Abstract
‘Taishanzaoxia’ fruit rapid softening and dehiscence during ripening stage and this process is very sensitive to endogenous ethylene. In this study, we cloned five ethylene signal transcription factors (ZMdEIL1, ZMdEIL2, ZMdEIL3, ZMdERF1 and ZMdERF2) and one functional gene, ZMdPG1, encoding polygalacturonase that could loose the cell connection which associated with fruit firmness decrease and fruit dehiscence to illustrate the reasons for this specific fruit phenotypic and physiological changes. Expression analysis showed that ZMdERF1 and ZMdEIL2 transcription were more abundant in ‘Taishanzaoxia’ softening fruit and dehiscent fruit and their expression was inhibited by an ethylene inhibitor 1-methylcyclopropene. Therefore, ZMdERF1 and ZMdEIL2 expression were responses to endogenous ethylene and associated with fruit softening and dehiscence. ZMdPG1 expression was induced when fruit softening and dehiscence but this induction can be blocked by 1-MCP, indicating that ZMdPG1 was essential for fruit softening and dehiscence and its expression was mediated by the endogenously occurred ethylene. ZMdPG1 overexpression in Arabidopsis led to silique early dehiscence while suppressing ZMdPG1 expression by antisense ZMdPG1 prevented silique naturally opening. The result also suggested that ZMdPG1 related with the connection between cells that contributed to fruit softening and dehiscence. ZMdERF1 was more closely related with ethylene signaling but it was not directly regulated the ZMdPG1, which might be regulated by the synergic pattern of ethylene transcription factors because of both the ZMdERF1 and ZMdERF2 could interact with ZMdEIL2.
Introduction
Fruit softening and dehiscence greatly reduce commercial value by influencing the fruit taste, flavor, out-looking and shelf life, which is common for certain apple cultivars, including Red Delicious Golden Delicious and ‘Taishanzaoxia’ [1]–[3]. It is especially the case for the apple cultivar ‘Taishanzaoxia’, which suffers from this fruit quality deterioration severer than any other apple cultivars, and which is softening very fast, accompanying with the fruit dehiscence during the fruit ripening [2]. The defect of this cultivar make it an ideal material for dissecting the mechanism underlying the easy softening and dehiscence, which is important for uncovering the mechanism for fruit quality formation, postharvest physiology and fruit breeding.
Previous research indicated that fruit softening was tightly connected with ethylene biosynthesis. Acceleration of fruit softening connect with the rapidly increase of ethylene production in some apple cultivars, so ethylene played an important role in this process [4], [5]. The findings in our group as well as many other international colleagues showed that 1-MCP treatment, which blocked ethylene biosynthesis, effectively prevented fruit softening and dehiscence, strongly demonstrating that ethylene was involved in this process [6]–[9]. In addition, the endo-ethylene accelerate the dehiscence process of flower organ even through ethylene doesn't initiate dehiscence in Arabidopsis [10].
Ethylene biological effects are discovered through the ethylene signaling pathway. Firstly, ethylene is perceived by the target cells through receptors (ETRs). Subsequently, the signal transmission would be regulated by the ethylene signaling negative regulator CTR1 (constitutive triple response 1) and positive regulators EIN2 (ethylene insensitive 2) and EIN3 (ethylene insensitive 3). In the end, the signal would be transmitted to ethylene responsive transcription factors(ERFs) [11].
EIN3/EILs(EIN3-like genes) belongs to a small transcription factors family including several DNA-binding domains such as acidic domain, proline-rich and basic domains[12], [13]. EIN3 gene is firstly identified from ein3 mutants of Arabidopsis. Subsequently, four LeEILs response for fruit ripening have been isolated from tomato [13], [14]. Antisense suppression of LeEILs reveals functional redundancy in tomato [13]. The function of EIN3/EILs is demonstrated at the protein level, the DNA-binding protein of this family directly binds to the primary response element in promoter of ERF1 (Ethylene response factor) to regulate ERF1 expression in Arabidopsis [15]. EIL genes have been also isolated from fruits such as tomato, melon, kiwifruit and apple [16]–[20]. In transgenic apple, MdEILs activate the MdPG1 promoter in the presence of ethylene [19]. CmEIL1 and CmEIL2 as ripening-related genes regulate the transcription of CmACO1in ripening melon fruits [16]. It is similar that AdEIL2 and AdEIL3 activate the expression of ripening-related genes AdACO1 and AdXET5 in kiwifruit [21]. However, much less is known response for EILs function in transcriptional level in fruit.
ERFs belongs to the large AP2/ERF superfamily including 122 members in Arabidopsis and 139 members in rice [22], and contains two conversed DNA-binding domains YRG element and RAYD element [23]. Referred to as the ethylene responsive element binding proteins (EREBPs), ERF was first isolated from tobacco by binding to the GCC motif in the promoter of functional genes [24]. Then, four ERF genes were identified which induced fruit ripening in tomato. Transgenic experiment result showed that antisense LeERF1 under the control of CaMV35 with longer postharvest life [25], and SlERF2 was shown to express predominantly in ripening fruits [26]. All four LeERFs have the ability binding to GCC-box element present in several defense responsive genes [27]. In kiwifruit, AdERFs protein did not bind to the AdEXP1 promoter containing a GCC-box, but the activation of AdXET5 was significantly suppressed by AdERF9 in interaction experiments which suggesting that fruit ripening might be regulated by unknown mechanism. Two MdERF genes had been isolated from ripening fruit which were regulated by ethylene [28]. Same as SlERF2, MdERF2 expressed exclusively in ripening fruit, and MdERF1 was expressed predominantly in ripening fruit. However, there is little research involved in the function of MdERF1 and MdERF2 in fruit, and the role of MdEILs and MdERFs is unknown.
Besides the ethylene signaling, another set of important ingredients correlate with softening and dehiscence are hydrolytic enzymes located at the cell wall, including PGs, because in essence the loosened or even broken cell connection cause fruit softening and dehiscence [29], [30]. PG was first cloned from ripening tomato cDNA library [31]. In tomato fruit, a correlation between endo-PG activity and softening has been observed in a number of cultivars [32], [33]. However, endo-PG activity in transgenic tomato plants is not the sole determinant of fruit softening [34], [35]. The relationship between PG and fruit softening has been presented in other plant species, such as apple, pears, kiwifruit and peach and certain PGs are also accountable for the organ abscission or dehiscence [36]–[39]. For example, repression of PG1 in apple brings about firmer fruit [40]. In ‘Gold Delicious’, softening is closely depended on the expression of MdPG with comparison of ‘Fuji’ [5]. PG overexpression in transgenic apple lead to premature leaf shedding because cell adhesion is reduced in leaf abscission zones [41]. MdPG1 is repressed in transgenic ‘Royal Gala’ apples while returned to wild-type levels with ethylene treatment [19]. Further, a MdEIN3-like transcription factor activates the promoter of MdPG1 by transient assays [19]. In addition, dehiscence occurs in wild siliques but not in adpg1 adpg2 qrt2 triple mutants in Arabidopsis [42].
In this research we characterized the ZMdERFs, ZMdEILs and ZMdPG1 in ‘Taishanzaoxia’ with the aim to uncover the heavily occurred softening and dehiscence. In this study, the expression of ethylene transcription factors and ZMdPG1 was significantly high and showed ripen-inducible pattern while their expression in ‘Liaofu’ was relatively stable and did not obvious change during fruit growth and developmental period. The ZMdPG1 induction was mediated by endogenously biosynthesized ethylene. BiFC assay showed that ZMdEIL2 interact with ZMdERF1 and ZMdERF2, respectively. Transgenic analysis showed that ZMdPG1 overexpression could result in cell connection broken as demonstrated by the silique dehiscence of ZMdPG1 overexpressed Arabidopsis transgenic plant. Therefore, ZMdPG1 expression may lead to fruit dehiscence in ‘Taishanzaoxia’, which is induced by ethylene.
Results
Ethylene promote the loss of fruit firmness
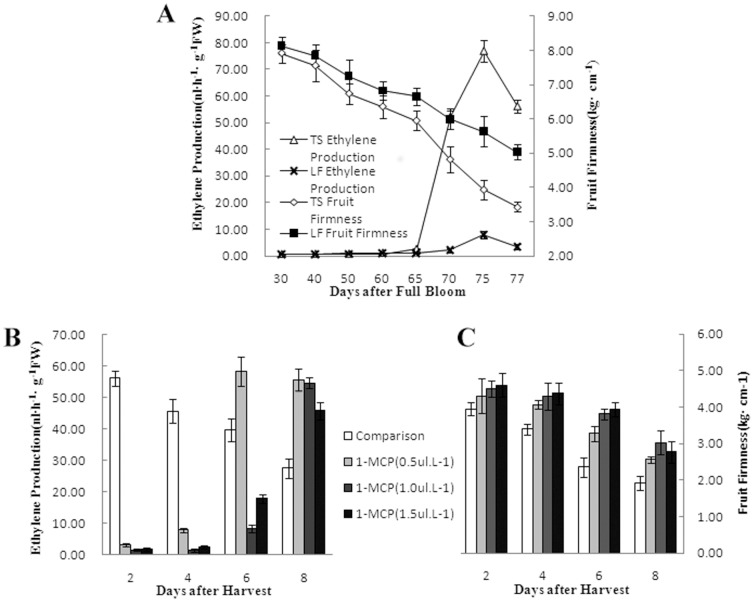
Fruit softening was closely associated with ethylene production [43]. As shown in Figure 1, there was a sharp increase of ethylene production in ‘Taishanzaoxia’, and the decline of fruit firmness was accelerated by the rapid collection of ethylene production that led to significant diversity in fruit firmness between ‘Taishanzaoxia’ and ‘Liaofu’. The change of fruit firmness was similar from 30 d to 60 d in two cultivars, whereas they assumed the difference after 65 d when ethylene biosynthesis began to increase in ‘Taishanzaoxia’ (Figure 1). The rapid increase of ethylene production might result in the fruit softening. After harvest, ‘Taishanzaoxia’ fruits were treated with 1-MCP. Fruit softening behavior was clearly limited associate with the significant inhibition of ethylene production. Compared with the control, fruit firmness of treatment was inhibited clearly at 6 d after harvest (Figure 1). Fruit dehiscence was also observed with the abundance of ethylene over the postharvest period.
Figure 1. The comparison of ethylene production and fruit firmness in ‘Taishanzaoxia’ and ‘Liaofu’ cultivar.
(A) Changes of fruit firmness and ethylene production in different apple cultivars during fruit development. (B) Effect of different levers 1-MCP on ethylene production in ‘Taishanzaoxia’. (C) Effect of different levers 1-MCP on fruit firmness in ‘Taishanzaoxia’. TS represent ‘Taishanzaoxia’ cultivar. LF represents ‘Liaofu’ cultivar.
Cloning and analysis of ZMdERFs, ZMdEILs and ZMdPG1
cDNA synthesized from ‘Taishanzaoxia’ ripening apple fruit was used as a template for RT-PCR, and the primers were designed from the nucleotide sequence among ‘Gold Delicious’ MdERFs. Then two cDNA full-length fragments corresponding to MdERF1 and MdERF2 were cloned and named as ZMdERF1 and ZMdERF2 (ZMdERF1, GenBank accession number KC128856; ZMdERF2, GenBank accession number KC128857). Alignments of amino acid showed both two genes contained the YRG and RAYD elements [23] (Figure S1A) indicating that they were the ethylene transcription factors in ‘Taishanzaoxia’. Phylogenetic analysis revealed that ZMdERF1 and ZMdERF2 were in the same cluster with ‘Gold Delicious’ MdERF1 and MdERF2, respectively (Figure S1B).
Three EIN3-like genes named MdEIL1, MdEIL2 and MdEIL3 (MdEIL1, GenBank accession number KC128859; MdEIL2, GenBank accession number KC128859; MdEIL3, GenBank accession number KC128860) were amplified from ‘Taishanzaoxia’ using the same strategy. They had the high similarity with each other (Figure S2A), sharing the commonly conserved domain with that of tobacco, Kiwifruit and tomato, which included a high acidic region, five basic domains and a proline-rich domain [12] (Figure S2B).
Functional gene ZMdPG1 (ZMdPG1, GenBank accession number KC128861) gene was also cloned using ‘Taishanzaoxia’ ripening fruit cDNA. ZMdPG1 gene encoded 460 amino acids. The predicted ZMdPG1 protein shared high similarity with known PGs in ‘Gold Delicious’ apple, pear, peach, kiwifruit and Arabidopsis (Figure S3A). NCBI (National Center of Biotechnology Information) assay indicated the homology between ZMdPG1 and ADPG1 was 67%. Phylogenetic analysis revealed that ZMdPG1 was in the same cluster with pGDPG-1 from ‘Gold Delicious’ cultivar and there was only two amino acid difference between them. ZMdPG1 was close to Arabidopsis ADPG1 and ADPG2 (Figure S3B).
ZMdQP, the promoter region of ZMdPG1, (ZMdQP, GenBank accession number KC128862) was identified in ‘Taishanzaoxia’ using high-TAIL PCR. Sequence analysis revealed that the ZMdQP was approximately 1.7 kb in length from the ZMdPG1 start cordon. The sequence 787-1697 bp in ZMdQP was high similar with the promoter of pGDGP-1 while there was significant difference in 1–786 bp (Figure S4). 29 elements in ZMdQP were analyzed, such as A-box, AT-rich element, CCGTCC-box and CGTCA-motif were involved in regulatory function, elicitor-mediated activation, meristem specific activation and MeJA-responsiveness. The traits TATA-box and CAAT-box at -303 bp and -244 bp from the ATG start cordon were identified in the upstream region.
We put the sequence of the genes cloned in our research into GDR (Genome Database for Rosaceae) database to compare with the apple genome. Many homologues were found in the apple genome (Table S1).
Expression profile of ZMdERFs, ZMdEILs and ZMdPG1
To understand the molecular mechanism of fruit softening and dehiscence, the expression of ZMdPG1 gene and five ethylene signaling components ZMdERF1, ZMdERF2, ZMdEIL1, ZMdEIL2 and ZMdEIL3 were investigated. As shown in Figure 2, ZMdERF1 was constitutively expressed during the development of ‘Taishanzaoxia’ and ‘Liaofu’ fruits but its abundance in ‘Taishanzaoxia’ was higher than that in ‘Liaofu’. ZMdEIL2 also showed a ripen-inducible trend in soften ‘Taishanzaoxia’ fruits while this trend was not obvious in ‘Liaofu’. In addition, the transcription of ZMdERF1 and ZMdEIL2 was more abundant in ‘Taishanzaoxia’ associated with the abundance of ethylene production in 65 d, whereas their expression in ‘Liaofu’ did not have obvious change during fruit development (Figure 2A). The expression of ZMdERF2 increased a little in the ripening and softening fruit of ‘Taishanzaoxia’. With 1-MCP treatment, fruit softening and dehiscence, ethylene production and the expression of ethylene-induced genes were suppressed. Transcript levels of ZMdPG1, ZMdERF1, ZMdEIL1 and ZMdEIL2 were substantially inhibited compared with control which indicated that the transcription of four genes was closely connected in ethylene during fruit softening and dehiscence stages. 1-MCP treatment has little effect on the reduction of ZMdERF2 and ZMdEIL3 (Figure 2B). Unlike in ‘Taishanzaoxia’ fruits, ZMdEIL2 and ZMdPG1 expressions in ‘Liaofu’ fruit maintained a same level during the whole growth and development period.
Figure 2. The expression pattern of ZMdPG1 and ethylene signal transcription factors.
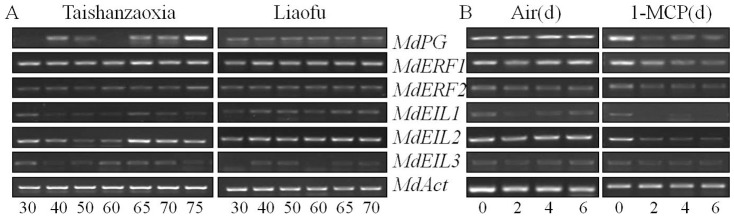
(A) Expression of the ZMdPG1 and ethylene signal transcription factors during fruit development. Data obtained from ‘Taishanzaoxia’ and ‘Liaofu’ apple fruit are shown in order from left to right. Data for different ripening stages are shown in order from left to right. Numbers below each lane indicate the number of ripening days after full bloom. Data from three repeats are provided. (B) Expression of the ZMdPG1 and ethylene signal transcription factors in ‘Taishanzaoxia’ apple fruit after harvest. Fruit were held at 24°C and treated with air (Air) or 1.0 µl.L-1 1-MCP (1-MCP) for 24 h. Data are shown in order from left to right. Data for different stages are shown in order from left to right. Numbers below each lane indicate the number of days after harvest. Data from three repeats are provided.
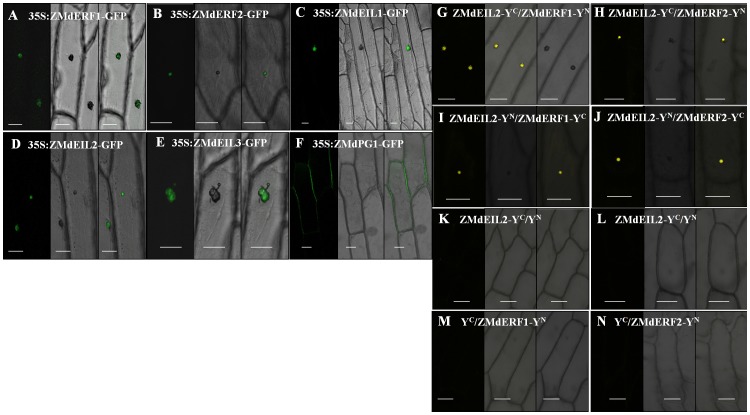
To confirm the functions of ZMdERF1, ZMdERF2, ZMdEIL1, ZMdEIL2 and ZMdEIL3 in cell, those five genes fused with the GFP gene under control of the CaMV35S promoter were transferred into onion epidermal cell. Localization of the fusion protein was determined by visualization with a confocal microscope. We found that ZMdERF1, ZMdERF2, ZMdEIL1, ZMdEIL2 and ZMdEIL3 proteins were accumulated in cell nucleus (Figure 3A)
Figure 3. Functional characterization of ZMdEILs, ZMdERFs and ZMdPG1.
(A) ZMdEIL2 interacts with ZMdERF1and ZMdERF2 in vivo in the BiFC assay. YFP fluorescence signals are detected in 2 d. (B) Transient expression assays showed that ZMdPG1 protein localized in the cell wall. ZMdERF1, ZMdERF2, ZMdEIL1, ZMdEIL2 and ZMdEIL3 localize in the cell nucleus. GFP fluorescence signals are detected in 2 d. bar = 50 µm
Overexpression of ZMdPG1 in transgenic plants
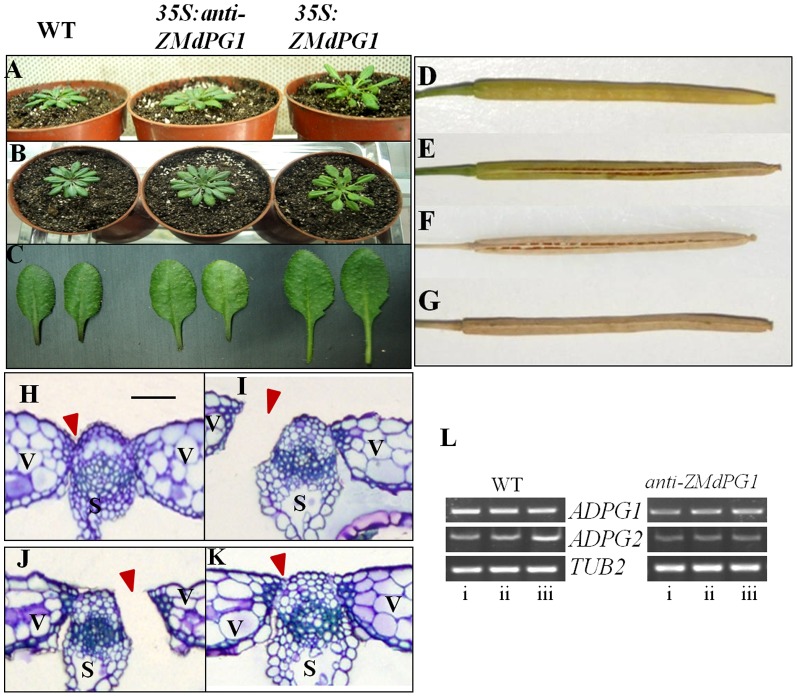
To understand the biological role of ZMdPG1, the sense and antisense ZMdPG1 transgenic Arabidopsis under the control of CaMV35 promoter were performed. As shown in Figure 4. Most overexpressed ZMdPG1 plants displayed similar phenotype such as loosened and slant growing habit, strait-angled branches and longer petioles. In contrast, Antisense ZMdPG1 plants showed the tight and regular phenotype, leaves grew parallel to and horizontal plane and petioles was short. In addition, the overexpression of ZMdPG1 in Arabidopsis resulted in earlier dehiscence fruit. Overexpressed ZMdPG1 Arabidopsis revealed split siliques in early stage 18 (Figure 4E), while that was observed in stage 19 in wide-type control plants (Figure 4F). It was in coincidence with the report that silique dehiscent normally in stage 19 in wide Arabidopsis [44]. But there was not dehiscence in antisense transgenic siliques (Figure 4G). Semi-quantitative RT-PCR analysis showed that the expression of ADPG1 and ADPG2 in antisense ZMdPG1 transgenic Arabidopsis were less than in wide type. The ADPG1 expression was partly inhibited and the ADPG2 expression was significantly inhibited (Figure 4L).The result was further demonstrated by organization experiment. Cross sections revealed that cell separation occurred in the DZ (Dehiscence Zone) in overexpressed ZMdPG1 siliques when siliques turned light yellow (Figure 4I). The same phenomenon occurred in mature and dry wide siliques (Figure 3J) but not in antisense transgenic plants (Figure 4K). The result indicated that dehiscence was caused by cell separation in the DZ. In the protein location assay, we found that the expressed ZMdPG1-GFP fusion protein was precisely localized at the cell wall (Figure 3A) which suggested that the expressed ZMdPG1 sited at the cell wall could play an important role in promoting fruit dehiscence.
Figure 4. The functional pattern of ZMdPG1 in transgenic Arabidopsis.
The plants showing loosened, slant growth and long petiole phenotype in overexpressed ZMdPG1 Arabidopsis but not in antisense Arabidopsis. Silique dehiscence and cell separation occurred in faint yellow silique of overexpressed ZMdPG1 Arabidopsis while that occurred in mature and dry wide siliques but not in antisense transgenic Arabidopsis. Triangle represents DZ. (A) The profile of phenotype. (B) The profile of phenotype. (C) The phenotype of petiole (D) The faint yellow silique of wild Arabidopsis, there is not split (early of stage 18); (E) The faint yellow dehiscence silique of transgenic Arabidopsis containing 35 S:ZMdPG1 (early of stage 18); (F) The mature and dry dehiscence silique of wild Arabidopsis (stage 19); (G) The mature and dry silique of transgenic Arabidopsis containing anti-ZMdPG1 (stage 19); (H) Transverse section of wide-type stained with Toluidine blue corresponding to (D); (I) Transverse section of overexpressed transgenic Arabidopsis stained with Toluidine blue corresponding to (E); (J) Transverse section of wide-type stained with Toluidine blue corresponding to (F); (K) Transverse section of antisense transgenic Arabidopsis stained with Toluidine blue corresponding to (G). Arrowheads indicate the DZ, bar = 50 µm; (L) The expression pattern of ADPG1 and ADPG2 in antisense ZMdPG1 transgenic Arabidopsis. (i) developing siliques in stage 17, (ii) yellow siliques in stage 18, (iii) fully matured siliques in stage 19.
To investigate ZMdPG1expression pattern, the sense and antisense tissues showing GUS staining under the control of CaMV35 promoter were developed in transgenic Arabidopsis and transgenic apple callus, and the construction of ZMdQP fused with the GUS gene was also performed in transgenic Arabidopsis and transgenic apple callus. As shown in Figure 5. GUS signaling was detected in matured silique valve DZs, seeds and ovule funiculus in overexpressed ZMdPG1 plants and ZMdQP-GUS plants (Figure 5A–B). GUS signaling was also observed in leaf and petiole in overexpressed ZMdPG1 plants (Figure 5C). Further more, overexpressed GUS signals were detected in few region of apple callus while ZMdQP-GUS signals were observed only in part regions of apple callus (Figure 5D).
Figure 5. The expression pattern of ZMdPG1 in transgenic Arabidopsis and cell callus.
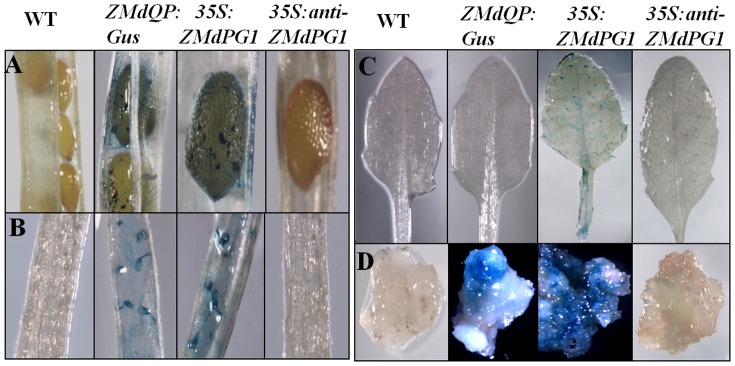
The expression of ZMdPG1 is detected in silique valve DZs (A), seeds (A), ovule funiculus (B) and apple calli (D). The overexpression of ZMdPG1 is detected in leaf and petiole of Arabidopsis (C) and apple callus (D).
ZMdEIL2 interacts with ZMdERF1 and ZMdERF2 physically
In this study, BiFC [45] assays were performed to investigate the function of ZMdEILs and ZMdERFs. ZMdEIL1, ZMdEIL2, ZMdEIL3, ZMdERF1 and ZMdERF2 were fused with the N-terminal fragment and C-terminal fragment of yellow fluorescent protein (YFP), respectively. As a result, strong yellow fluorescent signals were observed in cells containing ZMdERF1/ZMdEIL2, ZMdEFR2/ZMdEIL2 (Figure 3B). Meanwhile, there were not fluorescent signal was observed in other combinations and in control. The results indicated that ZMdERF1 and ZMdERF2 interacted with ZMdEIL2 specifically in cells.
ZMdERF1 did not bind to the ZMdPG1 promoter
To examine whether the nuclear protein ERF1 cloud bind to ZMdQP, electrophoretic mobility shift assay (EMSA) was performed. The recombinant His-ZMdERF1 protein was induced by isopropyl-β- D-thiogalactopyranoside (IPTG) and identified via Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then abundant His-ZMdERF1 protein purified with His Trap TM FF crude [46]. In addition, ten overlapping fragments covering 1.7 kb upstream sequence from the ZMdPG1 translation initiation site were labeled with biotin for chemiluminescence. But slower migrating band was not observed after ten probes were incubated with ZMdERF1 protein (Figure 6). The result demonstrated that ZMdERF1 protein did not bind to ZMdQP.
Figure 6. ZMdERF1 doesn't directly regulate the promoter of the ZMdPG1.
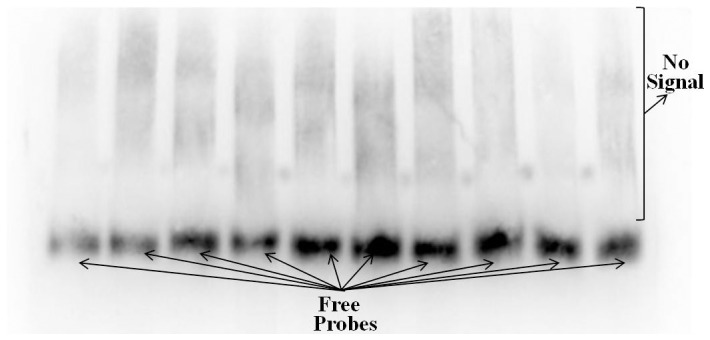
Ten probes were performed. Bracket indicates where binding signal has not occurred.
Discussion
Fruit softening and dehiscence are greatly blocked fruit quality formation and fruit breeding. In this research, the ‘Taishanzaoxia’ cultivar provides a good model for uncovering the molecular mechanism because of the easy softening and dehiscence. Previous work suggested that ‘Taishanzaoxia’ fruit was sensitive to endogenous ethylene [2], [8]. Unlike many other firmness apple fruits such as ‘Liaofu’ and ‘Fuji’ which had little ethylene production [28], the fruit initially and rapidly soften of ‘Taishanzaoxia’ species followed by a ethylene burst. After treatment of 1-MCP, ‘Taishanzaoxia’ fruit ethylene production was significantly inhibited which associated with clear limit of fruit softening behavior (Figure 1).Those results suggested that the loss of ‘Taishanzaoxia’ apple fruit firmness had a strong response to ethylene biosynthesis and it was sensitive to endogenous ethylene. Compared with control, the fruit dehiscence was inhibited with little ethylene biosynthesis after the 1-MCP treatment which also suggested that it was sensitive to endogenous ethylene. So we supported the hypothesis that the regulation for ethylene to the physiological change in fruit firmness and dehiscence.
To investigate the role of ZMdERFs and ZMdEILs, the feature of five ethylene signal factors was confirmed firstly. In this research, ZMdERF1 and ZMdERF2 showed high similarity at conserved region and shared two characteristic ERF elements, YRG and RAYD (Figure S1). Three EIL genes, named ZMdEIL1, ZMdEIL2 and ZMdEIL3 which showed high identity with the EILs in other plant species (Figure S2). ZMdPG1 has the high similarity with the known PG1 (Figure S3). WAAEIRD box, α-helix and β-sheet [47] were also observed in YRG and RAYD elements of ZMdERFs. However, little was known about the function of YRG and RAYD element in apple fruit. In Arabidopsis, YRG bound to DNA via its β-sheet for the highly basic in this region [47]. The α-helical structure in RAYD might interact with the major groove of DNA or regulated protein–protein interactions [23]. It was inferred that ZMdERFs might regulated downstream function genes via YRG or RAYD elements. It was predicted that the WAAEIRD motifs may be responsible for DNA binding sequence [23]. The conserved structure of ZMdEILs from ‘Taishanzaoxia’ fruit including highly acidic region, proline-rich regions but less was known about their function. The similar regions was only described as transcriptional activation domains in Arabidopsis [48]. The coil-basic motif was also observed in ZMdEILs which might mediate DNA binding.
ZMdERF1 gene was one of ethylene transcription factors, whose expression was constitutively higher during ‘Taishanzaoxia’ fruit ripening and softening and it can be induced when ethylene biosynthesis began to increase. This trend fit with those from other species such as ‘Golden Delicious’, where MdERF1 expression pattern paralleled the ethylene rise in ripening fruit [28]. However, 1-MCP treatment inhibited the increase of ZMdERF1 expression which was associated with the delay of the loss of fruit firmness and the fruit dehiscence (Figure 2). These results raised the possibility that ZMdERF1 expression was connect in fruit softening and dehiscence. Another finding was the difference in ZMdERF2 expression pattern. ZMdERF2 was very similar in conservative amino acid sequence to ZMdERF1, but their expression patterns were different response to ethylene in developing and softening apple. ZMdERF2 showed little ethylene response, but its expression pattern was stronger than that in ‘Liaofu’ at the late stage of fruit development associated with fruit softening, and with 1-MCP treatment, ZMdERF2 showed relatively lower expression levels consistency in delayed softening (Figure 2). The result suggested that ZMdERF1 was likely to be associated with softening in ‘Taishanzaoxia’ fruit. Like the EIL genes in ‘Royal Gala’ apple and kiwifruit [19], [21], ZMdEIL2 gene showed ripen-inducible trend and exhibited response to ethylene, it was constitutively expressed in ‘Taishanzaoxia’ fruit during ripening and harvesting stages. This agreed with the finding of ethylene-dependent activity with EIL2 in transgenic apple [19]. The result implied that ZMdEIL2 was necessary for regulation to fruit softening and dehiscence.
Previous reports have confirmed that ERFs is considered to have the ability to regulate functional genes, such tomato LeERFs [27]. In order to uncover the regulation role of ZMdERF1, ripening related gene ZMdPG1 was selected for promoter isolation. We know that PG activity was connected with fruit softening in many species. In this study, we found the high level expression of ZMdPG1 was not only led to fruit softening but also resulted in fruit dehiscence during ‘Taishanzaoxia’ ripening period. This trend was closely similar with the ZMdERF1 gene expression. However, the EMSA assay did not proved ZMdERF1 protein has the ability that directly binding to the ZMdPG1 promoter (Figure 6). It is well established that ERF1 is a GCC-box-binding protein [15], [49]. However, there is not GCC-box element in ZMdPG1 promoter. The result suggested that ZMdPG1 was not the target gene of ZMdERF1 protein, and ZMdPG1 might be activated by some additional regulatory mechanism. In other species, different mechanisms referred to ethylene regulation were demonstrated. In Arabidopsis, AtEBP protein interact with a basic Leu zipper transcription factor to regulate the expression of functional genes [50]. The activity of AdXET5 promoter was significantly suppressed by AdERF9 [21]. MdEIN3-like transcription factor activated the expression of MdPG1 in transient assays [19]. ZMdPG1 from ‘Taishanzaoxia’ fruit might regulate by potential unknown molecular mechanism. BiFC experiment evidenced that ZMdERF1 and ZMdERF2 interacted physically with ZMdEIL2 (Figure 3B), and the transcription of ZMdERF1 and ZMdEIL2 was more abundant in ‘Taishanzaoxia’. Such activated assays might suggest that ZMdPG1 was regulated by the synergic pattern of ethylene transcription factors. The regulation of ethylene signal factors to ZMdPG1 remains to be determined.
PGs play critical roles in cell separation during plant organ abscission or dehiscence processes, and the regulation of PG to fruit softening has been presented in many plant species [29], [34], [42], [43]. In this research, the expression of ZMdPG1 was observed in softening fruits and showed ripen-inducible pattern. After 1-MCP treatment, the activation of ZMdPG1 was inhibited and then fruit dehiscence was suppressed. So ‘Taishanzaoxia’ fruit softening and dehiscence were regulated by ZMdPG1. For further investigating the role of ZMdPG1, the transgenic Arabidopsis was provided because of apple transformation is a long process. ZMdPG1 overexpression in Arabidopsis led to seed silique early dehiscence while in antisense ZMdPG1Arabidopsis, the expression of ADPG1 and ADPG2 which were essential for silique dehiscence were inhibited and silique naturally opening was prevented (Figure 4). The ectopic expression of ZMdPG1 was involved in cell separation and contributed to fruit dehiscence. Similar results have been shown in other researches. Genetic analysis demonstrates that ADPG1 and ADPG2 contribute to silique dehiscence in Arabidopsis [42]. PG overexpression in transgenic apple resulted in premature leaf shedding [41]. Leaf abscission was delayed when PGs were silenced in tomato [51]. These data raised the confirmation that ZMdPG1 was one of important role which led to apple fruit dehiscence. In addition, PG involve in cell wall change resulted in soften fruit and loosened flesh [5], [34]. In this study, the firmness of fruit was obtained when ZMdPG1 expression was inhibited by 1-MCP treatment. The phenotype of overexpressed Arabidopsis was looser than that in control and antisense transgenic plants, and the petiole in sense Arabidopsis was longer than that in wide and antisense plants. These results showed that ZMdPG1 was involved in cell change and the loss of apple fruit firmness. This trend was consistent in pear that the accumulation of PG gene was also paralleled with the fruit softening [43]. So ZMdPG1expression led apple fruit softening and dehiscence. The primary molecular elucidation for apple fruit dehiscence and softening provided important information for the breeding.
Conclusion
In this research, we cloned two ethylene signaling components, ZMdERF1 and ZMdERF2, three EIL-likes genes ZMdEIL1, ZMdEIL2 and ZMdEIL3, and one ZMdPG1 gene in the easily soften and dehiscence apple cultivar. ZMdERF1, ZMdEIL2 and ZMdPG1 expressions were associated with fruit softening and dehiscence. ZMdERF1 was more closely related with ethylene signaling but it was not directly regulated the ZMdPG1, and both the ZMdERF1 and ZMdERF2 could interact with ZMdEIL2. ZMdPG1 overexpression in Arabidopsis led to silique early dehiscence. In contrast, suppressing ZMdPG1 expression by antisense ZMdPG1 prevented silique naturally opening. ZMdPG1 related with the connection between cells that contribute to fruit softening and dehiscence.
Materials and Methods
Plant materials
Two apple cultivars ‘Taishanzaoxia’ and ‘Liaofu’ were obtained from fruit Corp. Liaocheng, Shandong, China. To analysis the spatiotemporal expression, apple fruits were picked at different developmental stages. In ‘Taishanzaoxia’, three postharvest treatments with 0.5 µl·L−1, 1.0 µl·L−1 and 1.5 µl·L−1 1-MCP for 24 h and a control treatment (air) were supplied and stored at 25°C. Ethylene production and fruit firmness were recorded. Four replicates (two fruit for each replicate) were performed to exam ethylene production, and 8 replicates (one fruit for each replicate) were used for fruit firmness.
Callus of ‘Taishanzaoxia’ were induced in vitro on Murashige and Skoog (MS) medium containing 0.6 mg·L−1 6-BA and 0.5 mg·L−1 IAA. Arabidopsis were cultivated in light incubators under 16/8-h (day/night, 22°C/21°C) photoperiod. 1/2 MS medium was used for selection of transgenic plants.
Determination of firmness and measurements of ethylene
The firmness of unpeeled apples was measured with the TA.XT plus texture analyzer (Stable Microsystems, Surrey, U.K.) [52]. The ethylene concentration of fruits was tested with a gas chromatograph (Shimadzu, Kyoto, Japan) equipped with a flame ionization detector.
RNA isolation and gene cloning
RNA was extracted from the fruits and postharvest fruits following the introduction of Bioteke kit (Bioteke, Beijing, China) with DNAse treatment (Fermentas, Hanover, ZMD, USA). Three replicates for each sample were sectioned to reduce the material variability. First-strand cDNA was synthesized using oligo(dT)18 primer and Revert Aid TM first strand cDNA synthesis kit (Fermentas, Hanover, ZMD, USA). Genomic DNA was isolated from young leaves using the genomic DNA purification kit (QIAGEN, Shanghai, China). Primers were designed with the Primer5 software according to the homologous nucleotide sequence in other apple cultivars. High efficiency thermal asymmetric interlaced (high-tail) PCR was also performed to clone promote [53]. The details of the primers are described in Table S2. PLACE and Plant CARE were performed to analysis cis-acting elements and binding motif.
Semi-quantitative PCR analysis
For the semi-quantitative RT-PCR, nucleotide primers were designed according to each gene's conversed region with Primer5, and PCR reactions were performed in final volumes of 25 µL following the thermal profile: 5 min at 95 °C, then followed by 28 cycles of 30 s at 95 °C, 30 s at 56°C and 30 s at 72 °C, a final extension 5 min at 72 °C. Three biological replicates for each sample were provided. Malus×domestica actin gene (Mdactin, Genebank accession number CN938023) as the internal control was used to quantify cDNA abundance. Arabidopsis TUB2 gene (TUB2, Genebank accession number XM_002864767.1) as the internal control was used to quantify cDNA abundance. All of the primers used in this study are listed in Table S3.
Transformation of Arabidopsis and cell callus
The expression analysis was performed. ZMdPG1 and anti-ZMdPG1 were recombined into PBI121 vector through XbaI and BamHI sites. So they were fused with Gus tag under the control of CaMV35S promoter. The ZMdPG1 promoter fused with GUS gene was also recombined into pBI121 vector through BamHI and EcoRI sites. Subsequently, they were transformed into Agrobacterium tumefaciens strain LBA4404 and introduced into Arabidopsis (Columbia O) using the floral dipping method [54]. T1 seeds were selected on half-strength MS medium containing kanamycin (100 mg·L−1). After 2 weeks, resistant plants were grown on soil in light incubators under 16/8-h (day/night, 22°C/21°C) photoperiod on matrix. Then T2 seeds were selected as the same method. RT-PCR was also used in T1 line and T2 line of Arabidopsis for further verification. Sense ZMdPG1, antisense ZMdPG1 and ZMdQP were also transformed into cell callus of ‘Taishanzaoxia’ using dipping method. Resistant materials were selected on MS medium containing kanamycin (100 mg·L−1). Primers used for these constructs were shown in Table S4–S5. To investigate expression pattern, GUS histochemical staining assay was performed as described by Sieburth and Meyerowitz [55]. Tissues were fixed, cleared and stained. Then, the stained materials photographed using an Olympus JM dissecting microscope.
Light microscopy
Tissue was fixed in 4% glutaraldehyde in 100 mM sodium phosphate buffer at pH 7.0, vacuum infiltrated for 30 minutes, and incubated at 4°C overnight. Tissue was briefly flushed in 100 mM sodium phosphate buffer, pH 7.0, and dehydrated in an ethanol series. Then tissue was run through JB4 (A+C)/ethanol mix (1∶1) and immersed in JB4 (A+C) for two days, finally embedded in JB4 (A+C)+(B). Individual tissue in resin blocks were sectioned (2 µm) on a Leica Ultracut R microtome. The sections were dried onto glass microscope slides and stained with a 2% (w/v) aqueous Toluidine Blue solution for 30 s and dried on a hotplate for observation by light microscopy.
Subcellular localization
Full-length coding sequences of ZMdERF1,ZMdERF2,ZMdEIL1,ZMdEIL2,ZMdEIL3 and ZMdPG1 were cloned into the P-58 vector with GFP tag through XcmI sites [56]. All constructs were transformed into Agrobacterium tumefaciens strain LBA4404. Primers used for plasmid construction were presented in Table S3. The Agrobacterium tumefaciens strains containing different constructs were incubated in infiltration buffer with 10 mM MES, 0.2 mM acetosyringone, and 10 mM MgCl2 to an ultimate concentration of OD600 = 0.5. Subsequently, Agrobacterium tumefaciens strains were transferred into the same onion epidermis cells. Plants were placed at 24°C for 48 h before detection of GFP fluorescence. The GFP expression in the onion epidermis cells was examined using a Leica confocal microscope (Deerfield, IL, German). Primers used for these constructs were shown in Table S6.
Bimolecular fluorescence complementation assay (BiFC)
Full-length coding sequences of ZMdERF1,ZMdERF2,ZMdEIL1,ZMdEIL2 and ZMdEIL3 were respectively recombined into the binary YFP BiFC vectors [57], so that they were fused with N- or C-terminal fragment of YFP (nYFP or cYFP), and ZMdERF1/ZMdERF2/ZMdEIL1/ZMdEIL2/ZMdEIL3-nYFP and cYFP-ZMdERF1/ZMdERF2/ZMdEIL1/
ZMdEIL2/ZMdEIL3 plasmids were generated. Primers used for plasmid construction are presented in Table S7. All constructs were transferred into Atumefaciens tumefaciens strain LBA4404. After incubation, different combinations were co-infiltrated into the same onion epidermis cells. Onion epidermises were cultured at 24°C for 48 h before detection of YFP fluorescence. The YFP signals were examined in the onion epidermis cells using a Leica confocal microscope (Deerfield, IL, German).
Electrophoretic mobility shift assays (EMSA)
EMSA was performed by the Lightshift Chemiluminescent EMSA kit (Pierce, Rockford, IL, USA). A 1.6-kb fragment of ZMdQP was divided into ten linear DAN fragments (100 bp–200 bp). Then they were labeled using an EMSA Probe Biotin Labeling kit (Pierce). The recombinant His-ZMdERF1 protein was purified with His Trap TM FF crude (GE Healthcare, Sweden). The binding reaction was carried out in final volumes of 20 µL containing 1 pmol of labelled probe, 50 ng of purified protein, 25 mm EPES-KOH (pH 7.5), 100 mm KCI, 0.1 mm ethylene diamine tetraacetie acid (EDTA), 17% glycerol, 1 mm DTT and 4 mg of poly (dI–dC). The reactive solution was incubated at room temperature for 30 min. The mixtures were layered on non-denaturing 6% acrylamide gels to electrophorese in 0.5% TBE buffer for 2 h. Then the DNA was transferred to positively charged nylon membranes in the 0.5% TBE buffer for 2 h (Hybond N+; Amersham, Little Chalfont, Buckinghamshire, UK), and the signal was detected with the chemiluminescent nucleic acid detection method (Pierce). Primers used for plasmid construction are listed in Table S8.
Supporting Information
Homologous assay of ZMdERFs and ERFs in other species. (A) Amino acid sequence alignment between ZMdERFs and ERFs in other species. Identical amino acids are highlighted in dark gray and similar amino acids in pink and green. Arrows represent conserved YRG and RAYD elements. The accession numbers of these proteins in the GenBank database are as follows: ZMdERF1(KC128856), ZMdERF2(KC128857), RpERF1(AEQ58797.1), LeERF2(NP_001234308.1). (B) Phylogenetic relationship of ZMdERFs and other ERFs protein. The accession numbers of these proteins in the GenBank database are as follows: AaERF1(AEQ93554.1), AaERF2(JN162092.1),RcAP2(XP_002511013.1),EjERF1(AFG26326), PsERF1b(ACM49848.1),GhERF8(AFB35653.1),AdERF12(ADJ67441.1),GhEREB2(AAX68525),GhEREB3(AAX68526), LeERF2(NM_001247379.1), MdERF1(BAF43419.1), MdERF2(BAF43420.1),NtERF2(Q40479.1),RcERF2(F968116.1), RpERF1(AEQ58797.), ZMdERF1(KC128856),ZMdERF2(KC128857).
(TIF)
Homologous assay of ZMdILs and EILs in other species. (A) Comparison of the amino acid sequences of ZMdEILs and EILs in other species. Identical amino acids are highlighted in dark gray and similar amino acids in pink and green. Arrows represent BDI, BDII, BDIII, BDIV and BDV domains. AD represent N-terminal acidic region. PR represent proline-rich region. The accession numbers of these proteins in the GenBank database are as follows: ZMdEIL1(KC128858), ZMdEIL2(KC128859), ZMdEIL3 (KC128860), RpERF1(AEQ58797.1), LeERF2 (NP_001234308.1), NtEIL1(AAP03997.1), AdEIL2(ACJ70675.1), LeEIL3 (NP_001234546.1). (B) Phylogenetic relationship of ZMdEILs and other EILs protein. The accession numbers of these proteins in the GenBank database are as follows: AdEIL2(ACJ70675.1),CmEIL2 (BAB64345.1),DcEIL (BAI44821.1),CsEIL1 (ADI40102.1), NtEIL1(AAP03997.1),pEIL2(ABK35086.1),NtEIL5(AAP04001.1),RcEIN3(XP_002530192.1),VvEIN3(XP_002276380.1), EIN3A (XP_002312841.1),EIN3B (XP_002328098.1),EIN3C (XP_002315400.1),EIN3D(XP_002310961.1), LeEIL3(NP_001234721.1),MdEIL1(ADE41153.1),MdEIL2(ADE41154.1),MdEIL3(ADE41155.1),ZMdEIL1(KC128858), ZMdEIL2(KC128859), ZMdEIL3(KC128860).
(TIF)
Homologous assay of ZMdPG1 and PGs in other species. (A) Alignment of the ZMdPG1 protein with other PG proteins. Identical amino acids are highlighted in dark gray and similar amino acids in pink and green. GRO represent Gly-rich octapeptide, GS represent potential glycosylation site. The accession numbers of these proteins in the GenBank database are as follows: ZMdPG1(KC128861), Pgdpg-1(P48978.1), PcPG1 (AB066350.1), PcPG2(AB067641.1), PpPG(x77231), AdPG(AAF71160), ADPG1(NP_191310.1). (B) Phylogenetic relationship of ZMdPG1 and other PG protein. The accession numbers of these proteins in the GenBank database are as follows: ZMdPG1(KC128861), pGDPG-1(P48978.1), PcPG1 (AB066350.1), PcPG2(AB067641.1), PpPG(x77231), AdPG (AAF71160), ADPG1(NP_191310.1), ADPG2(NP_850359.1), QPT2(NP_187454.2), LePG2(NP_001234021.1), LePG1 (225933), CpPG(FJ007644), NtPG1(CAA50335).
(TIF)
Alignment of ZMdQP and rMdPQ. ZMdQP is the promoter of ZMdPG1 from ‘Taishanzaoxia’. rMdPQ is the promoter of MdPG1 from ‘Royal Gala’. The accession numbers of these proteins in the GenBank database are as follows: ZMdQP (KC128862), rMdPQ (AF031233.1).
(TIF)
Comparison of ZMdPG1, ZMdERFs and ZMdEILs genes with the apple genome.
(TIF)
Primers used in PCR amplification.
(TIF)
Primers used for Semi-quantitative PCR.
(TIF)
Primers used for transformation of Arabidopsis.
(TIF)
Primers used for GUS Staining.
(TIF)
Primers used for Subcellular Localization.
(TIF)
Primers used for BiFC assay.
(TIF)
Primers used for EMSA assay.
(TIF)
Acknowledgments
We are grateful to Xiansheng Zhang and Yujin Hao for generously sharing the biological resource. We thank Shujing Wu, Xiaoliu Chen and Xiaojiao Han for critical reading of the manuscript.We appreciate Baoxing Ye, Xiankui Guo, Yang Song, Mei Cui, Jingli Wei, Chuanzeng Wang, LiXia Wang and Yingchao Wei for various assistance during the lab setup.
Funding Statement
This work was supported by grants from the National Key Basic Research Program of China (2011CB100606 and the National Natural Science Foundation of China (31171932 and 31272132). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schupp JR, Greene DW (2004) Effect of aminoethoxyvinylglycine (AVG) on preharvest drop, fruit quality, and maturation of 'McIntosh' Apples. I. concentration and timing of dilute applications of AVG. HortScience 39(5): 1030–1035. [Google Scholar]
- 2. Liu CC, Wei JL, Xu YT, Jiao QQ, Sun HB, et al. (2011) Preliminary study on firmness and related physiological indices of three early-ripening apple cultivar during late development of the fruit. Acta Horticulturae Sinica 38(1): 133–138. [Google Scholar]
- 3. Yuan R, Carbaugh DH (2007) Effects of NAA, AVG, and 1-MCP on ethylene biosynthesis, preharvest fruit drop, fruit maturity, and quality of ‘Golden Supreme’ and ‘Golden Delicious’ apples. HortScience 42(1): 101–105. [Google Scholar]
- 4. Wang A, Tan D, Tatsuki M, Kasai A, Li T, et al. (2009) Molecular mechanism of distinct ripening profiles in ‘Fuji’ apple fruit and its early maturing sports. Postharvest Biol Tec 52(1): 38–43. [Google Scholar]
- 5. Wakasa Y, Kudo H, Ishikawa R, Akada S, Senda M, et al. (2006) Low expression of an endopolygalacturonase gene in apple fruit with long-term storage potential. Postharvest Biol Tec 39(2): 193–198. [Google Scholar]
- 6. Saftner RA, Abbott JA, Conway WS, Barden CL (2003) Effects of 1-methylcyclopropene and heat treatments on ripening and postharvest decay in 'Golden Delicious' apples. J Am Soc Hortic Sci 128(1): 120–127. [Google Scholar]
- 7. Mir NA, Curell E, Khan N, Whitaker M, Beaudry RM (2001) Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of 'Redchief Delicious' apples. J Am Soc Hortic Sci 126(5): 618–624. [Google Scholar]
- 8. Liu MY, Wei JL, Liu J, Fang L, Song Y, et al. (2012) The regulation of 1-methylcyclopropene on softening and expression of relevant genes in‘Taishan Zaoxia'apple. Acta Horticulturae Sinica 39(005): 845–852. [Google Scholar]
- 9. Toivonen P, Changwen L (2005) Studies on elevated temperature, short-term storage of 'Sunrise'Summer apples using 1-MCP to maintain quality. J Hortic Sci Biotechnol 80(4): 439–446. [Google Scholar]
- 10. Patterson SE, Bleecker AB (2004) Ethylene-dependent and -independent processes associated with floral organ abscission in Arabidopsis. Plant Physiol 134(1): 194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kendrick MD, Chang C (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11(5): 479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, et al. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89(7): 1133–1144. [DOI] [PubMed] [Google Scholar]
- 13. Tieman DM, Ciardi JA, Taylor MG, Klee HJ (2001) Members of the tomato LeEIL (EIN3-like) gene family are functionally redundant and regulate ethylene responses throughout plant development. Plant J 26(1): 47–58. [DOI] [PubMed] [Google Scholar]
- 14. Yokotani N, Tamura S, Nakano R, Inaba A, Kubo Y (2003) Characterization of a novel tomato EIN3-like gene (LeEIL4). J Exp Bot 54(393): 2775–2776. [DOI] [PubMed] [Google Scholar]
- 15. Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Gene Dev 12(23): 3703–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang S, Sawaki T, Takahashi A, Mizuno S, Takezawa K, et al. (2010) Melon EIN3-like transcription factors (CmEIL1 and CmEIL2) are positive regulators of an ethylene- and ripening-induced 1-aminocyclopropane-1-carboxylic acid oxidase gene (CM-ACO1). Plant Sci 178(3): 251–257. [Google Scholar]
- 17. Yin XR, Zhang B, Li X, Chen KS (2009) Ethylene signal transduction during fruit ripening and senescence. Acta Horticulturae Sinica 36(1): 133–140. [Google Scholar]
- 18. Yin XR, Chen KS, Allan AC, Wu RM, Zhang B, et al. (2008) Ethylene-induced modulation of genes associated with the ethylene signalling pathway in ripening kiwifruit. J Exp Bot 59(8): 2097–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tacken E, Ireland H, Gunaseelan K, Karunairetnam S, Wang D, et al. (2010) The role of ethylene and cold temperature in the regulation of the apple POLYGALACTURONASE1 gene and fruit softening. Plant Physiol 153(1): 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rieu I, Mariani C, Weterings K (2003) Expression analysis of five tobacco EIN3 family members in relation to tissue-specific ethylene responses. J Exp Bot 54(391): 2239–2244. [DOI] [PubMed] [Google Scholar]
- 21. Yin XR, Allan AC, Chen KS, Ferguson IB (2010) Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol 153(3): 1280–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140(2): 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Okamuro JK, Caster B, Villarroel R, Van Montagu M, Jofuku KD (1997) The AP2 domain of APETALA2 defines a large new family of DNA binding proteins in Arabidopsis . Proc Natl Acad Sci 94(13): 7076–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ohme-Takagi M, Shinshi H (1995) Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell 7(2): 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li YC, Zhu BZ, Xu WT, Zhu HL, Chen AJ, et al. (2007) LeERF1 positively modulated ethylene triple response on etiolated seedling, plant development and fruit ripening and softening in tomato. Plant Cell Rep 26(11): 1999–2008. [DOI] [PubMed] [Google Scholar]
- 26. Pirrello J, Jaimes-Miranda F, Sanchez-Ballesta MT, Tournier B, Khalil-Ahmad Q, et al. (2006) Sl-ERF2, a tomato ethylene response factor involved in ethylene response and seed germination. Plant Cell Physiol 47(9): 1195–1205. [DOI] [PubMed] [Google Scholar]
- 27. Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F, et al. (2003) New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. Febs Lett 550(1-3): 149–154. [DOI] [PubMed] [Google Scholar]
- 28. Wang A, Tan D, Takahashi A, Li TZ, Harada T (2007) MdERFs, two ethylene-response factors involved in apple fruit ripening. J Exp Bot 58(13): 3743–3748. [DOI] [PubMed] [Google Scholar]
- 29. Cara B, Giovannoni JJ (2008) Molecular biology of ethylene during tomato fruit development and maturation. Plant Sci 175(1-2): 106–113. [Google Scholar]
- 30. Wei J, Ma F, Shi S, Qi X, Zhu X, et al. (2010) Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol Tec 56(2): 147–154. [Google Scholar]
- 31. DellaPenna D, Alexander DC, Bennett AB (1986) Molecular cloning of tomato fruit polygalacturonase: analysis of polygalacturonase mRNA levels during ripening. Proc Natl Acad Sci 83(17): 6420–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Speirs J, Lee E, Brady CJ, Robertson J, McGlasson WB (1990) Endopolygalacturonase: messenger RNA, enzyme and softening in the ripening fruit of a range of tomato genotypes. J Plant Physiol 135(5): 576–582. [Google Scholar]
- 33. Dellapenna D, Lincoln JE, Fischer RL, Bennett AB (1989) Transcriptional analysis of polygalacturonase and other ripening associated genes in rutgers, rin, nor, and nr tomato fruit. Plant Physiol 90(4): 1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47(1): 311–339. [PubMed] [Google Scholar]
- 35. Tieman DM, Handa AK (1994) Reduction in pectin methylesterase activity modifies tissue integrity and cation levels in ripening tomato (Lycopersicon esculentum Mill.) fruits. Plant Physiol 106(2): 429–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Atkinson R (1994) A cDNA clone for endopolygalacturonase from apple. Plant Physiol 105(4): 1437–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hiwasa K, Nakano R, Hashimoto A, Matsuzaki M, Murayama H, et al. (2004) European, Chinese and Japanese pear fruits exhibit differential softening characteristics during ripening. J Exp Bot 55(406): 2281–2290. [DOI] [PubMed] [Google Scholar]
- 38. Peace C, Crisosto C, Gradziel T (2005) Endopolygalacturonase: a candidate gene for freestone and melting flesh in peach. Mol Breeding 16(1): 21–31. [Google Scholar]
- 39. Wang ZY, MacRae EA, Wright MA, Bolitho KM, Ross GS, et al. (2000) Polygalacturonase gene expression in kiwifruit: relationship to fruit softening and ethylene production. Plant Mol Biol 42(2): 317–328. [DOI] [PubMed] [Google Scholar]
- 40. Atkinson R, Schaffer R, Gunaseelan K, Schroder R, inventors S (2008) Methods and compositions for increasing storage-life of fruit. New Zealand Patent No.NZ570886. [Google Scholar]
- 41. Atkinson RG, Schroder R, Hallett IC, Cohen D, MacRae EA (2002) Overexpression of polygalacturonase in transgenic apple trees leads to a range of novel phenotypes involving changes in cell adhesion. Plant Physiol 129: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogawa M, Kay P, Wilson S, Swain SM (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis . Plant Cell 21(1): 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bapat VA, Trivedi PK, Ghosh A, Sane VA, Ganapathi TR, et al. (2010) Ripening of fleshy fruit: Molecular insight and the role of ethylene. Biotechnol Adv 28(1): 94–107. [DOI] [PubMed] [Google Scholar]
- 44. Roeder AHK, Yanofsky MF (2006) Fruit development in Arabidopsis . The Arabidopsis Book/American Society of Plant Biologists 4: e0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weinthal D, Tzfira T (2009) Imaging protein-protein interactions in plant cells by bimolecular fluorescence complementation assay. Trends Plant Sci 14(2): 59–63. [DOI] [PubMed] [Google Scholar]
- 46. Sawada K, Nishibori M, Nakaya N, Wang Z, Saeki K (2002) Purification and characterization of a trypsin-like serine proteinase from rat brain slices that degrades laminin and type IV collagen and stimulates protease-activated receptor-2. J Neurochem 74(4): 1731–8. [DOI] [PubMed] [Google Scholar]
- 47. Allen MD, Yamasaki K, Ohme-Takagi M, Tateno M, Suzuki M (1998) A novel mode of DNA recognition by a β-sheet revealed by the solution structure of the GCC-box binding domain in complex with DNA. EMBO J 17(18): 5484–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mitchell PJ, Tjian R (1989) Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science 245(4916): 371–378. [DOI] [PubMed] [Google Scholar]
- 49. Chakravarthy S, Tuori RP, D'Ascenzo MD, Fobert PR, Després C, et al. (2003) The tomato transcription factor Pti4 regulates defense-related gene expression via GCC box and non-GCC box cis elements. Plant Cell 15(12): 3033–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Büttner M, Singh KB (1997) Arabidopsis thaliana ethylene-responsive element binding protein (AtEBP), an ethylene-inducible, GCC box DNA-binding protein interacts with an ocs element binding protein. Proc Natl Acad Sci 94(11): 5961–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang CZ, Lu F, Imsabai W, Meir S, Reid MS (2008) Silencing polygalacturonase expression inhibits tomato petiole abscission. J Exp Bot 59(4): 973–979. [DOI] [PubMed] [Google Scholar]
- 52. Camps C, Guillermin P, Mauget J, Bertrand D (2005) Data analysis of penetrometric force/displacement curves for the characterization of whole apple fruits. J Texture Stud 36(4): 387–401. [Google Scholar]
- 53. Liu YG, Chen YL (2007) High-effciency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques 43(5): 649–656. [DOI] [PubMed] [Google Scholar]
- 54. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . Plant J 16(6): 735–743. [DOI] [PubMed] [Google Scholar]
- 55. Sieburth LE, Meyerowitz EM (1997) Molecular dissection of the AGAMOUS control region shows that cis elements for spatial regulation are located intragenically. Plant Cell 9(3): 355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen S, Songkumarn P, Liu J, Wang GL (2009) A versatile zero background T-vector system for gene cloning and functional genomics. Plant Physiol 150(3): 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Song S, Qi T, Huang H, Ren Q, Wu D, et al. (2011) The Jasmonate-ZIM domain proteins interact with the R2R3-MYB transcription factors MYB21 and MYB24 to affect jasmonate-regulated stamen development in Arabidopsis . Plant Cell 23(3): 1000–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Homologous assay of ZMdERFs and ERFs in other species. (A) Amino acid sequence alignment between ZMdERFs and ERFs in other species. Identical amino acids are highlighted in dark gray and similar amino acids in pink and green. Arrows represent conserved YRG and RAYD elements. The accession numbers of these proteins in the GenBank database are as follows: ZMdERF1(KC128856), ZMdERF2(KC128857), RpERF1(AEQ58797.1), LeERF2(NP_001234308.1). (B) Phylogenetic relationship of ZMdERFs and other ERFs protein. The accession numbers of these proteins in the GenBank database are as follows: AaERF1(AEQ93554.1), AaERF2(JN162092.1),RcAP2(XP_002511013.1),EjERF1(AFG26326), PsERF1b(ACM49848.1),GhERF8(AFB35653.1),AdERF12(ADJ67441.1),GhEREB2(AAX68525),GhEREB3(AAX68526), LeERF2(NM_001247379.1), MdERF1(BAF43419.1), MdERF2(BAF43420.1),NtERF2(Q40479.1),RcERF2(F968116.1), RpERF1(AEQ58797.), ZMdERF1(KC128856),ZMdERF2(KC128857).
(TIF)
Homologous assay of ZMdILs and EILs in other species. (A) Comparison of the amino acid sequences of ZMdEILs and EILs in other species. Identical amino acids are highlighted in dark gray and similar amino acids in pink and green. Arrows represent BDI, BDII, BDIII, BDIV and BDV domains. AD represent N-terminal acidic region. PR represent proline-rich region. The accession numbers of these proteins in the GenBank database are as follows: ZMdEIL1(KC128858), ZMdEIL2(KC128859), ZMdEIL3 (KC128860), RpERF1(AEQ58797.1), LeERF2 (NP_001234308.1), NtEIL1(AAP03997.1), AdEIL2(ACJ70675.1), LeEIL3 (NP_001234546.1). (B) Phylogenetic relationship of ZMdEILs and other EILs protein. The accession numbers of these proteins in the GenBank database are as follows: AdEIL2(ACJ70675.1),CmEIL2 (BAB64345.1),DcEIL (BAI44821.1),CsEIL1 (ADI40102.1), NtEIL1(AAP03997.1),pEIL2(ABK35086.1),NtEIL5(AAP04001.1),RcEIN3(XP_002530192.1),VvEIN3(XP_002276380.1), EIN3A (XP_002312841.1),EIN3B (XP_002328098.1),EIN3C (XP_002315400.1),EIN3D(XP_002310961.1), LeEIL3(NP_001234721.1),MdEIL1(ADE41153.1),MdEIL2(ADE41154.1),MdEIL3(ADE41155.1),ZMdEIL1(KC128858), ZMdEIL2(KC128859), ZMdEIL3(KC128860).
(TIF)
Homologous assay of ZMdPG1 and PGs in other species. (A) Alignment of the ZMdPG1 protein with other PG proteins. Identical amino acids are highlighted in dark gray and similar amino acids in pink and green. GRO represent Gly-rich octapeptide, GS represent potential glycosylation site. The accession numbers of these proteins in the GenBank database are as follows: ZMdPG1(KC128861), Pgdpg-1(P48978.1), PcPG1 (AB066350.1), PcPG2(AB067641.1), PpPG(x77231), AdPG(AAF71160), ADPG1(NP_191310.1). (B) Phylogenetic relationship of ZMdPG1 and other PG protein. The accession numbers of these proteins in the GenBank database are as follows: ZMdPG1(KC128861), pGDPG-1(P48978.1), PcPG1 (AB066350.1), PcPG2(AB067641.1), PpPG(x77231), AdPG (AAF71160), ADPG1(NP_191310.1), ADPG2(NP_850359.1), QPT2(NP_187454.2), LePG2(NP_001234021.1), LePG1 (225933), CpPG(FJ007644), NtPG1(CAA50335).
(TIF)
Alignment of ZMdQP and rMdPQ. ZMdQP is the promoter of ZMdPG1 from ‘Taishanzaoxia’. rMdPQ is the promoter of MdPG1 from ‘Royal Gala’. The accession numbers of these proteins in the GenBank database are as follows: ZMdQP (KC128862), rMdPQ (AF031233.1).
(TIF)
Comparison of ZMdPG1, ZMdERFs and ZMdEILs genes with the apple genome.
(TIF)
Primers used in PCR amplification.
(TIF)
Primers used for Semi-quantitative PCR.
(TIF)
Primers used for transformation of Arabidopsis.
(TIF)
Primers used for GUS Staining.
(TIF)
Primers used for Subcellular Localization.
(TIF)
Primers used for BiFC assay.
(TIF)
Primers used for EMSA assay.
(TIF)