Abstract
Background
Cancer patients who continue smoking are at increased risk for adverse outcomes including reduced treatment efficacy and poorer survival rates. Many patients spontaneously quit smoking after diagnosis; however, relapse is understudied. The goal of this study was to evaluate smoking-related, affective, cognitive, and physical variables as predictors of smoking after surgical treatment among lung and head/neck cancer patients.
Methods
A longitudinal study was conducted with 154 patients (57% male) who recently quit smoking. Predictor variables were measured at baseline (i.e., time of surgery); smoking behavior was assessed at 2, 4, 6, and 12 months post-surgery. Analyses of 7-day point prevalence were performed using a Generalized Estimating Equations (GEE) approach.
Results
Relapse rates varied significantly depending on pre-surgery smoking status. At 12-months post-surgery, 60% of patients who smoked during the week prior to surgery had resumed smoking, versus only 13% who were abstinent prior to surgery. Smoking rates among both groups were relatively stable across the 4 follow-ups. For patients smoking pre-surgery (N = 101), predictors of smoking relapse included lower quitting self-efficacy, higher depression proneness, and greater fears about cancer recurrence. For patients abstinent pre-surgery (N = 53), higher perceived difficulty quitting and lower cancer-related risk perceptions predicted smoking relapse.
Conclusion
Efforts to encourage early cessation at diagnosis, and increased smoking relapse-prevention efforts in the acute period following surgery, may promote long-term abstinence. Several modifiable variables are identified to target in future smoking relapse-prevention interventions for cancer patients.
Keywords: tobacco use, smoking relapse, head and neck cancer, lung cancer
Introduction
Cigarette smoking is responsible for 30% of all cancer-related mortalities.1 Lung and head/neck cancers are among the malignancies most strongly linked to tobacco use1 and a significant proportion of lung and head/neck cancer patients are current smokers at the time of diagnosis2, 3. Smoking cessation reduces morbidity and mortality in these patients4, 5, whereas continued smoking after diagnosis increases patients’ risk for other smoking related illnesses, (e.g. coronary heart disease), second primary tumors6, 7, and disease recurrence5, 6. Continued smoking also has more immediate adverse impact, including reduced cancer treatment efficacy8,9, higher rates of treatment complications and side effects10-13, greater treatment-related weight loss14, and poorer quality of life15-17.
The majority of patients smoking at the time of their diagnosis spontaneously quit smoking (e.g., 86% among lung cancer patients18, 84% among head/neck cancer patients19), with the greatest proportion of quit attempts occurring at diagnosis20. Furthermore, smoking cessation interventions for cancer patients have produced high short-term cessation rates2, 21-24. Among studies that have reported smoking relapse rates in cancer patients, estimates range from 13% to 60%18, 25-27. The majority of relapses occur after one month of abstinence; those who achieve 6 months of abstinence are unlikely to ever resume smoking15, 27.
Only a few studies have examined factors associated with smoking relapse among lung and head/neck cancer patients. Gritz et al.15 showed that head/neck cancer patients who relapsed versus those who remained abstinent were more likely to have less confidence in their ability to quit, greater withdrawal and addiction, and employed a gradual reduction method to quitting smoking. Negative affect was reported as the most common relapse precipitant. Other reported triggers included fatigue, thoughts about cancer, and craving. A prospective study conducted with lung and head/neck cancer patients28 found that relapsers had lower levels of self-efficacy as compared to those who quit at the follow-up or were abstinent. Finally, Walker et al.29 examined predictors of smoking relapse among lung cancer patients who smoked within 3 months before surgery. Patients who had quit for a shorter period before surgery and who held stronger expectations of the pleasure associated with smoking (i.e., appetitive cravings) were more likely to resume smoking.
The current study extends research on smoking relapse in lung and head and neck cancer patients in several ways. The majority of existing studies reported rates of relapse in the context of a smoking cessation treatment study, and thus it is unknown whether the findings generalize to patients who self-quit. Furthermore, prior prospective research is limited by small sample sizes, single follow-up assessments, and limited sets of predictors that were not modifiable and/or not unique to the cancer patient population (e.g., demographics, cravings). The aims of this prospective study were to (1) examine smoking trajectories among lung and head/neck cancer patients for 12 months following surgical treatment and (2) test potential predictors of relapse based on Witkiewitz and Marlatts’ model of relapse30 and our previous qualitative work with this population31. Selected predictors covered four primary domains (smoking-related, affective, cognitive, and physical), with an emphasis on potentially modifiable variables specifically relevant to cancer patients that could inform a future intervention for this population. They were assessed at the time of surgery (i.e., baseline), with follow-up assessments of smoking status that occurred at 2, 4, 6, and 12 months after surgery.
Method
Participants
Patients were recruited from the thoracic (TH) and head and neck (HN) clinics at Moffitt Cancer Center from March 1, 2008 to December 31, 2009. Eligible patients 1) were at least 18 years of age, 2) had a history of smoking at least 10 cigarettes per day for at least one year prior to diagnosis, and 3) were scheduled to undergo surgical cancer treatment. Additionally, all patients either 1) had recently quit smoking (≤ 6 months prior, confirmed biochemically via carbon monoxide breath sample), or 2) were smoking in the week prior to surgery but intended to quit immediately following surgery. All patients in the present study were abstinent ≥ 24 hours following surgery. All patients at the cancer center have access to a certified tobacco cessation specialist; however, no intervention was provided as part of the current study.
Procedure
Screening
Recruitment and study procedures have been described previously32. Patients scheduled for surgery were pre-screened for eligibility using the electronic clinic schedule and medical record. Preliminarily eligible patients were identified for an in-person screening by trained research assistants. Of eligible patients, 80% agreed to participate. In the HN clinic, patients were approached in the clinic waiting room on the day of their pre-operative appointment and taken to a private exam room to complete the screening. Due to differences in how the two clinics operated, it was not possible to screen TH patients at a pre-operative appointment. Rather, TH patients were approached for screening in their hospital rooms after their surgery at any time before discharge, generally within two to three days.
Enrollment and Baseline Assessment
Immediately after screening, participants provided informed consent and a breath sample (carbon monoxide, CO) to confirm their self-reported smoking status. They were then given the baseline assessment packet to complete (see measures below). HN patients completed this assessment in the clinic waiting room or an exam room. TH patients completed it during their hospital stay. Patients were compensated $25 upon completion of this assessment.
Follow-up Assessments
Research assistants conducted follow-up assessments with patients via telephone (or mail if they were unable to speak on the phone) at 2, 4, 6, and 12 months post-surgery. Patients were compensated $25 for completing each follow-up.
Measures
Assessment of smoking status
Smoking status at pre-surgery (i.e., baseline) and the follow-up time points (2, 4, 6, and 12 months after the date of surgery) was classified using 7-day point prevalence criteria (i.e., patients who reported not smoking in the 7 days prior to surgery were recorded as abstinent). At follow-ups, we collected biochemical confirmation of smoking abstinence, via breath CO, from a subsample of 44 participants who reported abstinence and were available to be seen at a hospital visit. Other patients were unavailable for biochemical verification due to logistics (e.g., patient lived far away and was not scheduled to visit the cancer center during a follow-up window) rather than refusal. Only one participant exhibited CO levels greater than 10 parts per million while self-reporting abstinence at the 6-month follow-up, and was therefore recoded as smoking.
Demographics, Smoking, and Alcohol Use
At baseline, participants provided demographics (age, gender, education, marital status, race, ethnicity, and household income), smoking history (age at smoking initiation, years smoked, previous quit attempts, and smoking cessation treatment history), and alcohol use information (frequency of drinking in the past 12 months and history of excess drinking or treatment for alcohol-related problems).
Smoking-Related Predictors
Nicotine dependence was assessed using the Fagerström Test for Nicotine Dependence (FTND)33, with items reworded to reflect pre-quit level of dependence for those patients who had already quit at baseline. Motivation to quit and self-efficacy were assessed using a previously validated three-item measure (Thoughts about Abstinence)34 in which desire for abstinence (TAS-Desire), expectation of success at abstinence (TAS-Quitting Self-Efficacy), and expected difficulty with abstinence (TAS-Perceived Difficulty Quitting) were each rated on a 10-point scale.
Affective Predictors
Participants reported if they had any history of treatment for depression, and rated how easily they become depressed (depression proneness) on a 7-point Likert-type scale. Depressive symptoms during the past week were assessed with the Center for Epidemiologic Studies Depression Scale (CES-D)35. Fear of cancer recurrence was assessed with four-items from the Fear of Relapse/Recurrence scale assessing beliefs and anxieties about possible cancer recurrence on a 5-point Likert scale36.
Cognitive Predictors
Perceptions of risk of resuming tobacco use (POR) were assessed with a modified version of the risk perception 6-item tool developed by Schnoll et al.28. We added an additional item to assess beliefs about how smoking affects current cancer treatment. The Impact of Event scale (IES)37, a 15-item instrument, was used to assess subjective distress about cancer-related concerns in the previous week. The control over the cause of their cancer and course of cancer subscales were used from the Cancer Locus of Control scale (CLOC)38.
Physical Predictors
Pain was assessed with the 15-item Brief Pain Inventory Short Form (BPI-SF)39, Fatigue was assessed with the 9-item Brief Fatigue Inventory (BFI)40.
Data analysis
Prior to conducting the primary analyses, descriptive statistics (means or frequencies) were computed to characterize the study sample. Chi-square (χ2) and analysis of variance (ANOVA) were used to examine differences in demographics and smoking characteristics by cancer type. Preliminary Generalized Estimating Equations (GEE) regression analyses were used to assess whether smoking status differed over the 4 follow-ups (2, 4, 6, and 12-months) and was predicted by pre-surgery smoking status.
The primary analyses used GEE to assess the smoking, affective, physical, and cognitive variables as predictors of post-surgery smoking for all patients. Each variable was tested individually. Variables with P values < .10 were entered into a backward stepwise regression analysis.
RESULTS
Participants
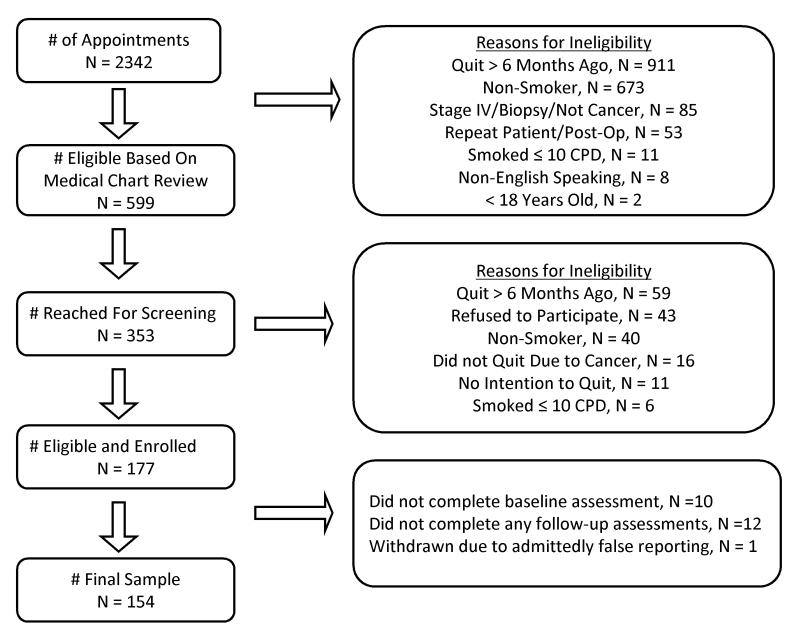
Of 177 patients who were eligible and enrolled, 154 completed the baseline and at least one of the follow-up assessments (see Figure 1). Completion rates for the 2, 4, 6, and 12-month follow-ups were 98%, 95%, 91%, and 86% respectively. Participant characteristics are reported in Table 1. As compared to the participants in the Thoracic Clinic, Head and Neck patients were more likely to be male, to be younger, and to have a less extensive smoking history.
FIGURE 1.
Participant Enrollment Flow Diagram
TABLE 1.
Demographic and smoking characteristics
| Demographic Variables |
All (N=154) |
Head/Neck (N=82) |
Thoracic (N=72) |
|---|---|---|---|
| Sex: Male *** | 57.1% | 69.5% | 43.1% |
| Age - M (SD) *** | 58.3 (11.0) | 55.1 (9.3) | 62.0 (11.6) |
| Race: | |||
| White/Caucasian | 96.1% | 96.3% | 95.8% |
| Black/African American | 2.0% | 2.4% | 1.4% |
| Other | 2.0% | 0% | 2.8% |
| Hispanic | 1.3% | 2.4% | 0% |
| Marital Status: | |||
| Single | 14.3% | 15.9% | 12.5% |
| Married | 54.5% | 50.0% | 59.7% |
| Divorced | 21.4% | 25.6% | 16.7% |
| Widowed | 9.7% | 8.5% | 11.1% |
| Education: Less than 12th grade | 19.5% | 23.2% | 15.3% |
| Household Income: Median category | $30K-$40K | $30K-$40K | $30K-$40K |
| Household Income: Modal category | < $20K | < $20K | < $20K |
| Smoking/Alcohol Variables | All | Head/Neck | Thoracic |
| Years smoking - M (SD) *** | 39.0 (12.4) | 35.0 (11.2) | 43.5 (12.4) |
| CPD average - M (SD) | 24.1 (11.8) | 24.3 (13.1) | 23.8 (10.3) |
| CPD maximum - M (SD) | 34.7 (15.3) | 36.2 (15.4) | 32.9 (15.1) |
| Fagerstrom Dependence - M (SD) | 5.8 (2.3) | 5.7 (2.4) | 5.9 (2.1) |
| Smoked the week before surgery*** | 65.6% | 79.3% | 50.0% |
| Spouse smokes (married, N=84) | 33.3% | 39.0% | 27.9% |
| Received Prior Tobacco Treatment | 59.7% | 56.1% | 63.9% |
| Another smoker in household | 42.2% | 48.8% | 34.7% |
| Alcohol Use: < 2 drinks/week | 54.9% | 51.9% | 58.3% |
| Alcohol abuse | 27.0% | 29.6% | 23.9% |
Note: For comparisons of Thoracic versus Head/Neck patients,
denotes P ≤.001.
denotes P ≤.01.
denotes P ≤.05.
Smoking Status
Based on 7-day point prevalence estimates, 101 patients (66%) smoked during the week prior to surgery. As shown in Table 1, a significantly higher percentage of head and neck cancer patients were smoking pre-surgery (P < .001). Pre-surgery smokers were also less likely to be married (47% versus 68%; P = .016) and more likely to have another smoker in the household (51% versus 26%; P = .004).
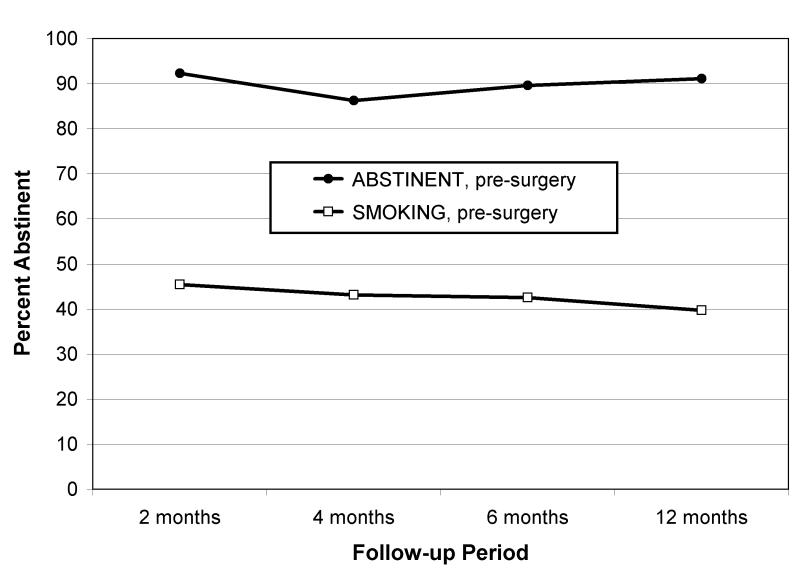
Figure 2 displays smoking rates for responders at 2, 4, 6, and 12 months post-surgery. Smoking rates exhibited high stability across the 4 follow-ups with phi coefficients greater than .76 (P’s < .0001). The preliminary GEE analysis of smoking at the 4 follow-ups revealed a small, but significant increase in smoking over the 12 months (OR = 1.03, 95% CI = 1.00, 1.06; P = .044). Analyses of demographic and smoking characteristics revealed and a significant effect of smoking status prior to surgery (OR = 11.4, 95% CI = 4.99, 26.0; P < .0001). Those who were abstinent in the week prior to surgery, compared to those who smoked, had significantly lower smoking relapse rates at all follow-ups (8-13% versus 53-60%; P’s < .0001). Analyses of demographic, smoking, alcohol, and medical characteristics (cancer stage*, type, and mortality) revealed that only prior alcohol treatment was related to smoking relapse above and beyond pre-surgery smoking status (P <.001).
FIGURE 2.
Relapse Rates over time by Patients’ Pre-Surgery Smoking Status
Predictors: Smoking-Related, Affective, Physical, and Cognitive
Table 2 presents descriptive statistics for target predictors for all patients and by smoking status in the week prior to surgery. The different trajectories based on pre-surgery smoking status prompted analysis of target predictors separately for these sub-groups. Not surprisingly, patients abstinent pre-surgery reported a greater desire to (stay) quit (P < .001), higher quitting self-efficacy, (P < .001), and less perceived difficulty quitting (P < .001). This group also reported stronger beliefs about having control over their cancer (P = .03) and higher perceptions of risks associated with continued smoking (P = .03). Below we present three sets of analyses conducted to predict relapse in: 1) all patients, 2) patients who were abstinent (≥ 7 days) pre-surgery, and 3) patients who were smoking (i.e. within 7 days) pre-surgery.
TABLE 2.
Descriptive statistics for predictors.
| Smoking-Related |
All
(N=154) |
Abstinent
(N=53) |
Smoking
(N=101) |
|---|---|---|---|
| Nicotine dependence - M (SD) | 5.8 (2.3) | 6.1 (2.2) | 5.6 (2.3) |
| TAS-1: Desire -M (SD)*** | 8.0 (1.7) | 8.7 (0.9) | 7.6 (1.8) |
| TAS-2: Quitting Self-efficacy - M (SD) *** | 7.6 (2.2) | 8.8 (0.8) | 7.0 (2.4) |
| TAS-3: Perceived Difficulty - M (SD) *** | 7.0 (2.9) | 5.8 (3.4) | 7.7 (2.3) |
| Affective | All | Abstinent | Smoking |
| Depression proneness - M (SD) | 3.3 (2.0) | 3.3 (2.1) | 3.2 (2.0) |
| CES-D -M (SD) | 18.5 (11.1) | 18.8 (10.4) | 18.3 (11.5) |
| Depression treatment | 30.5% | 32.1% | 29.7% |
| Fear of recurrence - M (SD) | 10.2 (4.0) | 9.9 (4.1) | 10.3 (3.9) |
| Physical | All | Abstinent | Smoking |
| Fatigue - M (SD) | 40.8 (25.1) | 42.6 (24.0) | 39.9 (25.7) |
| Pain-severity - M (SD) | 15.4 (9.8) | 14.5 (8.7) | 16.0 (10.4) |
| Pain-interference - M (SD) | 29.1 (20.4) | 30.3 (20.9) | 28.4 (20.1) |
| Cognitive | All | Abstinent | Smoking |
| Control over Cancer Cause - M (SD)* | 13.5 (4.1) | 14.7 (3.7) | 12.9 (4.1) |
| Control over Cancer Course - M (SD) | 24.1 (4.2) | 24.9 (3.6) | 23.7 (4.5) |
| Impact of event-Avoidance - M (SD) | 9.8 (5.9) | 10.3 (6.5) | 9.5 (5.5) |
| Impact of event-Intrusive - M (SD) | 8.7 (5.5) | 9.3 (5.5) | 8.4 (5.5) |
| Perceived risk - M (SD)* | 22.0 (4.8) | 23.3 (5.3) | 21.4 (4.4) |
Notes: For comparisons of Patients Abstinent versus Smoking pre-surgery,
denotes P ≤.001.
denotes P ≤.01.
denotes P ≤.05.
All Patients
Target predictors were examined individually using GEE in a model that included follow-up (Month) and pre-surgery smoking status. Smoking relapse was predicted by two smoking-related variables, a lower desire to quit (P = .017) and lower quitting self-efficacy (P < .001). With regards to the affective variables, higher ratings of depression proneness (P=.009), prior treatment for depression (P=.011), higher current depressive symptoms (P=.025), and greater reported fears about cancer recurrence (P=.001) were also significant predictors of resuming smoking. One physical variable, higher pain severity (P=.089), was a marginally significant predictor.
Table 3 displays the results of backward stepwise regression for models with Month and Pre-surgery Smoking as the base model. As can be seen in the top section of table, 2 of the 7 predictors initially entered into the regression remained significant. Participants were more likely to resume smoking post-surgery if they had lower quitting self-efficacy and higher ratings of depression proneness.
TABLE 3.
Final GEE Models Predicting Smoking Post-surgery following Backward Stepwise Regression
| All Patients (N=154) | ||
|---|---|---|
|
| ||
| Variable | P | Odds Ratio (95% CI) |
| Month (2, 4, 6, & 12) | .047 | 1.04 (1.00, 1.07) |
| Smoking Pre-surgery | <.0001 | 8.44 (3.38, 21.1) |
|
| ||
| Quitting Self-Efficacy | <.001 | 0.75 (0.64, 0.87) |
| Depression Proneness | .001 | 1.31 (1.12, 1.54) |
|
| ||
| Patients Abstinent Pre-surgery (N=53) | ||
|
| ||
| Variable | P | Odds Ratio (95% CI) |
| Month (2, 4, 6, & 12) | .654 | 1.02 (0.94, 1.11) |
| Perceived Difficulty Quitting | .012 | 1.23 (1.05, 1.44) |
| Perceived Risk | .002 | 0.86 (0.78, 0.94) |
|
| ||
| Patients Smoking Pre-surgery (N=101) | ||
|
| ||
| Variable | P | Odds Ratio (95% CI) |
| Month (2, 4, 6, 12) | .055 | 1.04 (1.00, 1.08) |
| Quitting Self-Efficacy | .029 | 0.83 (0.69, 0.98) |
| Depression Proneness | .037 | 1.25 (1.01, 1.53) |
| Fears About Cancer Recurrence | .028 | 1.15 (1.02, 1.30) |
Patients Abstinent Pre-surgery
A comparable analysis using GEE was conducted for the 53 participants who were abstinent pre-surgery. The two significant predictors were higher perceived difficulty quitting (P=.009) and lower perceived risk of the adverse effects of resuming smoking (P=.003). As can be seen in Table 3, both predictors were significant in the final model.
Patients Smoking Pre-surgery
In this group of 101 patients, smoking relapse was predicted by two smoking-related variables: a lower desire to quit (P=.031) and lower quitting self-efficacy (P<.001). With respect to the affective variables, significant predictors were higher depression proneness (P=.004), prior treatment for depression (P=.042), and greater fears about cancer recurrence (P<.001). One cognitive variable was a significant predictor: greater intrusive thoughts about cancer (P=.026). One physical variable, higher pain severity, was a marginally significant predictor (P=.093). These seven predictors were entered into a backward stepwise regression procedure. As can be seen in Table 3 (lower section), results indicated that lower quitting self-efficacy, higher levels of depression proneness, and greater fears of cancer recurrence remained significant predictors of relapse following backwards stepwise regression.
Discussion
The goals of this study were to examine rates and predictors of smoking relapse among lung and head/neck cancer patients who underwent surgical treatment. Relapse rates varied significantly depending on pre-surgery smoking status. At 12 months post-surgery, sixty percent (60%) of patients who smoked during the week prior to surgery had resumed smoking, versus only 13% of patients who were abstinent prior to surgery. Notably, these relapse rates are lower than among smokers in the general population (95%41), likely reflecting the high level of motivation and interest in smoking cessation expressed by cancer patients31, 42.
The immediate negative cancer outcomes for patients who continue to smoke underscore the need to further reduce the still substantial relapse rate found among patients who smoked prior to surgery. For these patients, lower self-efficacy for quitting, greater depression proneness, and greater fears about cancer recurrence were significant predictors of smoking relapse. The relationship between one’s expected success in quitting smoking (i.e., quitting self-efficacy) and relapse has been demonstrated in both the general smoking population and cancer patients15, 28. These results support the need for relapse-prevention interventions that address quitting self-efficacy. One potential challenge in targeting self-efficacy in this population is the extensive smoking history of cancer patients with tobacco-related malignancies. Testimonials from cancer patients who have long histories of smoking and have successfully maintained their abstinence post cancer treatment may prove effective. Depression proneness as a predictor of relapse was consistent with prior research showing that depression, and negative affect more generally, are risk factors for smoking initiation, maintenance, and relapse in the general population e.g., 43. Our finding that greater fear of cancer recurrence predicted smoking relapse is novel and unique to this population. The direction of this relationship suggests that rather than the fear of recurrence serving to motivate patients to maintain abstinence, it has negative consequences on smoking behavior, possibly due to the additional stress and negative affect associated with this fear. Therefore, both general and cancer-specific negative affect are important targets for smoking relapse-prevention interventions targeted at cancer patients.
For patients who were abstinent prior to surgery, only 13% resumed smoking at 12-months post-surgery. Two different factors predicted smoking relapse for this group of patients: higher perceived difficulty quitting and lower perceived links between smoking and cancer. These findings suggest additional efforts are warranted for patients who have already quit yet report difficulties with quitting. Such efforts may include provision of additional quitting resources and continued cessation support from oncology healthcare providers32, 44. Results also further support the need to assess and target cancer-related risk perceptions in future interventions.
This study informs the optimal timing for intervening. Given the significantly lower rates of relapse observed among those who quit prior to surgery, early cessation should be encouraged (i.e., at diagnosis). The notable stability in smoking status following the first 2 months, which is consistent with a prior study of lung cancer patients conducted by Walker et al.29, also suggests that intensifying intervention efforts in the acute period following surgery may promote long-term abstinence. Of note, our study extends this finding to head/neck patients as well.
The present study has several limitations. First, the generalizability of our study findings is limited to patients diagnosed with lung and head/neck cancer. Future research is needed to extend this work to a more a heterogeneous group of cancer patients. Other limitations related to generalizability include the predominately Caucasian sample and the focus on patients receiving surgical treatment for their cancer. More research is needed with more diverse patient samples with regards to ethnicity and cancer treatments. Another limitation was our fairly low rate of biochemical verification; however, we believe that our relapse rates are accurate as the lack of biochemical verification was due to logistics rather than refusal and only one patient was found to have misreported abstinence. Future research should consider using alternative methods of biochemical verification that do not require an in person visit, such as mailed cotinine samples. Finally, the difference in the timing of the baseline assessment between the head/neck (pre-surgery) and lung (post-surgery) patients may have influenced responses on some measures, such as pain and fatigue. Future research should examine post-surgical changes in these variables over time.
Given evidence that cancer patients are highly motivated and can be readily engaged in quitting smoking18, 19, 42, efforts aimed at helping cancer patients maintain tobacco abstinence could have dramatic public health implications. Receiving a cancer diagnosis represents a “teachable moment” for delivering smoking cessation and relapse prevention interventions45, 46. The current study further supports the important role of smoking status prior to surgery and identifies several potential modifiable variables to address in future relapse-prevention interventions targeted to cancer patients.
Acknowledgement
The authors wish to thank Angelina Fink, MPH, Elizabeth Railey, and Ellen Koltz for their contributions to the study.
Financial Support: National Cancer Institute R03 CA 126409
Footnotes
Four patients were not included in stage analyses; 3 patients upon surgery were found to have nonmalignant or premalignant disease, 1 patient refused surgery and could not be staged
References
- 1.USDHHS . The Health Consequences of Smoking: A Report of the Surgeon General: U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 2.Cox LS, Africano NL, Tercyak KP, Taylor KL. Nicotine dependence treatment for patients with cancer. Cancer. 2003;98:632–644. doi: 10.1002/cncr.11538. [DOI] [PubMed] [Google Scholar]
- 3.Park ER, Japuntich SJ, Rigotti NA, et al. A snapshot of smokers after lung and colorectal cancer diagnosis. Cancer. 2012 doi: 10.1002/cncr.26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerjes W, Upile T, Radhi H, et al. The effect of tobacco and alcohol and their reduction/cessation on mortality in oral cancer patients: Short communication. Head Neck Oncol. 2012;4:6. doi: 10.1186/1758-3284-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parsons A, Daley A, Begh R, Aveyard P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. doi: 10.1136/bmj.b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawahara M, Ushijima S, Kamimori T, et al. Second primary tumours in more than 2-year disease-free survivors of small-cell lung cancer in Japan: the role of smoking cessation. Br J Cancer. 1998;78:409–412. doi: 10.1038/bjc.1998.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–138. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 8.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med. 1993;328:159–163. doi: 10.1056/NEJM199301213280302. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Kamdar O, Le W, Rosen GD, Upadhyay D. Nicotine induces resistance to chemotherapy by modulating mitochondrial signaling in lung cancer. Am J Respir Cell Mol Biol. 2009;40:135–146. doi: 10.1165/rcmb.2007-0277OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zevallos JP, Mallen MJ, Lam CY, et al. Complications of radiotherapy in laryngopharyngeal cancer: effects of a prospective smoking cessation program. Cancer. 2009;115:4636–4644. doi: 10.1002/cncr.24499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arcavi L, Benowitz NL. Cigarette smoking and infection. Arch Intern Med. 2004;164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 12.Moller AM, Pedersen T, Villebro N, Schnaberich A, Haas M, Tonnesen R. A study of the impact of long-term tobacco smoking on postoperative intensive care admission. Anaesthesia. 2003;58:55–59. doi: 10.1046/j.1365-2044.2003.02788_2.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith JB, Fenske NA. Cutaneous manifestations and consequences of smoking. J Am Acad Dermatol. 1996;34:717–732. doi: 10.1016/s0190-9622(96)90002-x. quiz 733-714. [DOI] [PubMed] [Google Scholar]
- 14.Gritz ER. Smoking and smoking cessation in cancer patients. Br J Addict. 1991;86:549–554. doi: 10.1111/j.1360-0443.1991.tb01806.x. [DOI] [PubMed] [Google Scholar]
- 15.Gritz ER, Schacherer C, Koehly L, Nielsen IR, Abemayor E. Smoking Withdrawal And Relapse In Head And Neck Cancer Patients. Head Neck. 1999;21:420–427. doi: 10.1002/(sici)1097-0347(199908)21:5<420::aid-hed7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 16.Duffy SA, Terrell JE, Valenstein M, Ronis DL, Copeland LA, Connors M. Effect of smoking, alcohol, and depression on the quality of life of head and neck cancer patients. Gen Hosp Psychiatry. 2002;24:140–147. doi: 10.1016/s0163-8343(02)00180-9. [DOI] [PubMed] [Google Scholar]
- 17.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. Chest. 2004;126:1733–1741. doi: 10.1378/chest.126.6.1733. [DOI] [PubMed] [Google Scholar]
- 18.Gritz ER, Nisenbaum R, Elashoff RE, Holmes EC. Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer. Cancer Causes Control. 1991;2:105–112. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 19.Ostroff JS, Jacobsen PB, Moadel AB, et al. Prevalence and predictors of continued tobacco use after treatment of patients with head and neck cancer. Cancer. 1995;75:569–576. doi: 10.1002/1097-0142(19950115)75:2<569::aid-cncr2820750221>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Vander Ark W, DiNardo LJ, Oliver DS. Factors affecting smoking cessation in patients with head and neck cancer. Laryngoscope. 1997;107:888–892. doi: 10.1097/00005537-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Browning KK, Ahijevych KL, Ross P, Jr., Wewers ME. Implementing the Agency for Health Care Policy and Research’s Smoking Cessation Guideline in a lung cancer surgery clinic. Oncol Nurs Forum. 2000;27:1248–1254. [PubMed] [Google Scholar]
- 22.Wewers ME, Bowen JM, Stanislaw AE, Desimone VB. A nurse-delivered smoking cessation intervention among hospitalized postoperative patients--influence of a smoking-related diagnosis: a pilot study. Heart Lung. 1994;23:151–156. [PubMed] [Google Scholar]
- 23.Nayan S, Gupta MK, Sommer DD. Evaluating smoking cessation interventions and cessation rates in cancer patients: a systematic review and meta-analysis. ISRN Oncol. 2011;2011:849023. doi: 10.5402/2011/849023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev. 2006;15:2203–2208. doi: 10.1158/1055-9965.EPI-05-0880. [DOI] [PubMed] [Google Scholar]
- 25.Dresler CM, Bailey M, Roper CR, Patterson GA, Cooper JD. Smoking cessation and lung cancer resection. Chest. 1996;110:1199–1202. doi: 10.1378/chest.110.5.1199. [DOI] [PubMed] [Google Scholar]
- 26.Davison AG, Duffy M. Smoking habits of long-term survivors of surgery for lung cancer. Thorax. 1982;37:331–333. doi: 10.1136/thx.37.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker MS, Larsen RJ, Zona DM, Govindan R, Fisher EB. Smoking urges and relapse among lung cancer patients: findings from a preliminary retrospective study. Prev Med. 2004;39:449–457. doi: 10.1016/j.ypmed.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 28.Schnoll RA, James C, Malstrom M, et al. Longitudinal predictors of continued tobacco use among patients diagnosed with cancer. Ann Behav Med. 2003;25:214–222. doi: 10.1207/S15324796ABM2503_07. [DOI] [PubMed] [Google Scholar]
- 29.Walker MS, Vidrine DJ, Gritz ER, et al. Smoking relapse during the first year after treatment for early-stage non-small-cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2370–2377. doi: 10.1158/1055-9965.EPI-06-0509. [DOI] [PubMed] [Google Scholar]
- 30.Witkiewitz K, Marlatt GA. Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. Am Psychol. 2004;59:224–235. doi: 10.1037/0003-066X.59.4.224. [DOI] [PubMed] [Google Scholar]
- 31.Simmons VN, Litvin EB, Patel RD, et al. Patient-provider communication and perspectives on smoking cessation and relapse in the oncology setting. Patient Educ Couns. 2009;77:398–403. doi: 10.1016/j.pec.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons VN, Litvin EB, Unrod M, Brandon TH. Oncology healthcare providers’ implementation of the 5A’s model of brief intervention for smoking cessation: Patients’ perceptions. Patient Educ Couns. 2012;86:414–419. doi: 10.1016/j.pec.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 34.Hall SM, Havassy BE, Wasserman DA. Effects of commitment to abstinence, positive moods, stress, and coping on relapse to cocaine use. J Consult Clin Psychol. 1991;59:526–532. doi: 10.1037//0022-006x.59.4.526. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Greenberg DB, Kornblith AB, Herndon JE, et al. Quality of life for adult leukemia survivors treated on clinical trials of Cancer and Leukemia Group B during the period 1971-1988: predictors for later psychologic distress. Cancer. 1997;80:1936–1944. doi: 10.1002/(sici)1097-0142(19971115)80:10<1936::aid-cncr10>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 37.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41:209–218. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Henderson JW, Donatelle RJ, Acock AC. Confirmatory factor analysis of the cancer locus of control scale. Educ Psychol Meas. 2002;62:995–1005. [Google Scholar]
- 39.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in pain research and therapy, volume 12: issues in pain measurement. Raven Press; New York: 1989. pp. 391–403. [Google Scholar]
- 40.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 42.Cooley ME, Emmons KM, Haddad R, et al. Patient-reported receipt of and interest in smoking-cessation interventions after a diagnosis of cancer. Cancer. 2011;117:2961–2969. doi: 10.1002/cncr.25828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychol Bull. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- 44.Brandon TH, Unrod M. Tobacco use and cessation. In: Holland JC, Breitbart W, Jacobsen PB, Lederberg MS, McCorkle R, editors. Psycho-oncology. 2nd ed Oxford; New York: 2010. [Google Scholar]
- 45.Gritz ER, Dresler C, Sarna L. Smoking, the missing drug interaction in clinical trials: ignoring the obvious. Cancer Epidemiol Biomarkers Prev. 2005;14:2287–2293. doi: 10.1158/1055-9965.EPI-05-0224. [DOI] [PubMed] [Google Scholar]
- 46.McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control. 2003;10:325–333. doi: 10.1177/107327480301000407. [DOI] [PubMed] [Google Scholar]