Abstract
Characterization of N-glycans by liquid chromatography-positive electrospray ionization (ESI) tandem mass spectrometry (LC-MS/MS) using a microfluidic chip packed with porous graphitized carbon (PGC) represents a rapidly developing area in oligosaccharide analysis. Positive ion ESI-MS generates B/Y-type glycosidic fragment ions under collisional induced dissociation (CID). Although these ions facilitate glycan sequencing, they provide little information on linkage and positional isomers. Isomer identification in these cases is by retention on the PGC stationary phase where the specific structural isomers can, in principle, be separated. In this paper, we broaden the applicability of the PGC microfluidic chip/MS platform by implementing fluoride-mediated negative ESI-MS. Ammonium fluoride, added to the mobile phase, aids in the formation of pseudomolecular oligosaccharide anions due to the ability of fluoride to abstract a proton from the glycan structure. The negative charge results in the generation of C-type glycosidic fragments, highly informative A-type cross ring fragment ions and additional gas phase ion reaction products (e.g., D- and E-type ions), which, when combined, lead to in-depth oligosaccharide characterization, including linkage and positional isomers. Due to the separation of anomers by the PGC phase, comparison of oligosaccharides with an intact reducing terminus to their corresponding alditols was performed, revealing a more sensitive MS and, especially, MS/MS response from the glycans with a free reducing end. Fluoride also ensured recovery of charged oligosaccharides from the PGC stationary phase. Application to the characterization of N-glycans released from polyclonal human and murine serum IgG is presented to demonstrate the effectiveness of the chip/negative ESI approach.
Introduction
N-glycosylation is a diverse but physiologically important post-translational modification that modulates the physical, chemical and biological properties of proteins [1]. Structural elucidation of complex carbohydrates is necessary to gain a deeper understanding of the potential role of these post-translational modifications as functional modulators of therapeutic proteins [2, 3] and also as contributors to disease, e.g. cancer and inflammation [4, 5]. Liquid chromatographic and electrophoretic separation techniques, often coupled to mass spectrometry, have found widespread application for the characterization of protein N-glycosylation [6]. In particular, porous graphitized carbon (PGC) has become a popular LC stationary phase for hydrophilic oligosaccharides due to its ability to separate anomers and linkage and positional isomers [7]. Recently, a microfluidic chip containing an integrated nanoscale LC column packed with PGC has been introduced for nanoflow LC-MS (nLC-MS) analysis of oligosaccharides [8]. The chip offers the advantages of simplified operation and high reproducibility. Applications have been published demonstrating the characterization of oligosaccharides in the positive ion mode either as glycosylamines when coupled with an online PNGase F reactor or as alditols following off-line sample preparation [8–12].
Positive mode ionization of oligosaccharides results in the formation of [M+H]+ and [M+Na]+ pseudomolecular ions and adducts [13, 14]. Fragmentation of these ions by CID results in predominantly B/Y-type glycosidic cleavages [15]. These B/Y ions aid in the determination of the composition of the oligosaccharide; however, such ions generally do not allow elucidation of linkage and positional isomer information. Identification of these isomers is typically accomplished by retention differences on the PGC phase. Mass spectral structural analysis would clearly be useful to confirm the chromatographic identification, assuming an appropriate database was established. As an alternative approach, derivatization, such as permethylation, and multiple stages of tandem mass spectrometry have been used for isomer structure elucidation [16–18]. However, both the required sample amounts and associated method complexities are increased using such strategies. Furthermore, monosaccharide rearrangements during CID of protonated oligosaccharide cations, even following permethylation, have been reported which can complicate spectral annotation and structure assignment [19].
Negative ion ESI-MS of oligosaccharides has been developed using either elevated pH to promote glycan deprotonation or adduction with anions [20–22]. Anion adduction represents an attractive approach as it can be performed at neutral or acidic pH, an important feature since the basic pH limit of the microfluidic commercial chip used in this work was 8.0 [23]. Anions can form hydrogen bonds with hydroxyl groups present on the sugar rings, and, depending on the gas phase basicity of the specific anion, either stable negatively charged adducts can be formed [M + X]− where X is the anion, or a proton can be extracted by X− from the sugar ring generating [M − H]− with the neutral loss of HX [24–27].
Relative to positive ESI, analysis of glycans in the negative ion mode has several potential advantages. First, and most importantly, CID MS/MS spectra of oligosaccharide anions contain, besides B/Y ions, C-type glycosidic and A-type cross ring fragments as well as highly informative D- and E-type ions, which, when combined, facilitate annotation of linkage and positional isomers [13, 20–22, 28]. Secondly, monosaccharide rearrangements during CID fragmentation observed in positive ESI have generally not been seen for negative ESI [19]. Migration of sulfate was recently reported using a linear ion trap; however, the migration appeared to be overcome when converting from CID to HCD fragmentation [29]. On the other hand, the overall sensitivity in the negative ion mode is found to be lower than in the positive ion mode for neutral glycans [30]; however, higher sensitivity in the negative ion mode can be observed for negatively charged glycans, e.g. those with sialic acids.
In this paper, we have broadened the applicability of the PGC chip coupled to MS for the analysis of glycans using negative ESI. The performance of different ammonium anions added to the mobile phase was first examined, from which ammonium fluoride at neutral pH in a water/acetonitrile mobile phase was selected. The high gas phase basicity of the fluoride ion was utilized to promote anion attachment and proton extraction from the oligosaccharides [26]. Application of the method for the analysis of glycans with a free reducing end and their corresponding alditol was also investigated, revealing more sensitive MS and, especially, MS/MS response for the non-reduced oligosaccharides. The combination of PGC separation and MS analysis resulted in high confidence structural elucidation of linkage and positional isomers without the need for additional strategies such as exoglycosidase digestion [31]. The GlycoWorkBench platform [32] was successfully used for spectral interpretation and structural annotation. To demonstrate the applicability of the chip-based nLC-MS negative ESI platform, N-linked oligosaccharides present on polyclonal antibodies extracted from human and murine sera have been characterized, demonstrating the ability of the method to determine both linkage and positional isomers in complex oligosaccharide pools, including not only the traditional G0, G1/G1’ and G2 series but also the presence of galactose α-1-3 linked galactose residues and N-glycolyl neuraminic acid on the N-glycans liberated from the murine antibodies.
Experimental
Details regarding materials used and sample preparation are described in the Supporting Information.
Negative ion electrospray ionization mass spectrometry (nESI-MS)
Direct infusion, negative ionization electrospray mass spectrometry (nESI-MS) was performed on an Agilent 6520 Series Q-TOF instrument (Agilent Technologies, Palo Alto, CA) under the control of MassHunter Data Acquisition Software. Samples were prepared in 50% v/v water:acetonitrile containing 10 mM ammonium fluoride, ammonium chloride or ammonium bicarbonate. Samples were infused at a constant flow rate of 1 µL/min using a nanospray voltage of 1950 V with a drying gas of 4 L/min nitrogen at 325°C. The fragmentor voltage was set at 175 V, and the skimmer voltage was at 65 V. For tandem mass spectrometry, the instrument was operated using the automatic MS/MS switching mode, with the m/z range for MS from 400 to 2000 and 100 to 3000 for MS/MS spectral acquisition. The collision energy was set at 35 V. Data analysis details can be found in the supporting information.
LC-MS analysis
Oligosaccharides were separated on an Agilent Technologies HPLC Chip II (Waldbronn, Germany), consisting of a 40 nL enrichment column and a 75 µm i.d. × 43 mm separation column, both packed with porous graphitized carbon. Mobile phase A was 10 mM ammonium fluoride in 97% water and 3% acetonitrile, and mobile phase B was 10 mM ammonium fluoride in 10% water and 90% acetonitrile. Samples were transferred to the microfluidic chip in 100% mobile phase A using the capillary pump at a flow rate of 4 µL/min. The nanoflow pump was employed to generate the analytical gradient with a flow rate of 500 nL/min. The gradient for standard oligosaccharides separation was 0% B, 0−2.5 min; 0−16% B, 2.5−20 min; 16−44% B, 20−30 min; 44−100% B, 30−35 min; and 100% B, 35−45 min with a return to initial starting conditions in 1 minute, followed by an isocratic hold for 20 minutes to ensure complete column re-equilibration.
Results and Discussion
Utilization of a microfluidic chip packed with porous graphitized carbon (PGC) for LC-MS of glycans has become a popular approach because of the ease of operation and reproducibility. To date, the platform has operated in the positive ionization mode, which, as noted earlier, has limitations in the annotation of linkage and positional isomers. To broaden the applicability of the PGC chip/MS platform, we describe negative ESI LC-MS (Q-TOF) for the characterization of the N-glycans, demonstrating the effectiveness of the approach in the analysis of the N-glycans present on human and murine polyclonal serum antibodies. The PGC packing separates reducing end anomers, positional and linkage isomers, while negative ESI-MS yields in depth N-glycan analysis providing specific structural information not available by positive ESI-MS. Although the separated anomers can increase the complexity of the analysis, reduction can be performed for their removal. However, comparison of reducing sugars with their corresponding alditols revealed superior MS and MS/MS sensitivity for the reducing sugars (see below). The presence of fluoride in the mobile phase is not only demonstrated to successfully generate informative negative ESI but also to aid in the recovery of charged oligosaccharides from the retentive PGC phase.
Preliminary Investigation of anion-mediated negative ion using direct infusion Q-TOF MS
Governed by an upper operating pH limit of pH 8.0 on the current commercial microfluidic chip, we first identified a series of organic and inorganic anions, present in the anionic form at pH 7.0, for evaluation for anion-mediated negative ionization of oligosaccharides on the Q-TOF MS. Previous reports have recommended, among others, the use of nitrate and phosphate for static infusion anion mediated MS of oligosaccharides [20]. However, considering the addition of the anion to the LC mobile phase and the flow rates used (0.5 µL/min), these anions were not considered acceptable, given the potential of ion source and MS fouling. From the initial list of anions identified, we selected fluoride, chloride and bicarbonate for initial study by direct infusion, based on their high gas phase basicity and suitability for LC-MS. Although fluoride was previously reported in one case to yield high levels of in-source fragmentation [20], other studies found the anion to be effective in improving sensitivity for small molecules in negative ion LC-MS [26, 27, 33].
Mass spectrometric parameters affecting ion transmission were first optimized to ensure optimal transport of the [M−H]− and [M + anion adducts]− through the mass analyzer for each of the three anions investigated. The fragmentor voltage, (voltage governing ion transport through the instrument optics) was varied from 90 to 250 V in 10 V intervals. Negligible in-source fragmentation occurred for all three anions when transmission voltages less than 180 V were employed. Above 180 V, the ion signal decreased, presumably due to in-source fragmentation. Separately, the collision energy (CE) was optimized to maximize CID-MS/MS spectral sensitivity. At low CE values, minimal glycan fragmentation was observed while at high CE values, glycosidic ions and cross-ring fragments ions were found to undergo additional cleavage leading to loss of spectral information. An optimum collision energy of 35 V was selected for use in all subsequent experiments for all three anions.
In the case of ammonium chloride, it was found that infusion of 10 mM ammonium chloride prepared in 50/50 MeCN/H2O at 1 µL/min resulted in an intense series of ammonium chloride ion clusters which dominated the resulting MS spectra, in agreement with that reported by others when using FT-ICR-MS [34]. These intense adduct clusters reduced overall sensitivity, with the resulting MS/MS spectra containing product ions of multiple chloride adducts that complicated annotation (data not shown). Based on these results, chloride was eliminated from further study.
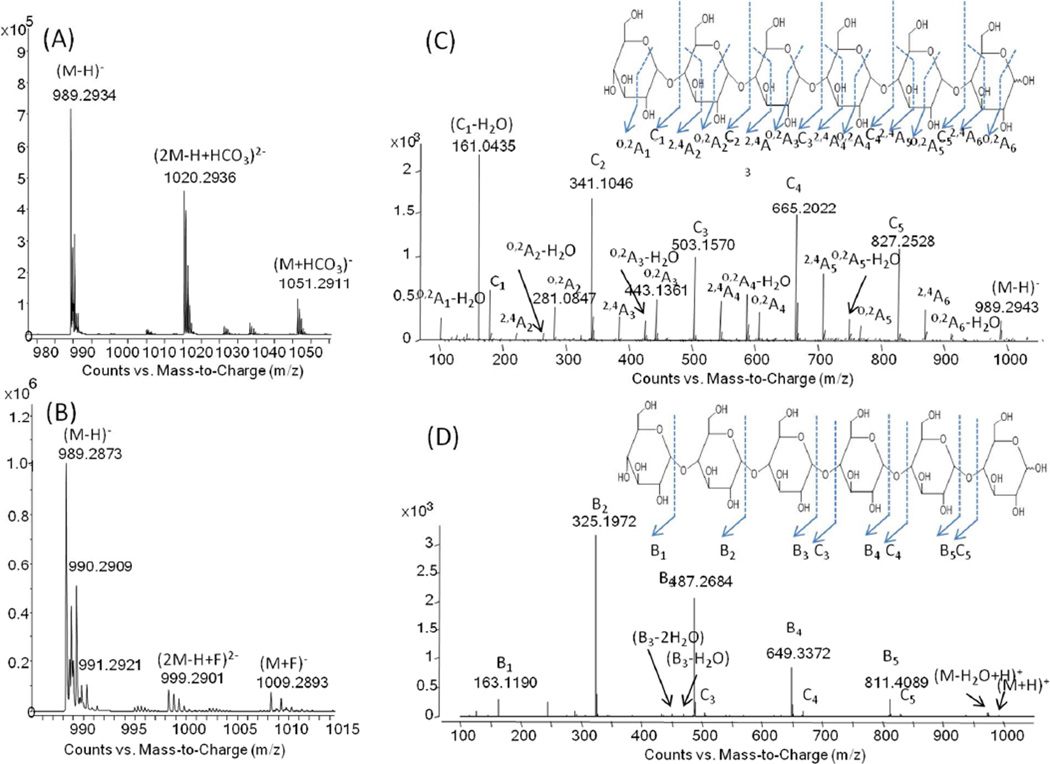
We next examined fluoride and bicarbonate, again by infusion of the oligosaccharides prepared in 10 mM solutions of anion dissolved in 50/50 acetonitrile/H2O. Although the use of ammonium bicarbonate yielded a buffer solution pH above the upper limit of the microfluidic chip, it was included as a benchmark based upon its use in previous studies [35]. The infusion results revealed that the levels of adduction present in the mass spectra with bicarbonate were significantly higher than when fluoride was employed, see Fig. 1 (A) vs. Fig. 1 (B). This trend was not surprising given the higher gas phase basicity of fluoride compared to bicarbonate [26, 27], resulting in fluoride more readily able to extract a proton from the oligosaccharide than bicarbonate. Given the higher levels of adduction in multiple forms for bicarbonate, the overall precursor MS sensitivity was 1.5 fold higher for fluoride as a dominant [M−H]− pseudomolecular oligosaccharide anion was produced, Fig. 1 (B). Based on these results, the focus of our study became fluoride-mediated negative mode ionization. The successful use of fluoride in the present study as opposed to other studies where fluoride was observed to yield significant in-source fragmentation [20] appears to be due to the different source configuration and associated energetics of the instrument used in this study [36].
Fig.1.
Positive and negative ESI ion CID analysis of infused maltohexaose glucose-α-1-4-Glucose (Dp6). Precursor ions of [M−H]− and adducts of Dp6 in negative ion analysis with (A) 10 mM ammonium bicarbonate and (B) 10 mM ammonium fluoride in 50% water and 50% acetonitrile; (C) annotated negative ion CID-MS/MS of the pseudomolecular anion of Dp6 [M−H]− in 10 mM ammonium fluoride in 50% of water and 50% of acetonitrile; (D) annotated positive ion CID-MS/MS of the protonated ion of Dp6 [M+H]+ in 0.1% formic acid in 50% of water and 50% of acetonitrile. The infusion flow rate was set at 1 µL/min and the fragmentor voltage at 175 V. Additional details can be found in the Experimental Section.
Infusing 10 mM ammonium fluoride in 50/50 (v/v) acetonitrile/H2O, the [M−H]− pseudomolecular anion of maltohexaose (glucose α-1-4 glucose) was fragmented by CID leading to a highly informative spectrum containing an intense C-type glycosidic series for determination of the oligosaccharide sequence, Fig. 1 (C). Importantly, and in contrast to the positive ion mode, intense 2,4A, O,2A and O,2A-H2O ion series, arising from cross-ring fragmentation, were observed, leading to the determination of the specific linkage. For comparison purposes, positive ion CID-MS/MS was also performed on the [M+H]+ pseudomolecular ion for maltohexaose. As expected, only B/Y-type glycosidic fragments were identified for positive ESI with no information rich cross ring fragments observed, see Fig. 1 (D). Sensitivity was approximately five times higher in the positive, relative to the negative ion mode, as expected for neutral glycans [8]; however, the overall level of information generated was higher in the negative mode, allowing for a more in-depth oligosaccharide characterization. Sensitivity would be expected to be higher in the negative ion mode for negatively charged glycans, e.g. sialylated oligosaccharides [13].
As further examples of the success in linkage determination in fluoride-mediated negative ESI, cellohexaose (glucose-β-1-4-glucose), glucose-α-1,6-glucose hexamer and laminarihexaose (glucose-β-1-3-glucose) were infused in the negative ion mode into the Q-TOF-MS. A strong C-type glycosidic ion series was observed for all three solutes, see Fig. SI-1. A-type cross ring fragments were obtained for the cellohexaose (2,4A, O,2A ions) and α-1-6-glucose hexamer (3,5A, O,3A ions), suggesting the ability to decipher linkage orientation of the glycosidic bond from the MS/MS spectra, see Figs. SI-1 (A) and (C), respectively. For the β-1-3 linked laminarihexaose, only C-type glycosidic ions were found in the CID-MS/MS spectrum of the [M−H]− pseudomolecular anion, see Fig. SI-1 (B). The presence of a free hydroxyl group at the C-3 position was previously noted to be required for initiation of cross-ring fragmentation [37]; thus, the absence of an A-ion series in the MS/MS spectrum of laminarihexose is consistent with earlier reports [20–22, 37].
When performing fluoride-mediated negative ionization for all the linear oligosaccharides, three precursor ions were observed: a dominant pseudomolecular [M−H]− anion and two lower level fluoride adducts [M+F]− and [2M−H+F]2− of lower intensity. The CID MS/MS spectra of each adduct was found to be identical to that observed for the pseudomolecular [M−H]− anion, see Fig. SI-2 and Fig. 1 (C), respectively. In contrast to other anions, no product ions were found that retained the anionic adduct in the MS/MS spectra. This lack of adduction of MS/MS product ions also simplified data analysis using GlycoWorkBench, wherein spectral annotation could be automatically performed without the need for custom modification to account for the incorporation of any potential fluoride adduction. The dominant production of [M−H]− compared to the fluoride adducts suggests that the gas phase basicity of the linear oligosaccharides is considerably lower than that of the highly basic fluoride anion, such that the extraction of a proton by fluoride with subsequent neutral loss as HF is facilitated [24–27, 38].
Fluoride-mediated negative ion nLC-MS/MS using a microfluidic chip
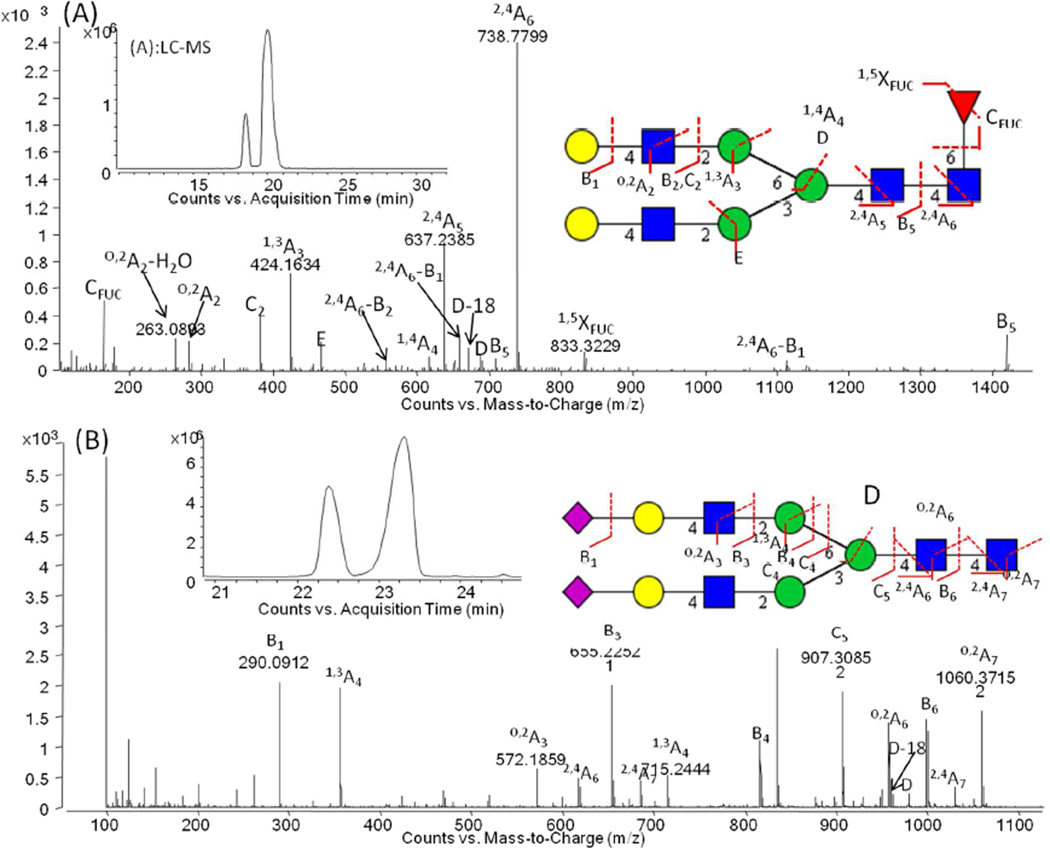
Given the success of the infusion results with fluoride in negative ESI, we next turned to utilize the fluoride containing solvent system for LC separation on the PGC chip. The application of PGC-based stationary phases for oligosaccharide LC-MS was recently reviewed [7]. A concern when using PGC phases is incomplete analyte elution, particularly for sialylated oligosaccharides [39, 40]. Therefore, the fluoride concentration in the nLC-MS mobile phase was investigated, in the range of 10 µM to 100 mM, using the neutral core fucosylated bi-antennary digalactosyl glycan (FA2G2) and the acidic bi-antennary digalactosyl disialylated glycan (A2G2S2) as model compounds to analyze recovery, ionization efficiency and spectral content. At low fluoride concentrations, A2G2S2 was retained on the PGC column (data not shown), while recovery was complete at fluoride concentrations greater than 10 mM. Thus, fluoride not only aids in negative analyte ionization but also in recovery from the PGC chip.
Under optimized conditions, both glycans eluted as a split peaks, due to the anomeric separation (α or β reducing-end anomers) by the PGC stationary phase, Fig. 2 (A) and (B) for FA2G2 and A2G2S2, respectively. Detection limits were estimated as 92 fmol and 12 fmol for FA2G2 and A2G2S2, respectively, determined as the sum of the two anomeric peaks with a S/N level of 3 for the lower level anomer. As expected for negative ESI, the sialylated species had a roughly 8 fold lower detection limit than the neutral species. Additionally, it is important to note that the detection limits can be further lowered using next generation Q-TOF instrumentation containing ion funnels for more efficient ion transmission into the mass spectrometer [41].
Fig.2.
Extracted ion chromatogram and negative CID-MS/MS spectra of (A) FA2G2 and (B) A2G2S2. Monosaccharide symbol key - blue square: GlcNAc, green circle: mannose, red triangle: fucose, yellow circle: galactose, pink diamond: N-acetylneuraminc acid (Neu5Ac), white diamond: N-glycolylneuraminic acid (Neu5Gc). The buffer contained 10 mM ammonium fluoride. Additional details in the Experimental Section.
CID-MS/MS fragmentation produced glycosidic ions and cross-ring fragments, as expected. The composition of the glycans was derived from the precursor ion for FA2G2 and A2G2S2, respectively, and fragment ions present in the resulting fluoride-mediated MS/MS spectra, Fig. 2 (A) and (B) for FA2G2 and A2G2S2, respectively. The MS/MS spectrum of the neutral FA2G2 N-glycan, Fig. 2 (A), was dominated by the 2,4A6 ion arising from cleavage across the reducing terminal GlcNAc residue, (m/z 738.7799, z=2). The composition of the antennae was confirmed based on the presence of the 1,3A3 (m/z 424.1634, z=1) and 0,2A2 − H20 (m/z 263.0893, z=1) fragments, the C2 glycosidic fragment (m/z 382.1526, z=1) and the D (m/z 688.2495, z=1), D-18 (m/z 670.2422, z=1) and E ions (m/z 466.1780, z=1). The presence of the fucose residue was confirmed by the CFUC (m/z 163.0701, z=1) and 1,5XFUC ions (m/z 833.3229, z=2).
The MS/MS spectrum of the acidic A2G2S2 oligosaccharide, Fig. 2 (B), showed ions of m/z 958.3197 (z=2) and 1059.8643 (z=2) with relative high intensity arising from O,2A cross-ring cleavage of the GlcNAc residues chitobiose core. 2,4A cross-ring fragments (m/z 618.5431, z=3 and 686.2323, z=3) of the chitobiose core were also observed. Ions present at m/z 979.3205 (z=1) and m/z 961.3167 (z=1) corresponded to the D and D-18 ion series, previously shown to be characteristic of the composition of the antenna extending from the α-1-6 linked antennary mannose residue [21, 22].
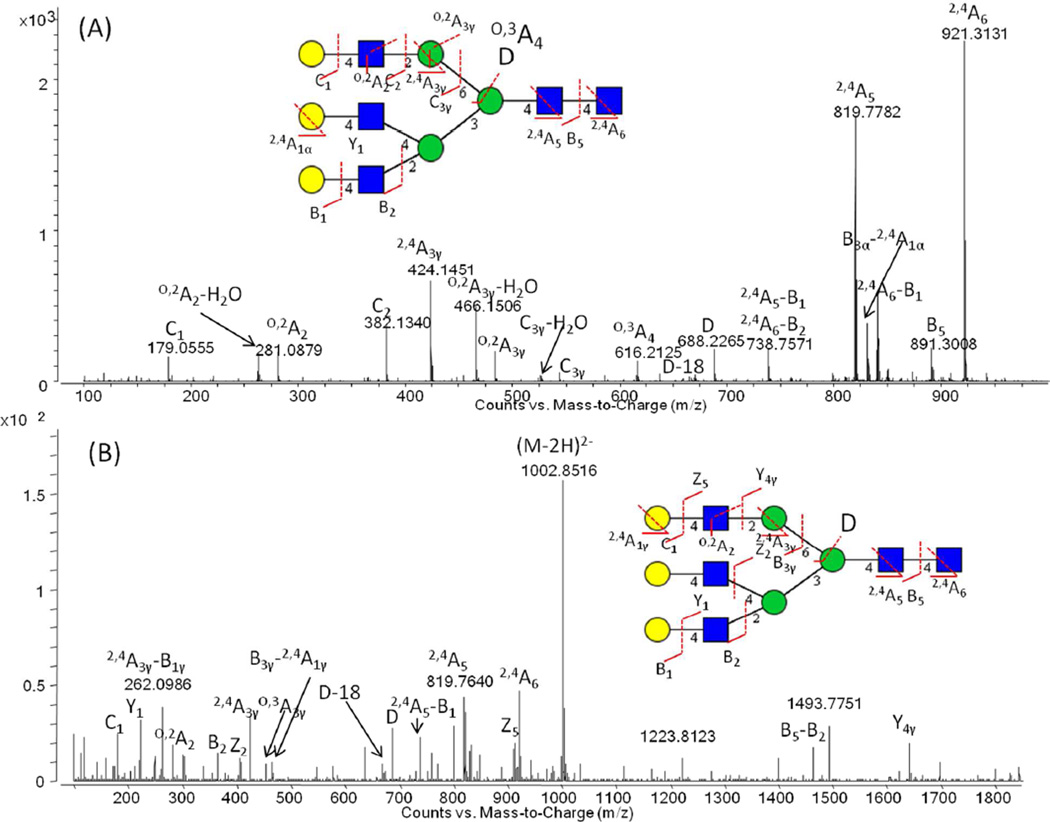
Given the separation of the anomers on the PGC packing, some researchers have reduced the oligosaccharides to generate single peak alditols [35]; therefore, we decided to compare the two approaches. Figs. 3 (A) and (B) show MS/MS spectra with a free reducing terminal GlcNAc (A) and the corresponding alditol (B) for the triantennary trigalatosyl glycan, A3G3. As can be seen in the figure, the aldose form is at least 10 fold higher in MS/MS sensitivity. The fragmentation spectrum for the aldose form is also seen to be considerably more informative due to the presence of more intense D- and D-18 ions. In addition, we found the precursor signal to be 4 or more fold higher for the aldose form. These differences in MS and MS/MS signals were found to be generally true for a variety of glycan structures. Furthermore, a much higher collision energy was required for the fragmentation of the alditols due to the increased stability of the [M−H]− pseudomolecular ion of this form. In addition, the generation of alditols results in increased sample preparation steps (reaction and clean up). Indeed, a recent study has recommended the use of released N-glycans with the native reducing aldose form because of its greater simplicity [42]. As a consequence of the above, we have used the aldose form in our studies, in spite of the anomeric peak splitting.
Fig.3.
Negative CID-MS/MS spectra of (A) the N-glycan with a free reducing terminal GlcNAc residue and (B) the corresponding alditol for the triantennary trigalactosyl N-glycan (A3G3). Monosaccharide symbols as described in Fig. 2. Further experimental details in Fig. 2.
Human and murine polyclonal serum IgG N-glycan analysis using fluoride-mediated negative ion LC-MS/MS
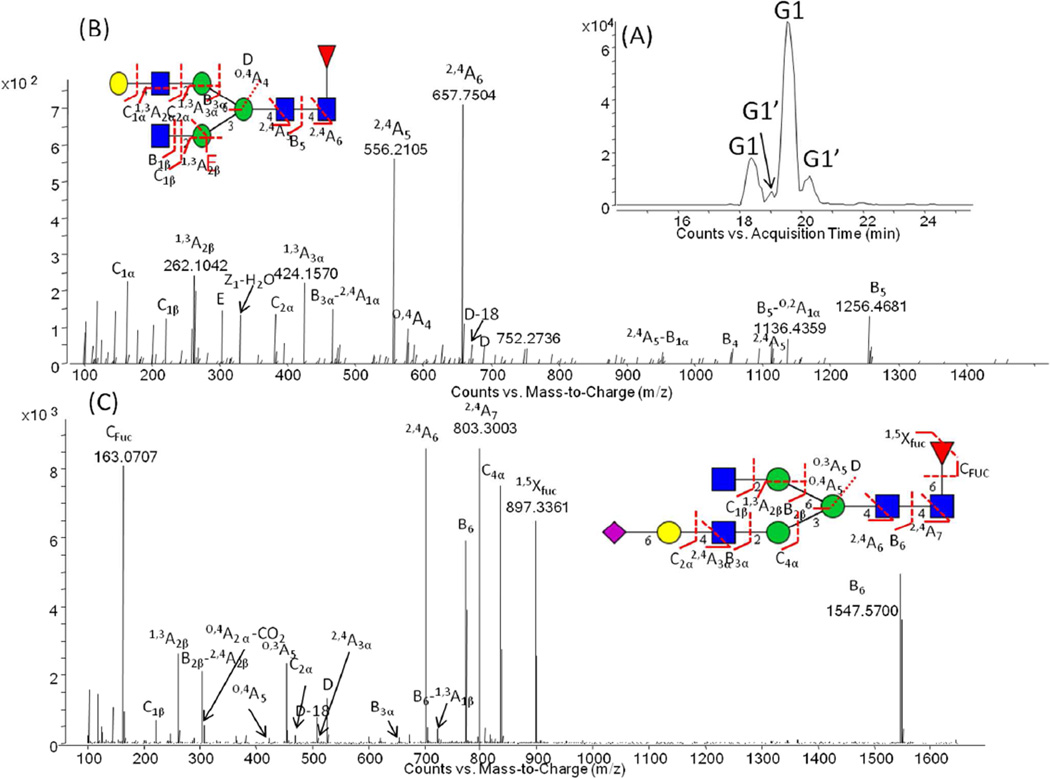
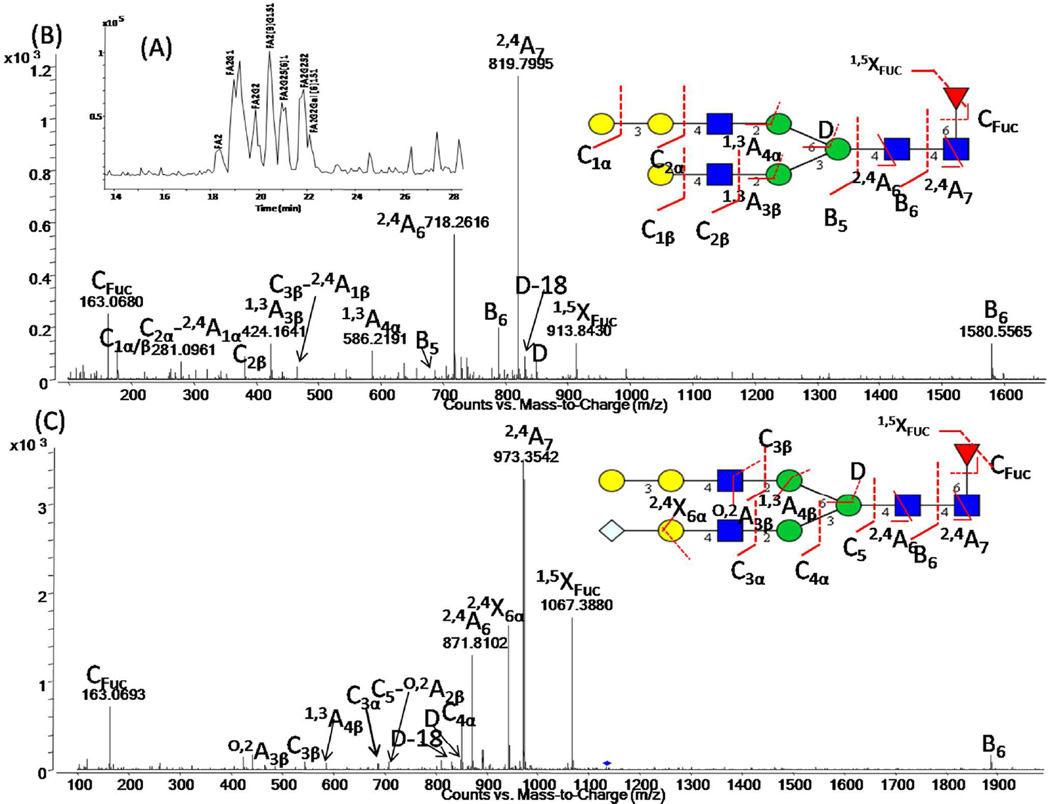
To demonstrate the power of the methodology, we applied our fluoride-mediated LC-MS platform for the characterization of the N-linked oligosaccharides present on polyclonal IgG extracted from human and murine serum. N-glycans present at asparagine 297 in the CH2 domain of an antibody play a crucial role in stabilizing the structure of the CH2 domain and also in modulating the interaction of the Fc region of the antibody with Igγ receptors present on the surface of cells of the innate immune system [43–45]. The ability to characterize these N-glycans in detail is important both from the regulatory point of view when considering monoclonal antibody therapeutics [3] and also as these N-glycans have been shown to be altered in disease [46]. Following Protein G enrichment, N-glycans from polyclonal human and murine serum IgGs were enzymatically liberated and analyzed using the fluoride-mediated negative ionization microchip LC-MS/MS platform. Previously, we performed an exhaustive analysis of both samples using ultrahigh-performance hydrophilic interaction liquid chromatography and capillary electrophoresis with laser induced fluorescence detection, with exoglycosidase digestion for structural annotation [31, 47]. Table SI-1 lists N-glycans, including low abundance afucosylated glycans, detected on polyclonal human serum IgG using fluoride-mediated accurate mass Q-TOF LC-MS/MS using the information rich glycosidic and cross ring fragments. Shown in Fig. 4 (A) is the separation of F(6)A2G[6]1 and F(6)A2G[3]1, more commonly referred to as G1 and G1’, respectively, demonstrating the ability to resolve these positional isomers on the short 43 mm PGC column on the microfluidic chip. Fig. 4 (B) depicts an annotated spectrum for F(6)A2G[6]1 wherein the position of the galactose residue is confirmed by the presence of the D and D-18 ion couple. The significance of these two ions, which reveal the composition of the antenna extending from the α-1-6 branching mannose, was previously discussed [21, 22]. In addition, linkage specificity could also be determined from the fluoride-mediated negative ion MS/MS spectra, as shown in Fig 4 (C). Here, the oligosaccharide structure was annotated as F(6)A2G[3]1S(6)1, based on the presence of the D and D-18 ion couple, the C2α glycosidic fragment and the O,4A2α−CO2, 2,5A3α and 0,3A5 cross ring fragments [22, 48]. The annotation of this structure is in agreement with that determined in our previous studies [31], without the need for exoglycosidase digestion.
Fig.4.
(A) Extracted ion chromatogram of F(6)A2G[6]1, more commonly known as G1 and F(6)A2G[3]1, more commonly known as G1’. (B) Annotated ESI negative ion CID MS/MS spectrum for F(6)A2G[6]1 at the retention time of 19.6 min. (C) Negative ion CID-MS/MS spectra of F(6)A2G[3]1S(6)1 at the retention time of 20.7 min , indicating diagnostic fragments for comprehensive structural annotation. Monosaccharide symbols as described in Fig. 2. Further experimental details in Fig. 2.
The analysis of the murine serum polyclonal N-glycans using fluoride-mediated negative ion LC-MS/MS revealed the presence of antigenic epitopes, including galactose-α-1-3-galactose (see Table SI-2) in agreement with our previous report [47] and also the presence of N-glycolylneuraminic acid (Neu5Gc) in a significantly higher quantity than N-acetylneuraminic acid (Neu5Ac) as the terminal monosaccharide present on the antennary chains of the oligosaccharides. Fig. 5 (A) depicts an example base peak chromatogram for the separation of the murine IgG N-glycan pool. Both glycan epitopes have an immunogenic potential in humans [49–52]. Fig. 5 depicts MS/MS spectra for two oligosaccharides containing galactose-α-1-3-galactose epitopes (B) and Neu5Gc residues (C), as determined in a position specific manner, based on the presence of D and D-18 ions and A ion cross ring fragments. The annotated structures are in agreement with those previously annotated using capillary electrophoresis with laser induced fluorescence detection and exoglycosidase digestion [47]. Importantly, the N-glycan structure, F(6)A2G2Gal[6]1S(Neu5Gc)[3]1, as annotated from the MS/MS spectrum in Fig. 5 (C), was determined to contain both α-1-3-galactose and Neu5Gc epitopes. The position of attachment was also revealed. Based on the MS/MS data, the α-1-3-galactose epitope was determined to be attached to the galactose residue on the antenna extending from the α-1-6-core mannose, whereas the Neu5Gc residue was found to be on the galactose residue on the antenna extending from the α-1-3-core mannose. This result suggests that the presence of acidic amino acids in the primary protein sequence of the CH2 domain play an important role in governing the processing of the glycans antenna [44, 46]. Table SI-2 contains details of all oligosaccharide structures annotated in the murine polyclonal IgG N-glycan pool by fluoride-mediated negative ion ESI. The results of the IgG glycan analysis of human and mouse demonstrate the potential of negative ESI glycan characterization including linkage and positional isomer determination.
Fig.5.
(A) Base peak chromatogram of murine IgG; annotated ESI negative ion CID-MS/MS spectra of (B) FA2G2Gal[6]1 at the retention time of 21.3 min and (C) F(6)A2G2Gal[6]1S(Neu5Gc)[3]1 at the retention time of 22.4 min. Monosaccharide symbols as described in Fig. 2. Further experimental details in Fig. 2.
Conclusion
We describe the use of fluoride-mediated negative ion LC-MS/MS for the in-depth characterization of N-linked oligosaccharides. Fluoride added to the mobile phase facilitates the generation of oligosaccharide pseudomolecular anions in the negative ion mode due to the high gas phase basicity of the fluoride anion. Ionization proceeds via a two-step process, the fluoride ion initially hydrogen bonds with the proton on C3 of the reducing terminal N-acetyl glucosamine ring, followed by proton abstraction and neutral loss of HF. The efficiency of proton abstraction by fluoride facilitates high sensitivity analysis due to the dominance of [M−H]− ions in the resulting MS spectrum. CID MS/MS analysis of these pseudomolecular oligosaccharide anions resulted in the generation of C-type glycosidic fragments, highly informative A-type cross ring fragment ions and D- and E-type ion reaction fragments, which, when combined, facilitated oligosaccharide compositional characterization. Furthermore, a comparison of the behavior of reducing sugars and their reduced alditol counterparts revealed significantly higher MS and MS/MS sensitivity for the oligosaccharides with the aldose compared to the alditol. Besides the higher LC-MS/MS sensitivity and thus higher information content of the aldose, omitting the reducing step simplifies the sample preparation. Additionally, use of the aldoses creates the possibility to ultimately use the PNGase F microfluidic chip thereby generating an automated glycan analysis platform.
The presence of fluoride in the mobile phase also ensured recovery of sialylated oligosaccharides from the PGC column. Overall method sensitivity was evaluated and found to be similar to levels attainable when using fluorescence detection of derivatized glycans. The combination of the orthogonal information provided through isomeric separation on the PGC phase, accurate mass Q-TOF MS analysis and information rich MS/MS spectra, all generated in a single analysis, facilitates detailed oligosaccharide structural elucidation. Indeed, the platform is complementary to traditionally used positive ion LC-MS.
The method has been applied to the characterization of polyclonal human and murine serum IgG N-glycans. The combination of PGC separation, with accurate mass fluoride-mediated negative ion Q-TOF-MS and CID-MS/MS analysis, allowed deep characterization of the N-glycans present, including the identification of glycans displaying the potentially immunogenic galactose-α-1-3-galactose epitope and Neu5Gc residues in both a linkage and positionally specific manner. We are currently investigating the application of the platform for the characterization of glycoproteins bearing larger N-glycans with higher degrees of sialylation.
Supplementary Material
Acknowledgements
This work was supported by NIH GM 15847. The authors also thank Dr. James Glick for experimental assistance and helpful comments and PhyNexus for the donation of the PGC packed PhyTips. Contribution number 1019 from the Barnett Institute.
References
- 1.Rudd PM, Dwek RA. Glycosylation: heterogeneity and the 3D structure of proteins. Crit Rev Biochem Mol Biol. 1997;32(1):1–100. doi: 10.3109/10409239709085144. [DOI] [PubMed] [Google Scholar]
- 2.Jefferis R. Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov. 2009;8(3):226–234. doi: 10.1038/nrd2804. [DOI] [PubMed] [Google Scholar]
- 3.Read EK, Park JT, Brorson KA. Industry and regulatory experience of the glycosylation of monoclonal antibodies. Biotechnol Appl Biochem. 2011;58(4):213–219. doi: 10.1002/bab.35. [DOI] [PubMed] [Google Scholar]
- 4.An HJ, et al. Glycomics and disease markers. Curr Opin Chem Biol. 2009;13(5–6):601–607. doi: 10.1016/j.cbpa.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao YY, et al. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci. 2008;99(7):1304–1310. doi: 10.1111/j.1349-7006.2008.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marino K, et al. A systematic approach to protein glycosylation analysis: a path through the maze. Nat Chem Biol. 2010;6(10):713–723. doi: 10.1038/nchembio.437. [DOI] [PubMed] [Google Scholar]
- 7.Ruhaak LR, Deelder AM, Wuhrer M. Oligosaccharide analysis by graphitized carbon liquid chromatography-mass spectrometry. Anal Bioanal Chem. 2009;394(1):163–174. doi: 10.1007/s00216-009-2664-5. [DOI] [PubMed] [Google Scholar]
- 8.Bynum MA, et al. Characterization of IgG N-glycans employing a microfluidic chip that integrates glycan cleavage, sample purification, LC separation, and MS detection. Anal Chem. 2009;81(21):8818–8825. doi: 10.1021/ac901326u. [DOI] [PubMed] [Google Scholar]
- 9.Aldredge D, et al. Annotation of a serum N-glycan library for rapid identification of structures. J Proteome Res. 2012;11(3):1958–1968. doi: 10.1021/pr2011439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu CS, et al. Profile of native N-linked glycan structures from human serum using high performance liquid chromatography on a microfluidic chip and time-of-flight mass spectrometry. Proteomics. 2009;9(7):1939–1951. doi: 10.1002/pmic.200800249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hua S, et al. Comprehensive native glycan profiling with isomer separation and quantitation for the discovery of cancer biomarkers. Analyst. 2011;136(18):3663–3671. doi: 10.1039/c1an15093f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ninonuevo M, et al. Nanoliquid chromatography-mass spectrometry of oligosaccharides employing graphitized carbon chromatography on microchip with a high-accuracy mass analyzer. Electrophoresis. 2005;26(19):3641–3649. doi: 10.1002/elps.200500246. [DOI] [PubMed] [Google Scholar]
- 13.Leymarie N, Zaia J. Effective use of mass spectrometry for glycan and glycopeptide structural analysis. Anal Chem. 2012;84(7):3040–3048. doi: 10.1021/ac3000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaia J. Mass spectrometry of oligosaccharides. Mass Spectrom Rev. 2004;23(3):161–227. doi: 10.1002/mas.10073. [DOI] [PubMed] [Google Scholar]
- 15.Domon B, Costello CE. A systematic nomenclature for crbohydrate fragmentation in FABMS MS spectra of glycoconjugates. Glycoconjugate Journal. 1988;5:397–409. [Google Scholar]
- 16.Costello CE, Contado-Miller JM, Cipollo JF. A glycomics platform for the analysis of permethylated oligosaccharide alditols. J Am Soc Mass Spectrom. 2007;18(10):1799–1812. doi: 10.1016/j.jasms.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang P, Mechref Y, Novotny MV. High-throughput solid-phase permethylation of glycans prior to mass spectrometry. Rapid Commun Mass Spectrom. 2008;22(5):721–734. doi: 10.1002/rcm.3395. [DOI] [PubMed] [Google Scholar]
- 18.Mechref Y, Kang P, Novotny MV. Solid-phase permethylation for glycomic analysis. Methods Mol Biol. 2009;534:53–64. doi: 10.1007/978-1-59745-022-5_4. [DOI] [PubMed] [Google Scholar]
- 19.Wuhrer M, Deelder AM, van der Burgt YE. Mass spectrometric glycan rearrangements. Mass Spectrom Rev. 2011;30(4):664–680. doi: 10.1002/mas.20337. [DOI] [PubMed] [Google Scholar]
- 20.Harvey DJ. Fragmentation of negative ions from carbohydrates: part 1. Use of nitrate and other anionic adducts for the production of negative ion electrospray spectra from N-linked carbohydrates. J Am Soc Mass Spectrom. 2005;16(5):622–630. doi: 10.1016/j.jasms.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Harvey DJ. Fragmentation of negative ions from carbohydrates: part 2. Fragmentation of high-mannose N-linked glycans. J Am Soc Mass Spectrom. 2005;16(5):631–646. doi: 10.1016/j.jasms.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Harvey DJ. Fragmentation of negative ions from carbohydrates: part 3. Fragmentation of hybrid and complex N-linked glycans. J Am Soc Mass Spectrom. 2005;16(5):647–659. doi: 10.1016/j.jasms.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Agilent HPLC-Chip: G4240-62003 Graphitized Carbon Care and Use Sheet. 2005 Available from: http://www.chem.agilent.com/Library/usermanuals/Public/G4240-90203_KitG4240-62003_HPLC-Chip_data-sheet.pdf.
- 24.Cai Y, et al. Evaluation of the role of multiple hydrogen bonding in offering stability to negative ion adducts in electrospray mass spectrometry. J Am Soc Mass Spectrom. 2002;13(12):1360–1369. doi: 10.1016/S1044-0305(02)00648-7. [DOI] [PubMed] [Google Scholar]
- 25.Guan B, Cole RB. MALDI linear-field reflectron TOF post-source decay analysis of underivatized oligosaccharides: determination of glycosidic linkages and anomeric configurations using anion attachment. J Am Soc Mass Spectrom. 2008;19(8):1119–1131. doi: 10.1016/j.jasms.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Y, Cole RB. Oligosaccharide analysis using anion attachment in negative mode electrospray mass spectrometry. J Am Soc Mass Spectrom. 2005;16(1):60–70. doi: 10.1016/j.jasms.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Cole RB. Enhanced collision-induced decomposition efficiency and unraveling of fragmentation pathways for anionic adducts of brevetoxins in negative ion electrospray mass spectrometry. Anal Chem. 2009;81(21):8826–8838. doi: 10.1021/ac901341c. [DOI] [PubMed] [Google Scholar]
- 28.Harvey DJ, et al. Structural and quantitative analysis of N-linked glycans by matrix-assisted laser desorption ionization and negative ion nanospray mass spectrometry. Anal Biochem. 2008;376(1):44–60. doi: 10.1016/j.ab.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 29.Kenny DT, Issa SMA, Karlsson NG. Sulfate migration in oligosaccharides induced by negative ion mode ion trap collision-induced dissociation. Rapid Commun Mass Spectrom. 2011;25:2611–2618. doi: 10.1002/rcm.5157. [DOI] [PubMed] [Google Scholar]
- 30.McAlister GC, et al. Analysis of the acidic proteome with negative electron-transfer dissociation mass spectrometry. Anal Chem. 2012;84(6):2875–2882. doi: 10.1021/ac203430u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mittermayr S, et al. Multiplexed analytical glycomics: rapid and confident IgG N-glycan structural elucidation. J Proteome Res. 2011;10(8):3820–3829. doi: 10.1021/pr200371s. [DOI] [PubMed] [Google Scholar]
- 32.Ceroni A, et al. GlycoWorkbench: a tool for the computer-assisted annotation of mass spectra of glycans. J Proteome Res. 2008;7(4):1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 33.Yanes O, et al. Expanding coverage of the metabolome for global metabolite profiling. Anal Chem. 2011;83(6):2152–2161. doi: 10.1021/ac102981k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boutegrabet L, et al. Attachment of Chloride Anion to Sugars: Mechanistic Investigation and Discovery of a New Dopant for Efficient Sugar Ionization/Detection in Mass Spectrometers. Chemistry. 2012 doi: 10.1002/chem.201103788. [DOI] [PubMed] [Google Scholar]
- 35.Karlsson NG, et al. Negative ion graphitised carbon nano-liquid chromatography/mass spectrometry increases sensitivity for glycoprotein oligosaccharide analysis. Rapid Commun Mass Spectrom. 2004;18(19):2282–2292. doi: 10.1002/rcm.1626. [DOI] [PubMed] [Google Scholar]
- 36.Gabelica V, De Pauw E. Internal energy and fragmentation of ions produced in electrospray sources. Mass spectrometry reviews. 2005;24(4):566–587. doi: 10.1002/mas.20027. [DOI] [PubMed] [Google Scholar]
- 37.Saad OM, Leary JA. Delineating mechanisms of dissociation for isomeric heparin disaccharides using isotope labeling and ion trap tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15(9):1274–1286. doi: 10.1016/j.jasms.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 38.Cai Y, Jiang Y, Cole RB. Anionic adducts of oligosaccharides by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal Chem. 2003;75(7):1638–1644. doi: 10.1021/ac0205513. [DOI] [PubMed] [Google Scholar]
- 39.Melmer M, et al. Solvent effects on the retention of oligosaccharides in porous graphitic carbon liquid chromatography. J Chromatogr A. 2010;1217(39):6092–6096. doi: 10.1016/j.chroma.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 40.Melmer M, et al. Effects of the redox state of porous graphitic carbon on the retention of oligosaccharides. J Chromatogr A. 2010;1217(39):6097–6101. doi: 10.1016/j.chroma.2010.06.069. [DOI] [PubMed] [Google Scholar]
- 41.Kelly RT, et al. The ion funnel: theory, implementations, and applications. Mass spectrometry reviews. 2010;29(2):294–312. doi: 10.1002/mas.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hua S, et al. Isomer-specific chromatographic profiling yields highly sensitive and specific potential N-glycan biomarkers for epithelial ovarian cancer. Journal of Chromatography A. 2013 doi: 10.1016/j.chroma.2012.12.079. Manuscript in Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nimmerjahn F, Ravetch JV. Fc-receptors as regulators of immunity. Adv Immunol. 2007;96:179–204. doi: 10.1016/S0065-2776(07)96005-8. [DOI] [PubMed] [Google Scholar]
- 44.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 45.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 46.Arnold JN, et al. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50. doi: 10.1146/annurev.immunol.25.022106.141702. [DOI] [PubMed] [Google Scholar]
- 47.Szabo Z, et al. Ultrasensitive capillary electrophoretic analysis of potentially immunogenic carbohydrate residues in biologics: galactose-alpha-1,3-galactose containing oligosaccharides. Mol Pharm. 2012;9(6):1612–1619. doi: 10.1021/mp200612n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wheeler SF, Harvey DJ. Negative ion mass spectrometry of sialylated carbohydrates: discrimination of N-acetylneuraminic acid linkages by MALDI-TOF and ESI-TOF mass spectrometry. Analytical chemistry. 2000;72(20):5027–5039. doi: 10.1021/ac000436x. [DOI] [PubMed] [Google Scholar]
- 49.Bosques CJ, et al. Chinese hamster ovary cells can produce galactose-alpha-1,3-galactose antigens on proteins. Nat Biotechnol. 2010;28(11):1153–1156. doi: 10.1038/nbt1110-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lammerts van Bueren JJ, et al. Anti-galactose-alpha-1,3-galactose IgE from allergic patients does not bind alpha-galactosylated glycans on intact therapeutic antibody Fc domains. Nat Biotechnol. 2011;29(7):574–576. doi: 10.1038/nbt.1912. [DOI] [PubMed] [Google Scholar]
- 51.Ghaderi D, et al. Implications of the presence of N-glycolylneuraminic acid in recombinant therapeutic glycoproteins. Nat Biotechnol. 2010;28(8):863–867. doi: 10.1038/nbt.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghaderi D, et al. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. 2012;28:147–175. doi: 10.5661/bger-28-147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.