Abstract
Oxidative stress and inflammation play a crucial role in Parkinson’s disease (PD) pathogenesis and may represent a target for treatment. Current PD drugs provide only symptomatic relief and have limitations in terms of adverse effects and inability to prevent neurodegeneration. Flavonoids have been suggested to exert human health benefits by its anti-oxidant and anti-inflammatory properties. Therefore, in the present study, using 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydro pyridine (MPTP)-induced mouse model of Parkinsonism, we investigated the neuroprotective potential of bioflavonoid compound Pycnogenol® (PYC), an extract of Pinus maritime bark. MPTP injected mice developed significantly severe oxidative stress and impaired motor coordination at day 1 and day 7 postinjection. This was associated with significantly increased inflammatory responses of astrocyte and microglia as assessed by ionized calcium binding adaptor molecule 1 (Iba 1) and glial fibrillary acidic protein (GFAP) immunohistochemistry, and nuclear transcription factor-κB (NF-kB), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) expression in the striata by Western blot. Additionally, there was significant upregulation of tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) expression in the striata of MPTP injected mice compared to saline controls. The MPTP-induced neuroinflammation, neurodegeneration and behavioral impairments were markedly repudiated by treatment with PYC. These results suggest that PYC protects dopaminergic neurons from MPTP toxicity in the mouse model of PD. Thus, the present finding of PYC-induced adaptation to oxidative stress and inflammation could suggest a novel avenue for clinical intervention in neurodegenerative diseases including PD.
Keywords: Pycnogenol®, MPTP, Oxidative stress, Neuroinflammation, Neurodegeneration
1. Introduction
Parkinson disease (PD) is a chronic progressive neurodegenerative disease. Severe dopaminergic neuronal loss in the substantia nigra and subsequent depletion of dopamine (DA) in the striatum lead to the characteristic movement impairment in PD. PD is characterized by the presence of degenerating dopaminergic neurons, Lewy bodies and activated glia in brain. Although, the cause of PD is not clear, exposure to environmental neurotoxic pollutant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is known to be associated with the pathology of human PD and in animal models of PD. Several reports support the role for increased oxidative stress and neuroinflammation in the pathogenesis of PD (Gao et al., 2008; Hirsch and Hunot, 2009; Jenner, 2003). Human postmortem studies have also suggested that oxidative damage to lipids, proteins and DNA occurs in the nigrostriatal regions of PD brains (Dexter et al., 1994; Jenner et al., 1992). Oxidative damage to lipid and protein can lead to structural and functional disruption of the cell membrane, inactivation of enzymes, and, finally, cell death and neurodegenerative disorders (Hald and Lotharius, 2005).
Chronic neuroinflammation plays a critical role in the progressive neurodegeneration in PD (Hirsch and Hunot, 2009; Joglar et al., 2009; Tansey et al., 2007). However, the precise implications of the inflammatory response for neurodegeneration have not been clearly elucidated. A current hypothesis considers that an extracellular insult to neurons could trigger the production of proinflammatory mediators such as cytokines/chemokines, enzymes like cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) by astrocyte and microglia. Moreover, nuclear transcription factor-κB (NF-κB) plays an important role in the pathogenesis of oxidative stress-associated neurodegeneration by inducing tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) expression (Hald and Lotharius, 2005). These cytokines and enzymes are known to be cytotoxic and could cause neuronal death. A number of studies have documented that suppressing neuroinflammation with anti-inflammatory drugs abrogates dopaminergic neurodegeneration in various experimental animal models of PD (Choi et al., 2005; Jin et al., 2008).
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a mitochondrial complex-1 inhibitor that causes parkinsonian features in humans and primates, and recapitulates dopaminergic degenerations in the nigrostriatal pathway in mice. Therefore, MPTP is widely used as a tool to study the molecular events that lead to degeneration of dopaminergic neurons in animal models of PD and to test potential neuroprotective agents (Jackson-Lewis et al., 2012).
Despite considerable advances in our understanding of the pathogenesis of neurodegeneration, attempts to develop effective therapeutic options are in the progress (Brotchie and Jenner, 2011). Pycnogenol (PYC; Horphag Research Ltd, UK) is a standardized extract from the bark of the French maritime pine (Pinus maritime) used as a dietary supplement to overcome inflammatory and degenerative diseases and wound healing (D’Andrea, 2010; Maimoona et al., 2011; Peng et al., 2000). The major constituents of PYC include phenolic monomers (catechin, epicatechin and taxifolin), flavonoids (procyanidins and proanthocyanidin) and phenolic acids (caffeic, ferulic and p-hydroxybenzoid acid) (D’Andrea, 2010). PYC is also used extensively in multi-vitamins and health products because of its efficient antioxidant activity (Bors and Michel, 2002; Rohdewald, 2002) and excellent radical scavenging properties (Guo et al., 1999). It has been claimed that PYC has diverse beneficial effects on diabetes, asthma, hypertension, hepatotoxicity, cancer, arthritis, attention deficit hyperactivity disorder, cognition improvement, immune diseases, and neurodegenerative diseases (Packer et al., 1999; Rohdewald, 2002; Yang et al., 2008). Previous study has shown that PYC could prevent MPTP-induced dopaminergic dysfunction through its antioxidative action (Khan et al., 2010). However, a most recent study indicated the current evidence is insufficient to support its use for chronic disorders (Schoonees et al., 2012) and suggests further studies. In the light of above findings, in the present study we have attempted to investigate the potential anti-inflammatory and neuroprotective effects of PYC on MPTP-induced mouse model of PD.
2. Materials and methods
2.1. Reagents
Assay kits for reduced glutathione (GSH), thiobarbituric acid reactive substance (TBARS), Glutathione peroxidase (GP), Glutathione reductase (GPx) and Super oxide dismutase (SOD), were purchased from Cayman Chemical Company (Ann Arbor, MI). Enzyme-linked immunosorbent assay (ELISA) kits for Dopamine (GenWay Bioteck Inc, CA) TNF-α, IL-1β (Biosource International, Camarillo, CA) were purchased. Polyclonal NF-κB p65 and cyclo-oxygenase-2 (COX-2) (Santa Cruz Biotechnology, Santa Cruz, CA), anti-inducible nitric oxide synthase (anti-iNOS) (Abcam, Cambridge, MA) antibodies, polyclonal secondary antibodies conjugated to horseradish peroxidase (Bio-Rad Laboratories, Hercules, CA), Rotamex Rotarod (Columbus Instruments, Columbus, OH), C57BL/6 mice (Harlan Sprague Dawley, Inc., Indianapolis, IN) and MPTP (Sigma, St. Louis, MO) were purchased.
2.2. MPTP and PYC treatments
C57BL/6 mice were maintained at The University of Iowa animal care facility and they were used in accordance with the guidelines approved by the IACUC and National Institutes of Health. Animals were randomly divided into six groups (n=6/group). The first group was saline-treated and served as a control group; the second group was MPTP-injected (four intraperitoneal injections of MPTP (18 mg/kg body weight in saline, at 2 hrs intervals) for one day only and third group received pretreatment of PYC 30 min before each MPTP injection and one additional injection on next day and then sacrifice, respectively. The fourth group was saline treated control for 7 days and the fifth group as only MPTP injected but the sixth group received pretreatment of PYC 30 min before each MPTP injection and continued once daily for 7 days. The total number of saline injections performed in the controls mice were 4 for both day 1 and day 7 controls. We have performed a dose-response effect (5, 10 and 20 mg/kg b.wt.) of PYC on MPTP model of PD in pilot studies to determine the optimal dose of PYC that provides the most neuroprotection against neurodegeneration. Additionally, the PYC dose (20 mg/kg body weight) and treatment regimen used in this study was also supported from previous animal studies showing that 20 mg/kg body weight dose provided the maximal neuroprotective effects in the treatment of different types of brain injuries (Jankyova et al., 2009; Khan et al., 2010; Yang et al., 2008). Moreover, we have used the commercial product Pycnogenol® as such for the intraperitoneal administration.
2.3. Behavioral studies
All behavioral tests were performed blindly with respect to drug administration. We have randomly divided the animals into different groups each having six mice. Behavioral studies were repeated two times with three different trials to validate the behavioral data.
2.3.1. Rota rod
Rotamex Rotarod was used to evaluate the motor coordination skill of mice of each group (Khan et al., 2010). The Rota rod unit consists of a rotating rod, which was divided into four parts by compartmentalization to permit the testing of four mice at a time. After twice daily training for two successive days (5 rpm on the first day and 8 rpm on second day) the rotational speed was increased to 10 rpm on the third day in a test session. The time for each mouse to remain on the rotating bar was recorded for three trials, at 15 min intervals with maximum trial length of 180 sec per trial. The motor deficiency was evaluated as the ability of the mouse to hold the rotating rod.
2.3.2. Grip test
Grip test was performed as described previously (Khuwaja et al., 2011). Briefly, the apparatus consist of a string of 50 cm length, pulled tight between two vertical supports and elevated 40 cm from a flat surface was used. Mouse was placed on the string at a point midway between supports and evaluated according to the following scale, 0 = fall off, 1 = hangs onto string by two forepaws, 2 = as for 1 but attempts to climb on string, 3 = hangs onto string by two forepaws plus one or both hind paws, 4= hangs onto string by all forepaws plus tail wrapped around string and 5 = escape.
2.3.3. Footprint analysis
The footprint analysis was performed as described previously (Tillerson et al., 2002) with slight modification. Briefly, the mice were trained to run toward an enclosed square box in an open-top runway. The forepaws and hindpaws were dipped with nontoxic paints, and the mice were immediately placed on one end of the sheet of paper opposite to the square box. The footprint patterns were analyzed for stride length by calculating from the mid-digit toe of the first step to the heel of the second step.
2.3.4. Drag test
This test was performed to measure the ability of the animal to balance its body posture using forelimbs in response to an externally imposed dynamic stimulus (Viaro et al., 2008). Each mouse was gently lifted using the tail (allowing the forepaws on the table) and dragged backwards at a constant speed (about 20 cm/sec) for a fixed distance (120 cm). The number of touches made by each forepaw was counted by two independent observers (mean between the two forepaws).
2.4. Tissue homogenate preparation
Animals were killed by cervical dislocation and the brains were removed to dissect out the striatum and then homogenised in phosphate buffered saline (PBS) supplemented with protease inhibitor (5 mM leupeptin, 1.5 mM aprotinin, 2 mM phenyl methyl-sulfonylfluoride (PMSF), 3 mM peptastatin A, and 10 mM EDTA). Tissue homogenates were centrifuged at 14,000 g for 20 min at 4°C to get post mitochondrial supernatant for used for the biochemical studies.
2.5. Biochemical studies
2.5.1. Lipid peroxidation determination
TBARS detection kit was used according to the manufacturer’s instructions to determine the TBARS level as a marker of lipid peroxidation. The results were expressed as nmol TBARS formed/mg protein.
2.5.2. GSH assay
Glutathione detection kit was used for GSH estimation according to the manufacturer’s instruction. GSH was calculated in terms of μmol DTNB conjugate formed/mg protein using a molar extinction coefficient of 13.6×103 M−1 cm−1.
2.5.3. Antioxidant Activities
Assay kits were used as per the manufacturer’s instructions to determine the activities of antioxidant enzymes GPx, GR and SOD from each treatment group. GPx and GR activities were calculated as nmol NADPH oxidized/min/mg/protein using molar extinction coefficient of 6.22 × 10−3 M−1 cm−1 and the SOD activity was calculated as Units/mg protein using molar extinction coefficient of 4.02 × 103 M−1 cm−1.
2.5.4. Dopamine estimation
DA ELISA kit was used for the estimation of DA level in the striata of each group of mice. Results were expressed as nmol/mg protein.
2.6. Immunohistochemistry for tyrosine hydroxylase (TH) and ionized calcium binding adaptor molecule 1 (Iba-1)
Immunohistochemistry of substantia nigra pars compacta (SNpc) and striatum were carried out as described previously (Thangavel et al., 2009). Briefly, 15 μm serial coronal brain sections were cut in cryostat (Leica, Germany) and incubated with 0.3% H2O2 and blocking reagent (5% normal goat serum, 3 % BSA and 0.3% Triton-X 100) followed by incubation with the primary antibody rabbit anti-goat TH (Millipore, Temecula, CA) or rabbit anti-goat Iba-1 (Wako, Richmond, VA) for overnight at 4 °C. The sections were then washed and incubated for 2 hrs with biotinylated secondary goat anti-rabbit antibody at room temperature (RT) followed by ABC standard kit solution and diaminobenzidine as a substrate (Vector Laboratories, Burlingame, CA). Finally the sections were dehydrated, cover slipped, viewed under a microscope and photomicrographed.
2.7. Immunofluorescence staining of glial fibrillary acidic protein (GFAP)
Immunofluorescence staining was performed to detect the expression of GFAP. Serial coronal sections were incubated with blocking reagent followed by primary antibody (rabbit anti-goat GFAP (Millipore) overnight at 4°C. After washing in PBS, the sections were incubated with Alexa Fluor-488 labeled secondary antibody (goat anti-rabbit) at RT for 1 hr. The sections were then cover-slipped in mounting media and stored in the dark at 4 °C until analysis.
2.8. Western blot analysis
Striata were collected from the mice of each group. Cytosolic and nuclear extracts were prepared as described previously (Khan et al., 2012). Samples of nuclear or cytoplasmic fractions containing equal amounts of protein (35 μg) were separated in 10 % SDS-polyacrylamide gel electrophoresis. Proteins were transferred onto PVDF membrane and incubated overnight at 4°C with specific primary rabbit polyclonal antibody against NF-κB p65 (1:200 dilutions), or goat polyclonal antibody against COX-2 (1:200 dilutions, Santa Cruz), or rabbit polyclonal antibody against iNOS (1:200 dilutions) followed by appropriate secondary antibodies conjugated to horseradish peroxidase (HRP). Proteins recognized by the antibody were visualized by enhanced chemiluminescence Femto kit (Thermo Scientific, Rockford, IL). Blots were stripped and reprobed for β-actin (Sigma) as a loading control. Bands intensity was measured by densitometry and quantified using NIH-Image J software.
2.9. ELISA
Mice striata were homogenized in tissue lysis buffer (50 mM Tris-Hcl, pH 8.0, 5 mM NaCl, and 1 % Triton X-100). Supernatants from homogenates were used for determination of TNF-α and IL-1β with commercial ELISA kits.
2.10. Statistical analyses
Results were expressed as mean ± SEM. One-way analysis of variance (ANOVA) was applied to calculate the statistical significance between various groups using GraphPad InStat software. Interaction of groups and time effects were analyzed by 2-way ANOVA followed by Bonferroni post-test. A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. Behavioral studies
3.1.1. Effect of PYC on behavioral recovery
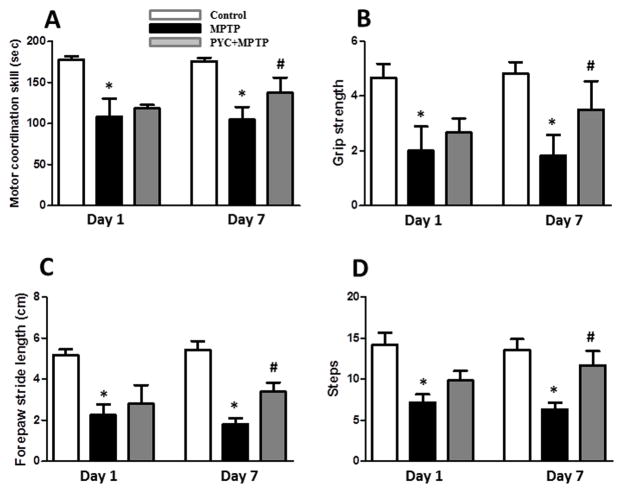
As assessed by Rota rod, a significant depletion (p<0.001) in motor coordination skill in MPTP injected groups as compared to their control groups was observed after day 1 and 7 (Fig. 1A). PYC (20 mg/kg) was found to be effective in partial recovery of motor coordination in 7 day PYC + MPTP injected group as compared to MPTP injected group. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.021, F=5.34) (Fig. 1A). A significant decrease (p<0.01) in motor strength as measured by grip strength test was observed in MPTP injected groups as compared with their controls. PYC treatment (7 days) significantly protected mice from the MPTP-induced decline in motor activity. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.049, F=3.95) (Fig. 1B). The forepaw step distance was significantly (p<0.01) decreased in the MPTP injected groups as compared with their control groups. The forepaw step distance was improved by 7 days PYC treatment when compared with the MPTP injected group. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.032, F= 6.448) (Fig. 1C). Number of steps in the drag test was significantly (p<0.01) decreased in MPTP groups as compared to saline injected controls. PYC treatment followed by MPTP injection for 7 days significantly improved the number of footsteps as compared to MPTP group. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.039, F= 5.65) (Fig. 1D). PYC treatment for 1 day did not significantly improve the motor coordination skill, grip strength, forepaw step distance and steps changes measured by drag test as compared to MPTP-injected group. In the Figures 2 to 8, we have provided the data of only one control group (day 7) since we did not find any significant difference between these two control groups (Day 1 and day 7) in the behavioral studies.
Figure 1. PYC treatment improves performances in the motor coordination skill, grip strength, foot print test and drag test in mice after MPTP injections.
(A) MPTP injection led to significant decrease in motor coordination skill as compared to control group, and significantly recovered in PYC + MPTP group as compared to only MPTP group (*p < 0.01, MPTP vs. control; #p < 0.01, PYC + MPTP vs. MPTP). (B) The grip strength decreased significantly in the MPTP injected animals as compared to control animals. Treating the animals with PYC followed by MPTP injections has protected (7 day) motor deficit as compared with only MPTP injected group (*p < 0.01, MPTP vs. control; #p < 0.05, PYC + MPTP vs. MPTP). (C) Forepaw stride distance was decreased significantly in MPTP group mice as compared to control group. PYC treatment increased the forepaw distance in PYC treated group mice (*p < 0.01, MPTP vs. control; #p < 0.05, PYC + MPTP vs. MPTP). (D) PYC treated MPTP group significantly increased the number of steps as compared with MPTP group, indicating a profound improvement (7 day) in sensory motor performance (*p < 0.01, MPTP vs. control; #p < 0.05, PYC + MPTP vs. MPTP). Results are expressed as mean ± SEM of six animals/group.
Figure 2. Effect of PYC treatment on TBARS (A) and GSH (B) levels in the striata of MPTP injected mice.
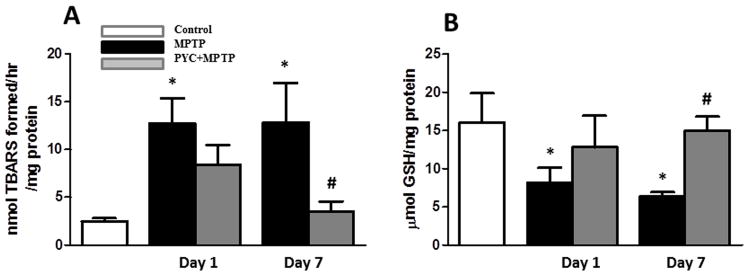
TBARS level was significantly increased while GSH level was significantly decreased in MPTP groups as compared to control group mice. PYC treatment followed by MPTP injection significantly prevented both the MPTP-induced elevation of TBARS (A) as well as MPTP-induced decrease in GSH (B) levels (*p < 0.05, MPTP vs. control; #p < 0.05, PYC + MPTP vs. MPTP.
Figure 8. TNF-α and IL-1β levels in the striata of MPTP injected group mice.
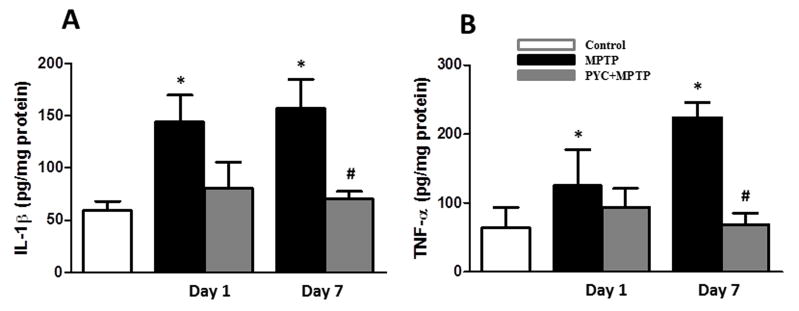
Striata levels of IL- 1β and TNF-α was significantly increased in MPTP injected group as compared to the control group. PYC treatment followed by MPTP injection significantly reduced IL-1β (A) and TNF-α (B) protein concentration in PYC+MPTP group as compared to only MPTP group. Values are mean ± SEM (*p < 0.01, MPTP vs. control; #p < 0.01, PYC + MPTP vs. MPTP).
3.2. Biochemical studies
3.2.1. Effect of PYC on TBARS and GSH content
TBARS content was measured to demonstrate the extent of oxidative damage to lipids and their protection by PYC. We found significant (p<0.05) increase of TBARS contents on day 1 (12.7±2.6 nmol/hr/mg protein) and day 7 (12.9±4.1 nmol/hr/mg protein) following MPTP administration as compared to the control group (2.5±0.3 nmol/hr/mg protein). PYC treatment followed by MPTP administration significantly prevented (p<0.05) the apparent increase in TBARS content (3.5±1.1 nmol/hr/mg protein) as compared to MPTP injected group. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.047, F= 4.58) (Fig. 2A). GSH content was reduced significantly (p<0.05) in MPTP groups at day 1 and 7 (8.2±1.9 and 6.3±0.6 μmol/mg protein), as compared to saline injected control group (16.0±3.9 μmol/mg protein) (Fig. 2B). The decrease in GSH content was significantly (p<0.05] protected in the PYC treated group (15.0±1.8 μmol/mg protein) (7 days) as compared to the MPTP injected groups. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.041, F= 5.83).
3.2.2. Effect of PYC on antioxidant enzymes activity
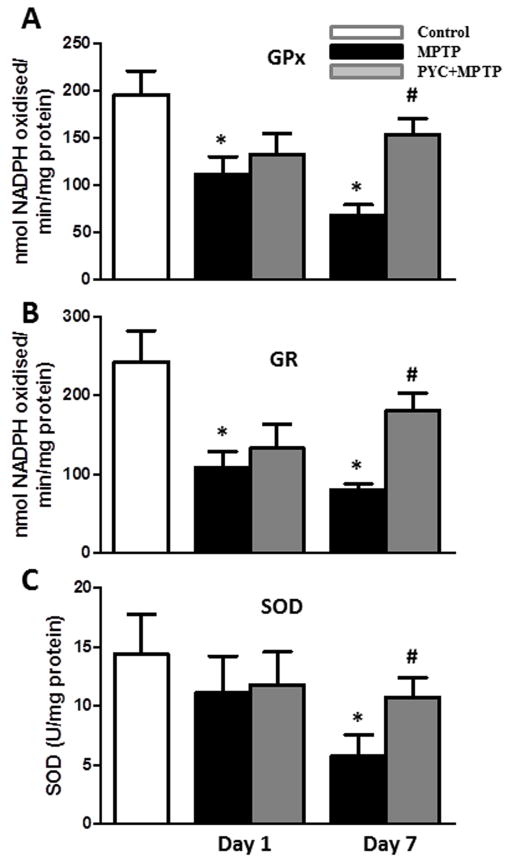
The activities of antioxidant enzymes GPx (Fig. 3A), GR (Fig. 3B), and SOD (Fig. 3C) in MPTP groups (68.7±10.7 nmol NADPH oxidised/min/mg protein, 80.9±7.3 nmol NADPH oxidised/min/mg protein and 5.7±1.8 U/mg protein, respectively) were decreased significantly (p<0.01 for GPx; p<0.01 for GR and p>0.01 for SOD) as compared to control groups (195.9±25.2 nmol NADPH oxidised/min/mg protein, 243.2±39.1 nmol NADPH oxidised/min/mg protein and 14.4±3.4 U/mg protein, respectively). PYC treatment for 7 days followed by MPTP injection preserved the activities (153.7±17.3 nmol NADPH oxidised/min/mg protein, 180.6±22.5 nmol NADPH oxidised/min/mg protein, and 10.8±1.6 U/mg protein, respectively) of these enzymes significantly (p<0.05 for GPx; p<0.05 for GR and p>0.05 for SOD) as compared to MPTP injected groups. On the other hand, PYC treatment for 1 day only was unable to prevent the loss of antioxidant enzyme activities (Fig. 3A, B and C). Two-way ANOVA showed that the interaction of group and time was significant [GPx (P<0.043, F= 4.79), GR (P<0.039, F= 5.13) and SOD (P<0.046, F= 4.90)].
Figure 3. Effect of PYC treatment on GPx (A), GR (B) and SOD (C) activities in MPTP injected mice.
Activities of antioxidant enzymes were significantly decreased in the MPTP group as compared to control group. PYC treatment significantly increased the activities in PYC + MPTP group mice as compared with only MPTP group (*p < 0.01, MPTP vs. control; #p < 0.05, PYC + MPTP vs. MPTP).
3.2.3. Effect of PYC on brain dopamine levels
MPTP injections caused significant decrease (p<0.01) in the level of dopamine in the striata of MPTP-injected mice (22.7±8.1 ng/mg protein) as compared to the saline-injected controls (174.7±45.4 ng/mg protein). The results in Fig. 4 show that the MPTP-induced dopamine depletion was attenuated in mice treated with PYC for 7 days (131.4±16.5 ng/mg protein) as compared to the MPTP-injected mice. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.027, F= 6.03).
Figure 4. Effect of PYC treatment on dopamine level in the striata of MPTP injected group mice.
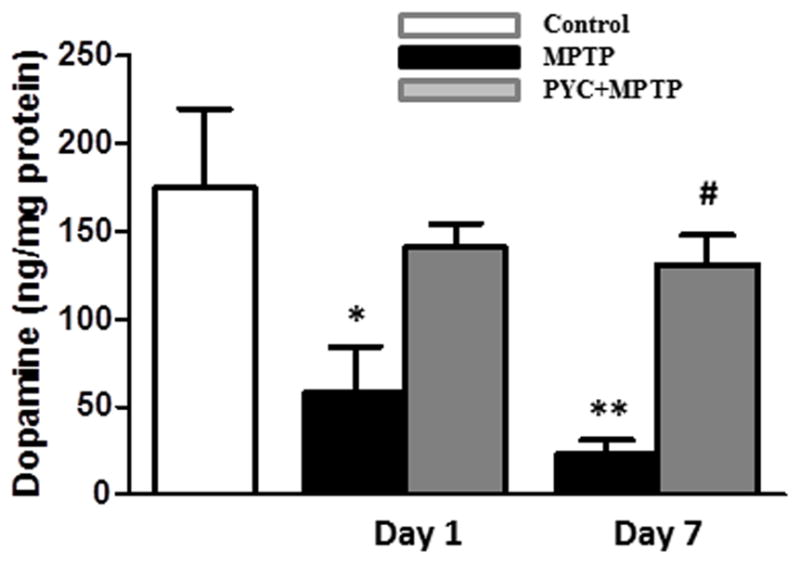
The MPTP injections led to a significant decrease in the level of dopamine in MPTP group as compared with the control group. Treatment with PYC followed by MPTP injection significantly protected the level of dopamine as compared with only MPTP group (*p < 0.01, MPTP vs. control; #p < 0.01, PYC + MPTP vs. MPTP).
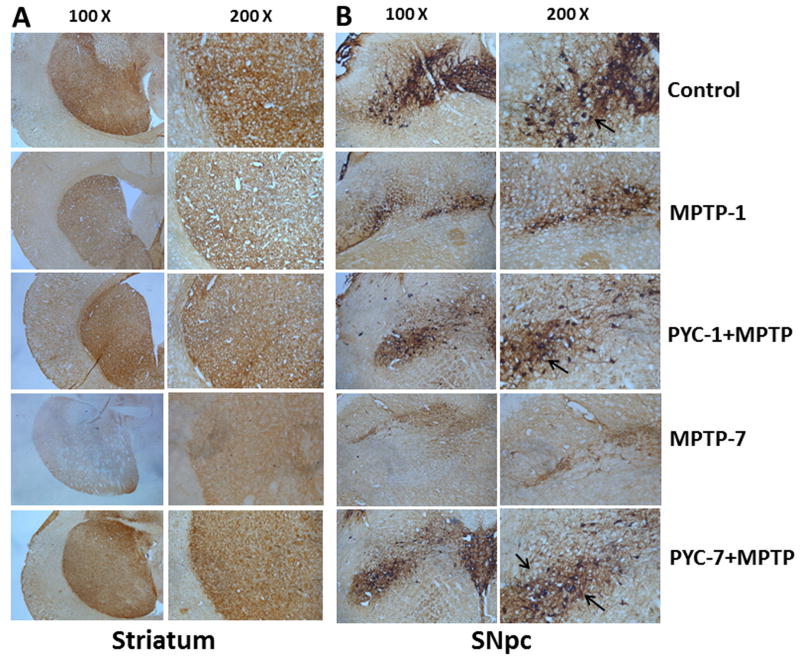
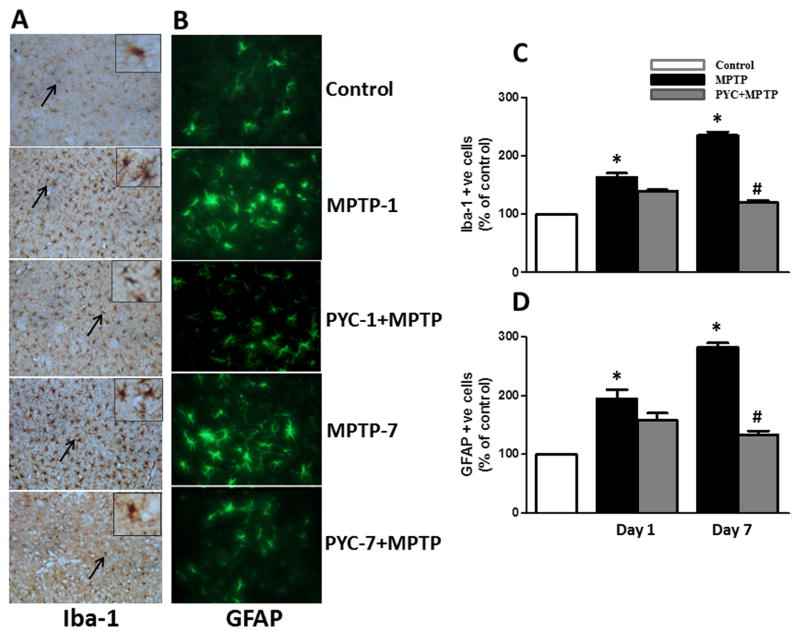
3.3. Effect of PYC on TH, Iba-1 and GFAP expression
We investigated glial activation and dopaminergic neurodegeneration in SNpc and striatum following MPTP injection in PYC-treated mice. PYC-treated mice show significantly reduced nigrostriatal dopaminergic neuron loss following MPTP injection (Fig. 5 A and B). Increased expression of Iba-1 indicating increased numbers and activation of microglia were observed as an index of inflammatory response in MPTP injected mice. PYC treatment for 7 days followed by MPTP administration significantly prevented the MPTP-induced increase in the number of microglia and their activation (Iba-1 expression, brown color) (Fig. 6A). Higher expressions of GFAP (green color) indicating increased number of astrocytes with astrocyte hypertrophy inflammatory response characteristics were seen in MPTP injected groups after day 1 and 7 as compared to control groups. PYC treatment attenuated higher expressions of GFAP in PYC + MPTP group as compared to MPTP groups (Fig. 6B). Boxed areas are the magnified areas (arrows) showing the morphology of microglia and astrocyte in the control and treated groups. We have also performed quantitative analysis of the staining by counting the number of microglia (C) as well as astrocytes (D) when compared to controls. Negative staining control slides incubated without the primary antibody did not produce any positive staining reactions for either Iba-1 or GFAP (data not shown).
Figure 5. Effect of PYC treatment on TH expression in striatum (A) and SNpc (B).
Representative microphotographs of the expression of TH was almost negligible in MPTP group as compared to control group, while the MPTP group treated with PYC has shown a moderate staining of TH. However, the control group has shown no discernible change in TH staining. Original magnifications at 100X and 200X.
Figure 6. Representative coronal brain sections from control, MPTP and PYC+MPTP treated groups stained for Iba-1 and GFAP.
The profound expression of (A) Iba-1 (brown color) and (B) GFAP (green color) were observed in MPTP group as compared to control group, while the MPTP group treated with PYC has shown a moderate staining of Iba-1 and GFAP. However, the control group has shown almost negligible staining. Boxed areas are the magnified areas (arrows) showing the morphology of microglia and astrocytes in the control and treated groups. We have also performed quantitative analysis of the staining by counting the number of microglia (C) and astrocytes (D). Original magnifications at 200X.
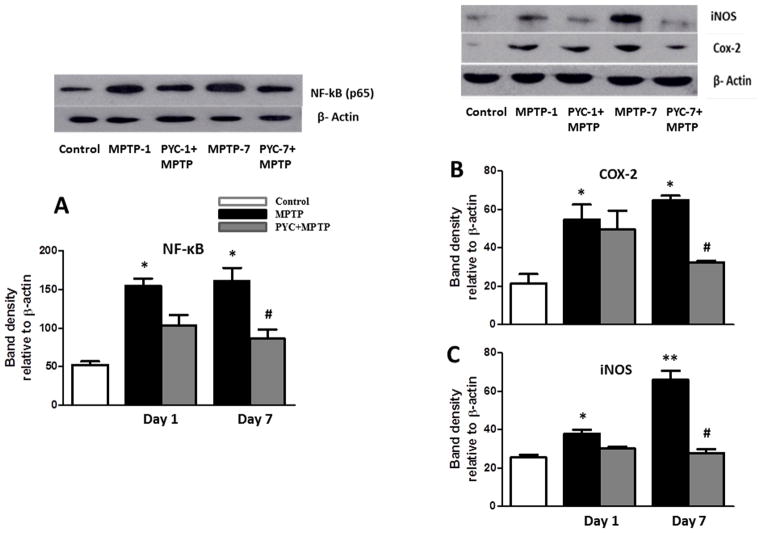
3.4. Effect of PYC on NF-κB, COX-2 and iNOS expression levels
To detect and quantify NF- κB activation, the translocation of NF- κB p65 to the nucleus was assayed in the striatum nuclear extracts using a subunit-specific anti-p65 antibody. MPTP-injected mice demonstrated significantly (p<0.01) higher levels of NF-κB p65 protein in nuclear extracts after 1 and 7 days compared with saline injected control mice. PYC treatment prior to MPTP injections significantly attenuated the activation of NF-κB. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.036, F= 7.73) (Fig. 7A). We further investigated expression of some of the NF-κB-responsive genes, such as COX-2 and iNOS by Western blotting of cytoplasmic fractions of the striatal tissue. Similar to NF-κB p65, we found significantly [(p<0.01) for COX-2 and (p<0.01) for iNOS] higher striata protein levels of COX-2 (Fig. 7B) and iNOS (Fig. 7C) in MPTP -injected mice as compared to controls. MPTP-induced expression of COX-2 and iNOS protein was almost completely blocked by PYC treatments for 7 days. Two-way ANOVA showed that the interaction of groups and time was significant (P<0.041, F= 6.09 for COX-2 and P<0.029, F= 8.52 for iNOS) (Fig. 7).
Figure 7. Expression level of NF-κB, COX-2 and iNOS in MPTP injected mice.
Expression level of striata NF-κB, COX-2 and iNOS were significantly increased in MPTP group mice as compared to the control group mice. PYC followed by MPTP injection significantly decreased the expression level of NF-κB (A), COX-2 (B) and iNOS (C) in the PYC+MPTP treated group as compared to only MPTP group mice. Values are expressed as mean ± SEM (n=3) (*p < 0.01, MPTP vs. control; #p < 0.01, PYC + MPTP vs. MPTP).
3.5. Effect of PYC on MPTP-induced TNF-α and IL-1β release
We analyzed IL-1β and TNF-α protein concentration by ELISA in the homogenate of striatum. As shown in Fig. 8, secretion of inflammatory cytokines was significantly increased (p<0.01 for IL-1β and p<0.01 for TNF-α] at day 1 and day 7 post MPTP-injections when compared with controls. The concentration of IL-1β at 1 and 7 days post MPTP-injections were 143.67 pg/mg protein and 157.14 pg/mg proteins, respectively, compared to 59.15 pg/mg protein in saline-injected controls (Fig. 8A). Similarly, the concentration of TNF-α at 1 and 7 days post MPTP-injections (Fig. 8B) were significantly higher (124.90 pg/mg protein and 223.82 pg/mg protein respectively) than found in control group (63.49 pg/mg protein). The MPTP-induced increase in the secretion of IL-1β (157.14 pg/mg protein in MPTP-injected mice versus 70.14 pg/mg protein in the PYC-treated mice) and TNF-α (223.82 pg/mg protein in MPTP-injected mice versus 67.96 pg/mg protein in the PYC-treated mice) was significantly blocked in PYC-treated mice at day 7 post-injections. Two-way ANOVA showed that the interaction of group and time was significant (P<0.0283, F= 5.437 for IL-1 β and P<0.0261, F= 6.34 for TNF-α).
4. Discussion
In the present study we demonstrate that PYC, a French maritime pine bark extract containing a mixture of polyphenols and procyanidins exerted protection of the dopaminergic neurons via amelioration of oxidative loads, suppression of glial cell activation and inhibition of inflammatory responses in the MPTP-induced mouse model of PD. These protections involve oxidative stress associated downregulation of NF-κB activation and its responsive genes TNF-α, IL-1β, iNOS and COX-2. PYC’s neuroprotective effects suggest that it is an efficient antioxidant and anti-inflammatory agent, corroborating previous studies (Ishrat et al., 2009; Khan et al., 2010; Kim et al., 2011; Peng et al., 2012).
It has been reported that MPTP administration leads to excessive generation of reactive oxygen species (ROS), leading to inflammation associated nigrostriatal DA neuronal damage (Chung et al., 2011). Indeed, our results in mice revealed that MPTP administration caused inflammation concomitant with ROS overproduction, while PYC blocked ROS accumulation and downstream events in the inflammatory cascade. Given that oxidative stress is an upstream event in the activation of inflammation, it is likely that PYC inhibited oxidative stress and inflammation, at least in part, by this demonstrated antioxidant and anti-inflammatory effects.
DA neurons possess reduced antioxidant capacity, as evidenced by low intracellular glutathione, which render DA neurons more vulnerable to oxidative stress. The antioxidant system uses reduced GSH, the most abundant non-protein thiol, which buffers free radicals in brain tissue (Dringen, 2000). GPx plays a predominant role in removing excess of free radicals and is a major defense system against oxidative threat in the brain (Khan et al., 2010). GR plays an important role in providing the pool for GSH which protects the membrane from toxicity. SOD converts superoxide into H2O2 (Freeman and Crapo, 1982). Since all antioxidant defenses are interconnected (Sun, 1990); hence disruption of one would disrupt the microenvironment and eventually could lead to a catastrophe. In the present study MPTP administration caused an overproduction of free radicals which, in turn, caused oxidative damages and ultimately lead to a decrease in GSH and the antioxidant enzymes GPx, GR and SOD. This oxidative neuronal damage in MPTP injected mice is consistent with previous reports (Cheng et al., 2008; Khan et al., 2010; Lee et al., 2011). PYC treatment reduced the oxidative damage as seen by the decrease in lipid peroxidation as well as the restoration of GSH level, and activities of antioxidant enzymes in the striatum following MPTP injections. The most prominent biochemical changes in the striatum of PD patients and MPTP-injected mice are the decreased levels of DA (Chung et al., 2011; Jackson-Lewis and Przedborski, 2007). Such deficits in striatal DA in MPTP-injected mice led to a decreased latency to fall on an accelerating rotarod apparatus, reflecting diminished coordination and balance (Moon et al., 2009). Since the behavioral effects are intertwined with the degree of neuronal dysfunction (Schwarting et al., 1991), its assessment is a more powerful endpoint in evaluating neuroprotection. Therefore, testing the behavioral function in the current study provides a sensitive evaluation of the PYC’s ability to provide neuroprotection. Our present results suggest acute MPTP injections caused severe motor deficits as assessed by rotarod, grip strength, foot print analysis and drag test in mice at two different time intervals. PYC was found to increase striatal DA levels after MPTP injection and improved motor deficits by day 7, but fails to improve the motor deficits after day 1. Our present findings are in agreement with the earlier reports that DA level and motor deficits in Parkinsonian mice have been attenuated by antioxidant supplementation (Chung et al., 2011; Khan et al., 2010; Moon et al., 2009). Restoration of antioxidants defense system and striatal DA content was further emphasized by the normalization of TH expression by the PYC. TH is a rate-limiting enzyme in the formation of DA, and its expression is the marker for the DA neuron survival. The immunohistochemical localization of TH in SNpc region further strengthens the protective action of PYC in MPTP induced PD, as reported in the present study.
Microglia mediated neuroinflammation has recently emerged as a key player in the pathogenesis of PD and microglial activation is considered a rapid cellular response to inflammation (Ghosh et al., 2007; Gordon et al., 2012; Hirsch et al., 2012). The up-regulation of Iba-1 expression following MPTP administration is an indicator of microglial activation. Experimental and postmortem studies also reveal the presence of activated microglia in the nigrostriatal regions of PD brains (Hirsch et al., 2003; Ouchi et al., 2009). Microglia activation leads to NF-κB nuclear translocation that upregulates the release of proinflammatory enzymes COX-2 and iNOS and TNF-α and IL-1β in PD (Kim and Joh, 2006; Mosley et al., 2006). Previous studies have shown that the blockade of microglia activation by selective inhibitors prevented MPTP-induced neurotoxicity (Chung et al., 2011; Wu et al., 2003). Here, through the Iba-1 and GFAP expression analysis, we show that PYC treatment suppressed the glial cell activation-induced inflammatory response and rescues dopaminergic neuron from MPTP-induced toxicity. In the neuroinflammatory conditions, nitric oxide, reactive nitric oxide species and ROS can initiate the redox-sensitive transcription factors such as NF-κB. The transcription factor, NF-kB plays an important role in the regulation of several proinflammatory mediators that are directly involved in the development of neuroinflammation/neurodegeneration. The inactive form of NF-kB is present in the cytoplasm in association with an inhibitory protein IkB, which prevents its nuclear translocation required for transcriptional activity. The phosphorylation followed by proteolytic degradation of IkB results in translocation of free NF-kB to the nucleus, where it binds to target DNA elements (gene promoters containing kB binding sites) and regulates transcription of several proinflammatory genes such as TNF-α, IL-1β, COX-2, iNOS, and adhesion molecules (Shen et al., 2010). Furthermore, MPTP is a potent inhibitor of mitochondrial function in PD (Acuna-Castroviejo et al., 2011). Due to the inhibition of mitochondrial enzyme complex activity, there is an increase in the production of superoxide anions which further up-regulate the NF-κB activation and its associated inflammatory cascade of events. Based on these finding, it is conceivable that drugs with an ability to prevent the generation of ROS may afford protection to the dopaminergic neurons from neurotoxins such as MPTP. We have observed that PYC treatment significantly ameliorated the free radical generation as well as inhibited the activation of NF-κB. It is well known that NF-κB activates proinflammatory cytokines/chemokine release from the activated glia and also induce iNOS expression in the MPTP-induced animal model of PD. Furthermore, iNOS expression in the brain has also been documented to play a role in the pathogenesis of PD (Dehmer et al., 2000). Glial activation, which is accompanied by the upregulation of iNOS, may have a pivotal role in PD (Hunot et al., 1997). In addition, inflammatory processes associated with increased expression of COX-2 and elevated level of prostaglandin E2 have been found to be involved in the cascade of deleterious events that lead to neurodegeneration in PD (Teismann et al., 2003). Many epidemiological studies showed that nonsteroid anti-inflammatory drugs such as COX-2 inhibitors may reduce the incidence of PD (Gupta et al., 2011; Teismann et al., 2003). Our present findings further suggest that PYC exerts its anti-inflammatory effects by inhibiting NF-κB expression and the subsequent release of COX-2, iNOS, TNF-α and IL-1β. Neurons are damaged under oxidative stress and high inflammatory responses may cause neurodegenerative conditions such as stroke, AD and PD (Gibson and Zhang, 2001). Therefore, drugs with antioxidative and anti-inflammatory characteristics are suggested to be effective therapeutic agent for the neurodegenerative diseases (Jin et al., 2005). Natural compounds containing flavonoids anti-inflammatory and are neuroprotective in neurodegenerative and demyelinating diseases (2012). There are several reports on i.p. administration of PYC in various disease models which reached the brain and provided neuro protection (Maritim et al., 2003; Ishrat et al., 2009; Khan et al., 2010; Parveen et al., 2012). The PYC regimen used in this study was determined from the previous studies showing that PYC administration via i.p route reaches to the brain in sufficient amount to provide the maximal protective effects in the treatment of various types of brain diseases. Additionally, IP administration has also been used as an effective route of administration to study the reinforcing properties of the drugs. This is a viable and effective technique for drug delivery.
5. Conclusions
In the present study, we showed that MPTP injection increases the expression of NF-κB, IL-1β, TNF-α, iNOS and COX-2 in the brain and these increases were attenuated by PYC treatment, suggesting the anti-inflammatory actions of PYC contribute to its neuroprotective effects. Further, our study demonstrated that PYC inhibited MPTP-induced impairment of behavioral functions, oxidative loads and activation of glial cells. We conclude that PYC may be a promising candidate for neuroprotection in the MPTP-induced animal model of PD.
HIGHLIGHTS.
Pycnogenol-treatment ameliorates clinical symptoms of MPTP-induced PD in mice
Pycnogenol limits MPTP-induced inflammation and neuronal death in mice
Pycnogenol mitigates MPTP-induced oxidative stress and behavioral deficits in mice
Acknowledgments
The authors thank Dr. Frank Schonlau, Horphag Research Ltd. (South Kensington, London, UK) for providing a gift pack of Pycnogenol for the study. This work was supported by the Department of Veterans Affairs Merit Review award (to A.Z.) and by the National Institute of Neurological Disorders and Stroke grants NS073670 (to A.Z.).
List of abbreviations used
- ANOVA
One-way analysis of variance
- COX-2
cyclooxygenase-2
- DA
dopamine
- ELISA
Enzyme-linked immunosorbent assay
- HRP
horseradish peroxidase
- GFAP
glial fibrillary acidic protein
- GP
Glutathione Peroxidase
- GPx
Glutathione reductase
- IL-1
interleukin-1
- iNOS
inducible nitric oxide synthase
- Iba-1
ionized calcium binding adaptor molecule 1
- MPTP
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydro pyridine
- NF-kB
nuclear transcription factor-κB
- PD
Parkinson’s disease
- PMSF
phenyl methyl-sulfonylfluoride
- PYC
Pycnogenol®
- SNpc
substantia nigra pars compacta
- SOD
Super oxide dismutase
- TBARS
thiobarbituric acid reactive substance
- TH
tyrosine hydroxylase
- TNF-alpha
tumor necrosis factor-α
Footnotes
Competing Interests:
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkinson disease: Could dietary flavonoids be protective against Parkinson disease? Nat Rev Neurol. 8:298. doi: 10.1038/nrneurol.2012.88. [DOI] [Google Scholar]
- 2.Acuna-Castroviejo D, Tapias V, Lopez LC, Doerrier C, Camacho E, Carrion MD, Mora F, Espinosa A, Escames G. Protective effects of synthetic kynurenines on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in mice. Brain Res Bull. 2011;85:133–40. doi: 10.1016/j.brainresbull.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Bors W, Michel C. Chemistry of the antioxidant effect of polyphenols. Ann N Y Acad Sci. 2002;957:57–69. doi: 10.1111/j.1749-6632.2002.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 4.Brotchie J, Jenner P. New approaches to therapy. Int Rev Neurobiol. 2011;98:123–50. doi: 10.1016/B978-0-12-381328-2.00005-5. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, He G, Mu X, Zhang T, Li X, Hu J, Xu B, Du G. Neuroprotective effect of baicalein against MPTP neurotoxicity: behavioral, biochemical and immunohistochemical profile. Neurosci Lett. 2008;441:16–20. doi: 10.1016/j.neulet.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 6.Choi DK, Pennathur S, Perier C, Tieu K, Teismann P, Wu DC, Jackson-Lewis V, Vila M, Vonsattel JP, Heinecke JW, Przedborski S. Ablation of the inflammatory enzyme myeloperoxidase mitigates features of Parkinson’s disease in mice. J Neurosci. 2005;25:6594–600. doi: 10.1523/JNEUROSCI.0970-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung YC, Kim SR, Park JY, Chung ES, Park KW, Won SY, Bok E, Jin M, Park ES, Yoon SH, Ko HW, Kim YS, Jin BK. Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology. 2011;60:963–74. doi: 10.1016/j.neuropharm.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 8.D’Andrea G. Pycnogenol: a blend of procyanidins with multifaceted therapeutic applications? Fitoterapia. 2010;81:724–36. doi: 10.1016/j.fitote.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem. 2000;74:2213–6. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- 10.Dexter DT, Holley AE, Flitter WD, Slater TF, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD. Increased levels of lipid hydroperoxides in the parkinsonian substantia nigra: an HPLC and ESR study. Mov Disord. 1994;9:92–7. doi: 10.1002/mds.870090115. [DOI] [PubMed] [Google Scholar]
- 11.Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–71. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- 12.Freeman BA, Crapo JD. Biology of disease: free radicals and tissue injury. Lab Invest. 1982;47:412–26. [PubMed] [Google Scholar]
- 13.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–98. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2007;104:18754–9. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson GE, Zhang H. Abnormalities in oxidative processes in non-neuronal tissues from patients with Alzheimer’s disease. J Alzheimers Dis. 2001;3:329–338. doi: 10.3233/jad-2001-3308. [DOI] [PubMed] [Google Scholar]
- 16.Gordon R, Anantharam V, Kanthasamy AG, Kanthasamy A. Proteolytic activation of proapoptotic kinase protein kinase Cdelta by tumor necrosis factor alpha death receptor signaling in dopaminergic neurons during neuroinflammation. J Neuroinflammation. 2012;9:82. doi: 10.1186/1742-2094-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Q, Zhao B, Packer L. Electron spin resonance study of free radicals formed from a procyanidin-rich pine (Pinus maritima) bark extract, pycnogenol. Free Radic Biol Med. 1999;27:1308–12. doi: 10.1016/s0891-5849(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A, Kumar A, Kulkarni SK. Targeting oxidative stress, mitochondrial dysfunction and neuroinflammatory signaling by selective cyclooxygenase (COX)-2 inhibitors mitigates MPTP-induced neurotoxicity in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:974–81. doi: 10.1016/j.pnpbp.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 19.Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp Neurol. 2005;193:279–90. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:214–28. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–97. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 22.Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–2. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 23.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–6. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishrat T, Parveen K, Hoda MN, Khan MB, Yousuf S, Ansari MA, Saleem S, Islam F. Effects of Pycnogenol and vitamin E on cognitive deficits and oxidative damage induced by intracerebroventricular streptozotocin in rats. Behav Pharmacol. 2009;20:567–75. doi: 10.1097/FBP.0b013e32832c7125. [DOI] [PubMed] [Google Scholar]
- 25.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–51. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 26.Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S183–5. doi: 10.1016/S1353-8020(11)70057-8. [DOI] [PubMed] [Google Scholar]
- 27.Jankyova S, Kucera P, Goldenberg Z, Yaghi D, Navarova J, Kyselova Z, Stolc S, Klimas J, Racanska E, Matyas S. Pycnogenol efficiency on glycaemia, motor nerve conduction velocity and markers of oxidative stress in mild type diabetes in rats. Phytother Res. 2009;23:1169–74. doi: 10.1002/ptr.2776. [DOI] [PubMed] [Google Scholar]
- 28.Jenner P, Dexter DT, Sian J, Schapira AH, Marsden CD. Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann Neurol. 1992;32(Suppl):S82–7. doi: 10.1002/ana.410320714. [DOI] [PubMed] [Google Scholar]
- 29.Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36–8. [DOI] [PubMed] [Google Scholar]
- 30.Jin DQ, Lim CS, Hwang JK, Ha I, Han JS. Anti-oxidant and anti-inflammatory activities of macelignan in murine hippocampal cell line and primary culture of rat microglial cells. Biochem Biophys Res Commun. 2005;331:1264–9. doi: 10.1016/j.bbrc.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Jin F, Wu Q, Lu YF, Gong QH, Shi JS. Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol. 2008;600:78–82. doi: 10.1016/j.ejphar.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, Rey P, Guerra MJ, Labandeira-Garcia JL. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: relevance to progression of the disease. J Neurochem. 2009;109:656–69. doi: 10.1111/j.1471-4159.2009.05999.x. [DOI] [PubMed] [Google Scholar]
- 33.Khan MM, Hoda MN, Ishrat T, Ahmad A, Khan MB, Khuwaja G, Raza SS, Safhi MM, Islam F. Amelioration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced behavioural dysfunction and oxidative stress by Pycnogenol in mouse model of Parkinson’s disease. Behav Pharmacol. 2010;21:563–71. doi: 10.1097/FBP.0b013e32833d4186. [DOI] [PubMed] [Google Scholar]
- 34.Khan MM, Gandhi C, Chauhan N, Stevens JW, Motto DG, Lentz SR, Chauhan AK. Alternatively-spliced extra domain a of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke. 2012;43:1376–82. doi: 10.1161/STROKEAHA.111.635516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khuwaja G, Khan MM, Ishrat T, Ahmad A, Raza SS, Ashafaq M, Javed H, Khan MB, Khan A, Vaibhav K, Safhi MM, Islam F. Neuroprotective effects of curcumin on 6-hydroxydopamine-induced Parkinsonism in rats: behavioral, neurochemical and immunohistochemical studies. Brain Res. 2011;1368:254–63. doi: 10.1016/j.brainres.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, Kim YA, Yokozawa T. Pycnogenol modulates apoptosis by suppressing oxidative stress and inflammation in high glucose-treated renal tubular cells. Food Chem Toxicol. 2011;49:2196–201. doi: 10.1016/j.fct.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Kim YS, Joh TH. Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp Mol Med. 2006;38:333–47. doi: 10.1038/emm.2006.40. [DOI] [PubMed] [Google Scholar]
- 38.Lee DH, Kim CS, Lee YJ. Astaxanthin protects against MPTP/MPP+-induced mitochondrial dysfunction and ROS production in vivo and in vitro. Food Chem Toxicol. 2011;49:271–80. doi: 10.1016/j.fct.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu FJ, Zhang TX, Lau BHS. Pycnogenol improves learning impairment and memory deficit in senescence-accelerated mice. J Anti-Aging Med. 1999;2:349–355. [Google Scholar]
- 40.Maimoona A, Naeem I, Saddiqe Z, Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J Ethnopharmacol. 2011;133:261–77. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 41.Maritim A, Dene BA, Sanders RA, Watkins JB., 3rd Source Effects of pycnogenol treatment on oxidative stress in streptozotocin-induced diabetic rats. J Biochem Mol Toxicol. 2003;17:193–199. doi: 10.1002/jbt.10078. [DOI] [PubMed] [Google Scholar]
- 42.Moon M, Kim HG, Hwang L, Seo JH, Kim S, Hwang S, Kim S, Lee D, Chung H, Oh MS, Lee KT, Park S. Neuroprotective effect of ghrelin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease by blocking microglial activation. Neurotox Res. 2009;15:332–47. doi: 10.1007/s12640-009-9037-x. [DOI] [PubMed] [Google Scholar]
- 43.Mosley RL, Benner EJ, Kadiu I, Thomas M, Boska MD, Hasan K, Laurie C, Gendelman HE. Neuroinflammation, Oxidative Stress and the Pathogenesis of Parkinson’s Disease. Clin Neurosci Res. 2006;6:261–281. doi: 10.1016/j.cnr.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S200–4. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 45.Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic Biol Med. 1999;27:704–24. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 46.Parveen K, Ishrat T, Malik S, Kausar MA, Siddiqui WA. Modulatory effects of Pycnogenol® in a rat model of insulin-dependent diabetes mellitus: biochemical, histological, and immunohistochemical evidences. Protoplasma. 2012 doi: 10.1007/s00709-012-0418-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Peng Q, Wei Z, Lau BH. Pycnogenol inhibits tumor necrosis factor-alpha-induced nuclear factor kappa B activation and adhesion molecule expression in human vascular endothelial cells. Cell Mol Life Sci. 2000;57:834–41. doi: 10.1007/s000180050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng YJ, Lee CH, Wang CC, Salter DM, Lee HS. Pycnogenol attenuates the inflammatory and nitrosative stress on joint inflammation induced by urate crystals. Free Radic Biol Med. 2012;52:765–74. doi: 10.1016/j.freeradbiomed.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 49.Rohdewald P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int J Clin Pharmacol Ther. 2002;40:158–68. doi: 10.5414/cpp40158. [DOI] [PubMed] [Google Scholar]
- 50.Schoonees A, Visser J, Musekiwa A, Volmink J. Pycnogenol(R) (extract of French maritime pine bark) for the treatment of chronic disorders((R)) for the treatment of chronic disorders. Cochrane Database Syst Rev. 2012;4:CD008294. doi: 10.1002/14651858.CD008294.pub4. [DOI] [PubMed] [Google Scholar]
- 51.Schwarting RK, Bonatz AE, Carey RJ, Huston JP. Relationships between indices of behavioral asymmetries and neurochemical changes following mesencephalic 6-hydroxydopamine injections. Brain Res. 1991;554:46–55. doi: 10.1016/0006-8993(91)90170-z. [DOI] [PubMed] [Google Scholar]
- 52.Shen H, Hu X, Liu C, Wang S, Zhang W, Gao H, Stetler RA, Gao Y, Chen J. Ethyl pyruvate protects against hypoxic-ischemic brain injury via anti-cell death and anti-inflammatory mechanisms. Neurobiol Dis. 2010;37:711–22. doi: 10.1016/j.nbd.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun Y. Free radicals, antioxidant enzymes, and carcinogenesis. Free Radic Biol Med. 1990;8:583–99. doi: 10.1016/0891-5849(90)90156-d. [DOI] [PubMed] [Google Scholar]
- 54.Tansey MG, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp Neurol. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100:5473–8. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thangavel R, Sahu SK, Van Hoesen GW, Zaheer A. Loss of nonphosphorylated neurofilament immunoreactivity in temporal cortical areas in Alzheimer’s disease. Neuroscience. 2009;160:427–33. doi: 10.1016/j.neuroscience.2009.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- 58.Viaro R, Sanchez-Pernaute R, Marti M, Trapella C, Isacson O, Morari M. Nociceptin/orphanin FQ receptor blockade attenuates MPTP-induced parkinsonism. Neurobiol Dis. 2008;30:430–8. doi: 10.1016/j.nbd.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:6145–50. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang YS, Ahn TH, Lee JC, Moon CJ, Kim SH, Jun W, Park SC, Kim HC, Kim JC. Protective effects of Pycnogenol on carbon tetrachloride-induced hepatotoxicity in Sprague-Dawley rats. Food Chem Toxicol. 2008;46:380–7. doi: 10.1016/j.fct.2007.08.016. [DOI] [PubMed] [Google Scholar]