFR) signaling in DUOX1-mediated epithelial wound responses. (A) Analysis of wound closure rates in scratched MTE monolayers in the absence or presence of the Src inhibitor PP2 (10 μM), the EGFR tyrosine kinase inhibitor AG1478 (10 μM), or a blocking EGFR monoclonal antibody (4 ng/ml). Data represent the mean ± SE (n = 3–5). (B) Western blot analysis of pEGFR (Y1068) and total EGFR in untreated MTE cells (Untr) or MTE cells transfected with DUOX1 siRNA or NS-siRNA, after 10-minute stimulation with exogenous ATP (100 μM) or epidermal growth factor (EGF; 10 ng/ml). Representative Western blots and relative band intensities from three separate experiments are presented. C, control; N, NS-siRNA; D, DUOX1 siRNA. *P < 0.05, compared with corresponding control samples. (C) Analysis of phosphorylated forms of EGFR and Src (pEGFR Y1068 and pSrc Y416) in lung-tissue homogenates of mice collected 0, 2, 7, or 14 days after an intraperitoneal injection of naphthalene. (D) Similar analysis of pEGFR (Y1068) or pSrc (Y416) in lung tissues obtained 2 days after naphthalene injection (2 DPN) or vehicle control (Ctl) and instillation of DUOX1 siRNA or NS-siRNA. Representative blots show results from two mice.
To establish the importance of these pathways in epithelial injury in vivo, we evaluated their activation during naphthalene-induced epithelial injury and regeneration. As shown in Figure 5C, we observed an increased phosphorylation of both Src at Tyr416 and EGFR at Tyr1068 in lung tissue, especially at earlier stages after naphthalene injury (2 DPN), indicating the activation of these pathways. Moreover, the activation of both Src and EGFR at 2 DPN was strongly attenuated in DUOX1 siRNA-treated mice compared with mice receiving NS-siRNA (Figure 5D), indicating a role for DUOX1 in the activation of both Src and EGFR as an important mechanism of epithelial regeneration by promoting cell migration.
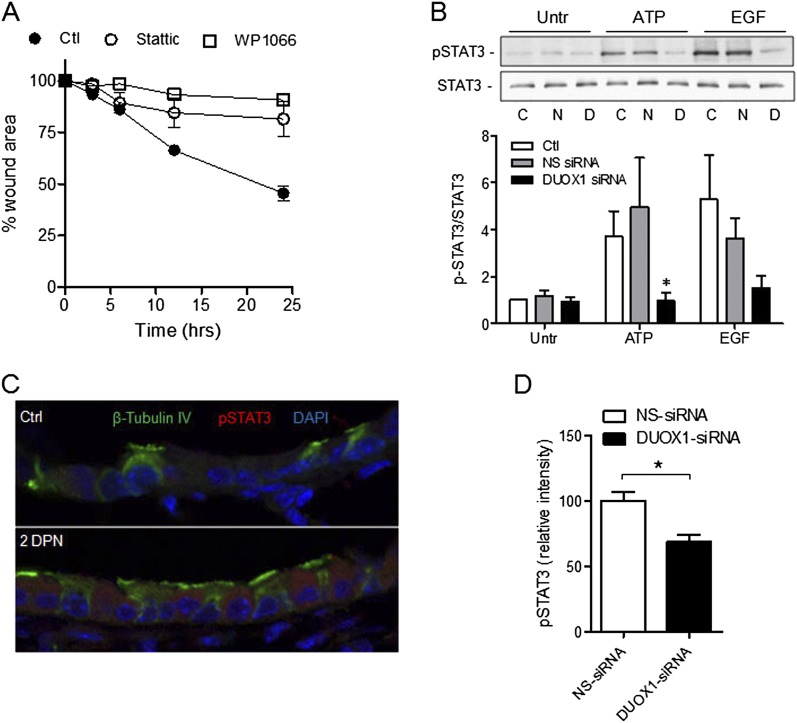
A critical factor in regulation of epithelial cell motility, migration, and invasiveness is the transcription factor STAT3. The activation of STAT3 is closely associated with Src and EGFR signaling (44), and was recently shown to be essential for airway epithelial cell migration and effective epithelial regeneration after lung epithelial injury induced by naphthalene (25). We therefore evaluated the involvement of DUOX1 in the activation of STAT3 in MTE cells and in vivo in response to naphthalene-induced injury. As shown in Figure 6A, MTE cell wound closure after injury was significantly attenuated in the presence of Stattic (10 μM; Calbiochem) or WP1066 (5 μM; Alexis, San Diego, CA), two structurally unrelated inhibitors of STAT3 (45, 46). Moreover, the tyrosine phosphorylation of STAT3 at Tyr705 was consistently attenuated after the siRNA silencing of DUOX1 in untreated or ATP-stimulated or EGF-stimulated MTE cells (reaching statistical significance in cases of ATP stimulation; Figure 6B), consistent with a role of DUOX1 in STAT3 activation. Immunofluorescence analysis also demonstrated increased STAT3 phosphorylation within the bronchiolar airways of naphthalene-injured mice (2 DPN; Figure 6C), and the intensity of STAT3 phosphorylation was significantly attenuated in mice receiving DUOX1 siRNA compared with corresponding control animals receiving NS-siRNA (Figure 6D). Therefore, our findings suggest that DUOX1-mediated epithelial repair is (at least in part) mediated by the activation of STAT3, which promotes the migration of remaining ciliated cells and resistant club cells, thereby facilitating reepithelialization.
Figure 6.
DUOX1-dependent activation of signal transducer and activator of transcription–3 (STAT3) phosphorylation in epithelial wound responses. (A) Analysis of wound closure rates in scratched MTE monolayers in the absence or presence of the two structurally unrelated inhibitors of STAT3, Stattic (10 μM), or WP1006 (5 μM). Data represent the mean ± SE (n = 3–5). (B) Western blot analysis of pSTAT (Y705) and total STAT3 in MTE cells after transfection with DUOX1 siRNA or NS-siRNA, and stimulation with ATP (100 μM) or EGF (10 ng/ml) for 10 minutes. Representative Western blots and relative band intensities from three separate experiments are shown. C, control; N, NS-siRNA; D, DUOX1 siRNA. *P < 0.05, compared with corresponding control samples. (C) Immunofluorescence imaging of pSTAT3 in lung-tissue sections obtained 2 days after vehicle (Ctl) or naphthalene injection (2DPN). (D) Quantification of pSTAT3 immunofluorescence intensity within the epithelium was performed on 2-day naphthalene-exposed mice receiving NS siRNA or DUOX1 siRNA, using Metamorph software. Data are based on average intensities, determined in at least three airways per mouse, and mean values ± SEs from five animals are shown. *P < 0.05, compared with NS-siRNA.
Discussion
Epithelial cells within the adult lung are largely quiescent and are replaced at a low rate, and mechanisms of epithelial regeneration after injury have been the subject of intense interest. These regenerative mechanisms within the lung vary depending on the regional compartment, based on differences in cellular composition, and are known to involve distinct progenitor cell populations that self-renew and replace injured cells (38, 39, 47). In the conducting airways, subsets of nonciliated, secretory (club) cells in neuroepithelial bodies and at airway–alveolar branch points have been identified as primary progenitor cells, and in the naphthalene model of airway injury, resistant populations of club cells are primarily responsible for epithelial self-renewal and reepithelialization. In addition, surviving ciliated cells are critical in early responses to injury, and cover denuded areas by cell spreading and migration (37). However, they most likely do not contribute significantly as progenitor cells, because they do not appear to proliferate or transdifferentiate as part of the repair process (48). Building on recent in vitro studies (15, 16), our present experiments indicate that the NADPH oxidase DUOX1 plays an important role in this epithelial repair process, by promoting epithelial cell migration as a critical early wound response to promote epithelial regeneration.
Since the discovery of the dual oxidases DUOX1 and DUOX2 within the respiratory epithelium, the functional properties of these enzymes in epithelial physiology or pathology remain incompletely understood. Studies in Drosophila or zebrafish have shown that their single DUOX protein is involved in direct mucosal host defense (8, 9, 49), but also in epidermal wound responses (18, 20), which are associated with both direct oxidative killing mechanisms and the activation of cell signaling pathways that coordinate injury responses (20). Analogous oxidative host-defense responses have been ascribed to DUOX2 within intestinal and respiratory epithelia (4, 11), and the present experiments, to our knowledge, are the first to demonstrate a critical role of DUOX1 in airway epithelial regeneration in vivo in mammalian systems. Therefore, whereas the single DUOX gene (e.g., in Drosophila or zebrafish) appears to be involved in both antimicrobial responses and injury-repair responses, these functions appear to have been segregated in higher organisms and seem to rely on the specific involvement of either DUOX1 or DUOX2, despite their close homology and simultaneous presence in epithelia. Such unique functions of DUOX1 and DUOX2 are also illustrated by the fact that they are subject to unique modes of transcriptional or posttranscriptional regulation (5, 6) and activation (50), consistent with the isoform-specific functions of DUOX1/2 in different aspects of epithelial biology or under different conditions.
Our experiments also provide insights into the mechanisms by which DUOX1 promotes epithelial cell migration and epithelial regeneration, and indicate that DUOX1 participates in the activation of Src, EGFR, and STAT3 signaling pathways regulating epithelial cell migration. The activation of EGFR and its ligands have been recognized as critical events in epithelial repair pathways (29, 40, 41), and in vitro studies indicate a prominent role for EGFR in epithelial cell migration in early wound responses (21, 51, 52), although EGFR is also likely to contribute to epithelial regeneration by stimulating proliferation (29, 40). The DUOX1-dependent activation of EGFR was reported to involve ligand-dependent mechanisms (12, 14), but may also involve the oxidant-induced activation of the nonreceptor tyrosine kinase Src, which promotes EGFR activation by a ligand-independent mechanism (43, 53), and is a critical mediator of epithelial cell spreading and motility (33, 54). Indeed, our studies suggest a role for DUOX1 in Src activation as a critical component of the epithelial wound response.
The importance of EGFR activation in naphthalene-induced lung injury was recently explored using the EGFR tyrosine kinase inhibitor gefitinib (24). The results suggested that EGFR inhibition prolongs lung injuries associated with increased neutrophil sequestration, especially at earlier stages of epithelial regeneration (24, 29). Although neutrophil infiltration is commonly associated with acute lung injury, neutrophils are also capable of promoting epithelial repair mechanisms (55). Indeed, studies in zebrafish indicated a critical role for DUOX-derived H2O2 in neutrophil recruitment as part of the epidermal wound response (18), which was suggested to involve direct neutrophil chemotaxis by H2O2 gradients (56). Alternatively, the epithelial activation of DUOX1 has also been linked to the EGFR-dependent induction of the neutrophil chemokine IL-8, as an alternative mechanism of neutrophil recruitment (14, 57). Although naphthalene-induced epithelial injury is known to be associated with neutrophil infiltration (24), the adverse effects of the systemic inhibition of EGFR on neutrophil infiltration and naphthalene-induced lung injury suggest a complex relationship between EGFR activation, neutrophil recruitment, and injury/repair pathways. Because our findings indicated a direct ability of DUOX1 and EGFR activation to promote epithelial cell migration in vitro, independent of neutrophils, we did not further address their involvement with DUOX1-dependent epithelial repair in the present study.
Our findings also build on the previously demonstrated role of the transcription factor STAT3 in wound healing responses (58) and in epithelial cell migration and epithelial regeneration in the naphthalene model of lung epithelial injury (25). Moreover, this study confirms the importance of STAT3 activation in epithelial cell migration, and demonstrates the involvement of DUOX1 in the activation of STAT3 during early stages of epithelial repair after injury. STAT3 is a critical regulator of cell motility, migration, and invasiveness, which involves both transcriptional regulation of (e.g., of MMPs) and the direct interactions of STAT3 with focal adhesion kinase (23) or cytosolic stathmin to regulate microtubule organization (22). Given the close association of Src and EGFR signaling with STAT3 activation (44), our experiments indicate a common role for DUOX1 in activating these signaling pathways, although the precise oxidant-dependent steps remain to be fully elucidated.
The mechanisms by which DUOX1 is activated during naphthalene-induced injury were not directly addressed in this study, but likely involve the actions of released DAMPs such as ATP from injured cells. Indeed, live cell imaging within pulmonary lung slices indicated the release of ATP from secretory vesicles in neuroepithelial bodies upon depolarization, and this was found to promote paracrine effects on surrounding club cells by activation of purinergic P2Y2 receptors (59). Because of the stem cell–like characteristics of these club cells and their critical role in epithelial regeneration (38), this purinergic signaling may be of great importance for epithelial repair after injury. However, such ATP release and purinergic signaling are not necessarily restricted to neuroepithelial bodies, and ATP release may be more broadly involved in naphthalene-induced club cell injury throughout the airways, and may promote the migration of ciliated cell types during initial wound responses after injury. Indeed, ATP-dependent P2Y receptor signaling plays a well-established role in epithelial cell migration and wound responses (15, 32) and in the EGFR activation and induction of wound response genes such as MMP-9 and IL-8 (32, 47, 51, 60). These responses are linked to the activation of DUOX1 (12, 14, 15). Although DUOX1 appears to be globally present throughout the airway epithelium, in both ciliated and secretory cell types (e.g., Figure 2A), to what extent DUOX1 is also expressed in neuroepithelial bodies remains unknown. Likewise, the precise cellular source of ATP release and the location of purinergic signaling in this injury model remain to be clarified, but our results suggest that ATP-mediated DUOX1 activation is mostly involved in early repair stages after club cell injury by promoting cell migration and the spreading of remaining ciliated cells.
The important contribution of epithelial DUOX1 to epithelial cell wound responses and epithelial regeneration after injury would imply that the inappropriate expression or activation of DUOX1 may be associated with ineffective or dysregulated epithelial repair mechanisms, thereby contributing to the pathology of chronic lung disease. Indeed, epithelial DUOX1 expression was found to be suppressed in smokers with or without chronic obstructive pulmonary disease (61), and this suppression may be associated with epithelial alterations in these subjects and ineffective epithelial repair as a contributing factor in the development of emphysema. Conversely, the excessive or persistent activation of epithelial DUOX1 may contribute to the chronic activation of EGFR–STAT3 pathways, and may thereby contribute to the chronic wound responses seen in asthmatic airways (2, 41, 62). Indeed, animal studies have shown that the persistent activation of epithelial EGFR (63) and STAT3 (64) promotes airway hyperresponsiveness or remodeling in murine models of asthma. Moreover, because airway concentrations of ATP are commonly elevated in asthmatic airways (65), and epithelial DUOX1 is inducible by Th2 cytokines such as IL-13 (5, 15), such persistent EGFR/STAT3 activation and airway remodeling may be driven by an excessive activation of DUOX1.
Supplementary Material
Acknowledgments
The authors thank Nicole Bishop of the UVM Microscopy Imaging Center for assistance with confocal microscopy analysis.
Footnotes
This work received research support from National Institutes of Health grant R01 HL085646 (A.v.d.V.), the Department of Pathology, College of Medicine, University of Vermont (A.v.d.V.), and a Young Clinical Scientist Award from the Flight Attendant Medical Research Institute (P.C.S.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2012-0393OC on December 13, 2012
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med 2012;18:684–692 [DOI] [PubMed] [Google Scholar]
- 2.Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, et al. Defective epithelial barrier function in asthma. J Allergy Clin Immunol 2011;128:549–556 [DOI] [PubMed] [Google Scholar]
- 3.Wijkstrom-Frei C, El-Chemaly S, Ali-Rachedi R, Gerson C, Cobas MA, Forteza R, Salathe M, Conner GE. Lactoperoxidase and human airway host defense. Am J Respir Cell Mol Biol 2003;29:206–212 [DOI] [PubMed] [Google Scholar]
- 4.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 2007;175:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, DUOX1 and DUOX2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 2005;579:4911–4917 [DOI] [PubMed] [Google Scholar]
- 6.Gattas MV, Forteza R, Fragoso MA, Fregien N, Salas P, Salathe M, Conner GE. Oxidative epithelial host defense is regulated by infectious and inflammatory stimuli. Free Radic Biol Med 2009;47:1450–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ha EM, Lee KA, Seo YY, Kim SH, Lim JH, Oh BH, Kim J, Lee WJ. Coordination of multiple dual oxidase–regulatory pathways in responses to commensal and infectious microbes in Drosophila gut. Nat Immunol 2009;10:949–957 [DOI] [PubMed] [Google Scholar]
- 8.Ha EM, Oh CT, Bae YS, Lee WJ. A direct role for dual oxidase in Drosophila gut immunity. Science 2005;310:847–850 [DOI] [PubMed] [Google Scholar]
- 9.Flores MV, Crawford KC, Pullin LM, Hall CJ, Crosier KE, Crosier PS. Dual oxidase in the intestinal epithelium of zebrafish larvae has anti-bacterial properties. Biochem Biophys Res Commun 2010;400:164–168 [DOI] [PubMed] [Google Scholar]
- 10.El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, et al. Dual oxidase 2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 2005;288:G933–G942 [DOI] [PubMed] [Google Scholar]
- 11.Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 2009;122:3522–3530 [DOI] [PubMed] [Google Scholar]
- 12.Koff JL, Shao MX, Ueki IF, Nadel JA. Multiple TLRs activate EGFR via a signaling cascade to produce innate immune responses in airway epithelium. Am J Physiol Lung Cell Mol Physiol 2008;294:L1068–L1075 [DOI] [PubMed] [Google Scholar]
- 13.Shao MX, Nadel JA. Dual oxidase 1–dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci USA 2005;102:767–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boots AW, Hristova M, Kasahara DI, Haenen GR, Bast A, van der Vliet A. ATP-mediated activation of the NADPH oxidase DUOX1 mediates airway epithelial responses to bacterial stimuli. J Biol Chem 2009;284:17858–17867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wesley UV, Bove PF, Hristova M, McCarthy S, van der Vliet A. Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 2007;282:3213–3220 [DOI] [PubMed] [Google Scholar]
- 16.Koff JL, Shao MX, Kim S, Ueki IF, Nadel JA. Pseudomonas lipopolysaccharide accelerates wound repair via activation of a novel epithelial cell signaling cascade. J Immunol 2006;177:8693–8700 [DOI] [PubMed] [Google Scholar]
- 17.Forteza R, Salathe M, Miot F, Forteza R, Conner GE. Regulated hydrogen peroxide production by DUOX in human airway epithelial cells. Am J Respir Cell Mol Biol 2005;32:462–469 [DOI] [PubMed] [Google Scholar]
- 18.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009;459:996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rieger S, Sagasti A. Hydrogen peroxide promotes injury-induced peripheral sensory axon regeneration in the zebrafish skin. PLoS Biol 2011;9:e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Juarez MT, Patterson RA, Sandoval-Guillen E, McGinnis W. DUOX, Flotillin-2, and SRC42A are required to activate or delimit the spread of the transcriptional response to epidermal wounds in Drosophila. PLoS Genet 2011;7:e1002424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maretzky T, Evers A, Zhou W, Swendeman SL, Wong PM, Rafii S, Reiss K, Blobel CP. Migration of growth factor–stimulated epithelial and endothelial cells depends on EGFR transactivation by ADAM17. Nat Commun 2011;2:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng DC, Lin BH, Lim CP, Huang G, Zhang T, Poli V, Cao X. STAT3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol 2006;172:245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silver DL, Naora H, Liu J, Cheng W, Montell DJ. Activated signal transducer and activator of transcription (STAT) 3: localization in focal adhesions and function in ovarian cancer cell motility. Cancer Res 2004;64:3550–3558 [DOI] [PubMed] [Google Scholar]
- 24.Harada C, Kawaguchi T, Ogata-Suetsugu S, Yamada M, Hamada N, Maeyama T, Souzaki R, Tajiri T, Taguchi T, Kuwano K, et al. EGFR tyrosine kinase inhibition worsens acute lung injury in mice with repairing airway epithelium. Am J Respir Crit Care Med 2011;183:743–751 [DOI] [PubMed] [Google Scholar]
- 25.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Muller W, Whitsett JA. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol 2008;172:1542–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu R, Smith D. Continuous multiplication of rabbit tracheal epithelial cells in a defined, hormone-supplemented medium. In Vitro 1982;18:800–812 [DOI] [PubMed] [Google Scholar]
- 27.Alcorn JF, Guala AS, van der Velden J, McElhinney B, Irvin CG, Davis RJ, Janssen-Heininger YM. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-beta1. J Cell Sci 2008;121:1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bove PF, Hristova M, Wesley UV, Olson N, Lounsbury KM, van der Vliet A. Inflammatory levels of nitric oxide inhibit airway epithelial cell migration by inhibition of the kinase ERK1/2 and activation of hypoxia-inducible factor–1alpha. J Biol Chem 2008;283:17919–17928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Winkle LS, Isaac JM, Plopper CG. Distribution of epidermal growth factor receptor and ligands during bronchiolar epithelial repair from naphthalene-induced Clara cell injury in the mouse. Am J Pathol 1997;151:443–459 [PMC free article] [PubMed] [Google Scholar]
- 30.Lawson GW, Van Winkle LS, Toskala E, Senior RM, Parks WC, Plopper CG. Mouse strain modulates the role of the ciliated cell in acute tracheobronchial airway injury: distal airways. Am J Pathol 2002;160:315–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 2000;275:23227–23233 [DOI] [PubMed] [Google Scholar]
- 32.Boucher I, Rich C, Lee A, Marcincin M, Trinkaus-Randall V. The P2Y2 receptor mediates the epithelial injury response and cell migration. Am J Physiol Cell Physiol 2010;299:C411–C421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Block ER, Tolino MA, Klarlund JK. Extracellular ATP stimulates epithelial cell motility through PYK2-mediated activation of the EGF receptor. Cell Signal 2011;23:2051–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. DUOX maturation factors form cell surface complexes with DUOX affecting the specificity of reactive oxygen species generation. FASEB J 2009;23:1205–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurd TR, DeGennaro M, Lehmann R. Redox regulation of cell migration and adhesion. Trends Cell Biol 2012;22:107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Subramani S. Protein import into peroxisomes and biogenesis of the organelle. Annu Rev Cell Biol 1993;9:445–478 [DOI] [PubMed] [Google Scholar]
- 37.Park KS, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, Whitsett JA. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol 2006;34:151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Invest 2012;122:2724–2730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annu Rev Cell Dev Biol 2011;27:493–512 [DOI] [PubMed] [Google Scholar]
- 40.Burgel PR, Nadel JA. Epidermal growth factor receptor–mediated innate immune responses and their roles in airway diseases. Eur Respir J 2008;32:1068–1081 [DOI] [PubMed] [Google Scholar]
- 41.Puddicombe SM, Polosa R, Richter A, Krishna MT, Howarth PH, Holgate ST, Davies DE. Involvement of the epidermal growth factor receptor in epithelial repair in asthma. FASEB J 2000;14:1362–1374 [DOI] [PubMed] [Google Scholar]
- 42.Khan EM, Heidinger JM, Levy M, Lisanti MP, Ravid T, Goldkorn T. Epidermal growth factor receptor exposed to oxidative stress undergoes Src- and caveolin-1–dependent perinuclear trafficking. J Biol Chem 2006;281:14486–14493 [DOI] [PubMed] [Google Scholar]
- 43.Zhuang S, Schnellmann RG. H2O2-induced transactivation of EGF receptor requires Src and mediates ERK1/2, but not Akt, activation in renal cells. Am J Physiol Renal Physiol 2004;286:F858–F865 [DOI] [PubMed] [Google Scholar]
- 44.Gao SP, Bromberg JF. Touched and moved by STAT3. Sci STKE 2006;2006:pe30. [DOI] [PubMed] [Google Scholar]
- 45.Iwamaru A, Szymanski S, Iwado E, Aoki H, Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al. A novel inhibitor of the STAT3 pathway induces apoptosis in malignant glioma cells both in vitro and in vivo. Oncogene 2007;26:2435–2444 [DOI] [PubMed] [Google Scholar]
- 46.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol 2006;13:1235–1242 [DOI] [PubMed] [Google Scholar]
- 47.Crosby LM, Waters CM. Epithelial repair mechanisms in the lung. Am J Physiol Lung Cell Mol Physiol 2010;298:L715–L731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA 2007;104:410–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host–microbe homeostasis. Trends Immunol 2010;31:278–287 [DOI] [PubMed] [Google Scholar]
- 50.Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, De Deken X. Activation of dual oxidases DUOX1 and DUOX2: differential regulation mediated by cAMP-dependent protein kinase and protein kinase C–dependent phosphorylation. J Biol Chem 2009;284:6725–6734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boucher I, Yang L, Mayo C, Klepeis V, Trinkaus-Randall V. Injury and nucleotides induce phosphorylation of epidermal growth factor receptor: MMP and HB-EGF dependent pathway. Exp Eye Res 2007;85:130–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim JS, McKinnis VS, Nawrocki A, White SR. Stimulation of migration and wound repair of guinea-pig airway epithelial cells in response to epidermal growth factor. Am J Respir Cell Mol Biol 1998;18:66–74 [DOI] [PubMed] [Google Scholar]
- 53.Filosto S, Khan EM, Tognon E, Becker C, Ashfaq M, Ravid T, Goldkorn T. EGF receptor exposed to oxidative stress acquires abnormal phosphorylation and aberrant activated conformation that impairs canonical dimerization. PLoS ONE 2011;6:e23240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giannoni E, Buricchi F, Raugei G, Ramponi G, Chiarugi P. Intracellular reactive oxygen species activate Src tyrosine kinase during cell adhesion and anchorage-dependent cell growth. Mol Cell Biol 2005;25:6391–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zemans RL, Briones N, Campbell M, McClendon J, Young SK, Suzuki T, Yang IV, De Langhe S, Reynolds SD, Mason RJ, et al. Neutrophil transmigration triggers repair of the lung epithelium via beta-catenin signaling. Proc Natl Acad Sci USA 2011;108:15990–15995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo SK, Starnes TW, Deng Q, Huttenlocher A. Lyn is a redox sensor that mediates leukocyte wound attraction in vivo. Nature 2011;480:109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakanaga T, Nadel JA, Ueki IF, Koff JL, Shao MX. Regulation of interleukin-8 via an airway epithelial signaling cascade. Am J Physiol Lung Cell Mol Physiol 2007;292:L1289–L1296 [DOI] [PubMed] [Google Scholar]
- 58.Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB. STAT3 regulates genes common to both wound healing and cancer. Oncogene 2005;24:3397–3408 [DOI] [PubMed] [Google Scholar]
- 59.De Proost I, Pintelon I, Wilkinson WJ, Goethals S, Brouns I, Van Nassauw L, Riccardi D, Timmermans JP, Kemp PJ, Adriaensen D. Purinergic signaling in the pulmonary neuroepithelial body microenvironment unraveled by live cell imaging. FASEB J 2009;23:1153–1160 [DOI] [PubMed] [Google Scholar]
- 60.Puchelle E, Zahm JM, Tournier JM, Coraux C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006;3:726–733 [DOI] [PubMed] [Google Scholar]
- 61.Nagai K, Betsuyaku T, Suzuki M, Nasuhara Y, Kaga K, Kondo S, Nishimura M. Dual oxidase 1 and 2 expression in airway epithelium of smokers and patients with mild/moderate chronic obstructive pulmonary disease. Antioxid Redox Signal 2008;10:705–714 [DOI] [PubMed] [Google Scholar]
- 62.Holgate ST, Roberts G, Arshad HS, Howarth PH, Davies DE. The role of the airway epithelium and its interaction with environmental factors in asthma pathogenesis. Proc Am Thorac Soc 2009;6:655–659 [DOI] [PubMed] [Google Scholar]
- 63.Le Cras TD, Acciani TH, Mushaben EM, Kramer EL, Pastura PA, Hardie WD, Korfhagen TR, Sivaprasad U, Ericksen M, Gibson AM, et al. Epithelial EGF receptor signaling mediates airway hyperreactivity and remodeling in a mouse model of chronic asthma. Am J Physiol Lung Cell Mol Physiol 2011;300:L414–L421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simeone-Penney MC, Severgnini M, Tu P, Homer RJ, Mariani TJ, Cohn L, Simon AR. Airway epithelial STAT3 is required for allergic inflammation in a murine model of asthma. J Immunol 2007;178:6191–6199 [DOI] [PubMed] [Google Scholar]
- 65.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 2007;13:913–919 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.