Abstract
The opportunistic bacterial pathogen Pseudomonas aeruginosa causes chronic lung infections in cystic fibrosis (CF) patients. Importantly, virulence factor expression and biofilm formation in P. aeruginosa is coordinated by quorum sensing (QS) and one of the key QS signaling molecules is 3-oxo-C12-HSL. Remarkably, a tetramic acid, (C12-TA), with antibacterial properties is formed spontaneously from 3-oxo-C12-HSL under physiological conditions. Seeking to better understand this relationship we sought to investigate if 3-oxo-C12-HSL and C12-TA may be contributing factors to the overall pathogenicity of P. aeruginosa in CF individuals and their detection and quantitation in sputum samples might be used as an indicator to assess disease states and monitor therapy success in CF patients. To this end, 3-oxo-C12-HSL and C12-TA concentrations were initially analyzed in P. aeruginosa flow cell biofilms using liquid chromatography coupled with mass spectrometry (LC-MS). A liquid chromatography tandem mass spectrometry (LC-MS-MS)-based method was then developed and validated for their detection and quantification in sputa of CF patients. We highlight that this is the first report to show the presence of both the quorum sensing molecule (3-oxo-C12-HSL) and its rearranged product (C12-TA) in human clinical samples such as sputum. A total of 47 sputum samples from 20 CF and 2 non-CF individuals were analyzed: 3-oxo-C12-HSL was detected and quantified in 45 samples with concentrations ranging from 20 nM to >1000 nM; C12-TA was found in 14 samples (13 – 900 nM). Based on our findings, quorum sensing autoinducers merit further investigation as biomarkers for infectious disease states.
Keywords: cystic fibrosis, sputum, quorum sensing, Pseudomonas aeruginosa, 3-oxo-C12-HSL, C12-TA, mass spectrometry
The ubiquitous environmental Gram-negative bacterium Pseudomonas aeruginosa is an opportunistic pathogen because of its ability to take advantage of hosts with weakened immune systems.1–3 For instance, P. aeruginosa causes bacteremia in severe burn victims, infections in injured cornea, and chronic lung disease in patients with cystic fibrosis (CF);1,2 the latter being an autosomal recessive disorder resulting from mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) chloride channel1,4–6. The clinical pathology of CF is characterized mainly by elevated sweat chloride concentrations, production of thick mucus, and loss of lung function, which is also the major cause of mortality and morbidity.4–6 Although Haemophilus influenza, Klebsiella pneumoniae, Staphylococcus aureus, and Streptococcus pneumoniae colonize the lungs in young CF patients, P. aeruginosa eventually dominates the microbial population,4,7,8 leading to chronic airway inflammation and obstruction followed by respiratory failure4,9.
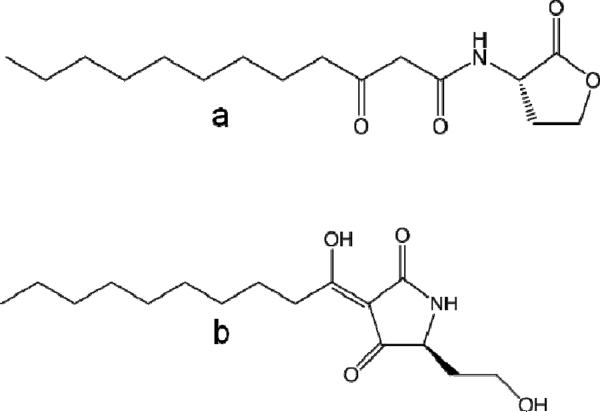
P. aeruginosa utilizes cell-to-cell communication, also known as “quorum sensing” (QS),10 to coordinate production of a multitude of virulence factors11,12 as well as biofilm formation13 in a cell density-dependent manner. Of note is that over time P. aeruginosa acquires a mucoid phenotype and exists as a biofilm in CF lungs.9 There are two N-acyl homoserine lactones (AHLs) based QS systems in P. aeruginosa utilizing N-3-oxododecaonyl homoserine lactone (3-oxo-C12-HSL; lasI/R) and N-butryl homoserine lactone (C4-HSL; rhlI/R).14 Besides its role in QS signaling, 3-oxo-C12-HSL has been demonstrated to act directly as a virulence factor capable of modulating innate immune responses in host, thus further exploiting their already compromised immunity.12,15,16 Furthermore, we have previously reported the discovery of a non-enzymatically formed metabolic product of 3-oxo-C12-HSL, specifically, a tetramic acid [3-(1-hydroxydecylidene)-5-(2-hydroxyethyl) pyrrolidine-2,4-dione; C12-TA; Figure 1).17,18
Figure 1.
Chemical structures of 3-oxo-C12-HSL (a) and the corresponding tetramic acid (C12-TA, b).
Tetramic acids (TAs) are a class of chemical compounds containing a 2,4-pyrrolidinedione ring system that represent a key structural motif of many natural products originating from various terrestrial and marine organisms, such as sponges, cyanobacteria, fungi and bacteria.19,20 These compounds are known to have an extraordinarily broad spectrum of biomedical activities ranging from antibacterial, antiviral, antifungal, antiulcerative, to anticancer activities. C12-TA also possesses biological properties like antibacterial activity17,18 and metal ion chelation;17,21 features that may confer competitive advantages to P. aeruginosa in natural and host environments. Thus, P. aeruginosa may employ a specific set of chemical compounds not only to gauge its number of “kin” and to synchronize gene expression, but also to potentially overwhelm and modulate host defense, as well as to ward off microbial competitors.
P. aeruginosa colonizes the lungs of CF patients over long periods of time, hence, an accumulation of 3-oxo-C12-HSL and C12-TA in the airway biofilms could be envisioned. Although 3-oxo-C12-HSL has been detected in various clinical samples from CF patients,9,22–25 the presence of C12-TA and potential correlation between each is yet to be reported. Because 3-oxo-C12-HSL is a prerequisite for successful initiation and establishment of infection, we conjectured that the presence of 3-oxo-C12-HSL and/or C12-TA in biological samples might lend itself as diagnostic or even predictive biomarker for P. aeruginosa colonization, pathogenicity and ultimately disease progression. Consequently, as a starting point to begin to test this hypothesis, we first set forth to detect the presence of 3-oxo-C12-HSL and C12-TA in P. aeruginosa biofilms, formed in vitro in flow cells, using a liquid chromatography coupled with mass spectrometry (LC-MS)-based method. Success of this initial research laid the foundation for the detection and quantitation of 3-oxo-C12-HSL and C12-TA in human clinical samples specifically, sputum from CF patients. For the latter purpose, a liquid chromatography tandem mass spec-trometry (LC-MS-MS)-based method was developed. Our findings imply that 3-oxo-C12-HSL could be used to monitor P. aeruginosa pathogenesis.
EXPERIMENTAL SECTION
Reagents, chemicals and synthesis
All reagents and chemicals used were of LC-MS grade. Methylene chloride (Optima), methanol (Optima), and water (Optima) were purchased from Fisher Scientific (Pittsburgh, PA, USA).
The four standard compounds were synthesized in-house: 3-oxo-C12-HSL, C12-TA, 13C labeled 3-oxo-C12-HSL, and 13C labeled C12-TA. 3-oxo-C12-HSL and C12-TA were synthesized as described previously.17 The synthetic procedures and spectral data for 13C labeled 3-oxo-C12-HSL and C12-TA are provided in the supporting information (synthetic scheme is shown in Figure S-1).
Analysis of P. aeruginosa biofilms using LC-MS
P. aeruginosa biofilms were grown at 37 °C in a FC-81 model flow cell system (BioSurface Technologies Corp., Bozeman, Montana) as described earlier.18 The dimensions of the flow chamber are 50 × 13 × 2.35 mm and it used microscope cover slips (60 × 24 mm; no. 2) and microscope slides (25 × 75 × 1 mm) as viewing windows. All the components were autoclaved at 120 °C for 15 min before use. A peristaltic pump was employed to maintain a steady flow of medium through the chamber at 0.01 mL/min. The flow-system was equilibrated with M9 minimal medium containing 200 μg/ mL carbenicillin before inoculation.
An overnight culture of P. aeruginosa PAO1 was prepared in 3 mL of PTSB medium containing 200 μg/ mL carbenicillinat 37 °C and 250 rpm. The overnight culture was diluted in M9 minimal medium containing 200 μg/ mL carbenicillin to an OD600 of 1.0. Using a sterile needle, a ~2 mL aliquot of the diluted culture was injected carefully into the flow chamber. Inoculated culture was kept static for 90 min or 24 h for initial attachment and then the peristaltic pump was turned on to maintain a steady and continuous flow of minimal medium through the chamber. After the desired length of days (3 or 6 days), the flow of culture medium was stopped and the culture medium in the flow chamber along with the biofilm cover slip was collected and then extracted. To enable complete extraction, the cover slip was first crushed carefully. The sample was extracted three times with at least an equal volume of acidified ethyl acetate (0.1% formic acid). The organic layer was separated, collected and dried using anhydrous magnesium sulfate and then removed under reduced pressure. The residue was reconstituted in 100 μL of 35% (v/v) methanol in water (0.1% formic acid) and analyzed with LC-MS.
An Agilent 1100 MSD LC/MS system, with electrospray ionization (ESI) as the ionization method, was used to analyze standards and biofilm extracts. The column used was Agilent Zorbax C8, 4.6 × 50 mm, 5 μm particles size with Phenomenex Security Guard cartridge (C8, double stacked, 4.0 × 3.0 mm). The solvent gradient was used with A: water (0.1 % formic acid) and B: methanol (0.1% formic acid) 0 min = 35% B, 2 min = 35% B, 15 min = 95% B, 17 min = 95% B) at a flow rate of 0.500 mL/min and 10 μL sample injection volume. Total run time was 17 min for each sample. The column was re-equilibrated for 4 min before another injection. The LC column was connected to an ESI chamber used in positive ion mode. The mass spectrometer was set in a selected ion monitoring (SIM) mode to record the most abundant ions for both compounds: [M + H]+, [M + Na]+, [M + H + H2O]+.
Individual 10 mM solutions of 3-oxo-C12-HSL and C12-TA were prepared in acidified methanol (0.1% formic acid). The pure solutions were used to prepare a set of standard solutions which contained both 3-oxo-C12-HSL and C12-TA each at the following concentrations (μM): 10, 1, 0.1, 0.01, 0.001 and 0.0001. Using the mixed standard solutions, dose-response curves with six calibration points were acquired for 3-oxo-C12-HSL and C12-TA over the concentration range 0.0001–10 μM. The LC-MS calibration curves were generated using the total peak area for the three ions {[M + H]+, [M + Na]+, [M + H + H2O]+} against a range of concentrations. Using unweighted linear regression, the equation for the line that fits the data was determined
Analysis of 3-oxo-C12-HSL and C12-TA in CF sputa using LC-MS-MS
Preparation of standard solutions
Individual 10 mM solutions of 3-oxo-C12-HSL and C12-TA were prepared in acidified methanol (0.1% formic acid). The pure solutions were used to prepare a set of solutions which contained both 3-oxo-C12-HSL and C12-TA each at the following concentrations (nM): 1100, 550, 275, 110, 55, 27.5, 11 and 5.5. 13C labeled 3-oxo-C12-HSL and C12-TA served as internal standards for all the standards mixtures and samples analyzed. Individual 10 mM solutions of the 13C labeled 3-oxo-C12-HSL and C12-TA were prepared in acidified methanol (0.1% formic acid). Using these pure solutions, an internal standards stock solution was prepared that contained both 13C labeled 3-oxo-C12-HSL and C12-TA each at 5.5 μM.
To 100 μL of solutions containing both 3-oxo-C12-HSL and C12-TA each at 1100, 550, 275, 110, 55, 27.5, 11 and 5.5 nM, a 10 μL of 5.5 μM internal standard stock solution was added. Thus, the eight concentration points in the calibration curve were (5–1000 nM): 5, 10, 25, 50, 100, 250, 500, 1000 nM. The concentration of both the internal standards in all the eight final calibration points was 500 nM. Similarly, 10 μL of 5.5 μM internal standards stock solution was added to 100 μL of acidified methanol to obtain 500 nM in the final solution, and the resulting solution served as a blank. All solutions were stored at −20 °C until analyzed.
LC-MS-MS
LC analysis was performed using an Agilent 1290 system. The column used was Agilent Extend-C18, 2.1 × 50 mm with 3.5 μm particle size. Both were purchased from Agilent (Santa Clara, CA, USA). The LC column was maintained at 50 °C by a column oven. Five microliters of blank, standards or samples in mobile phase acetonitrile-water (15:85, v/v) with 0.1% formic acid was injected in the column, at a flow rate of 0.35 mL/min. The elution method utilized for LC separation of 3-oxo-C12-HSL and C12-TA included an isocratic profile of acetonitrile in water (15:85, v/v) for 2 min, followed by a linear gradient from 15 to 98% acetonitrile in water over 13 min. A subsequent isocratic profile of 98% acetonitrile in water over 5 min. Total run time was 20 min for each sample. The column was re-equilibrated for 4 min before another injection.
The LC column was connected to an ESI chamber used in positive ion mode. Nitrogen was used as the drying gas with a flow rate of 10 L/min at 350 °C. The pressure for the nebulizing gas, nitrogen, and the temperature for the ESI housing were kept at 20 psi and 25 °C, respectively. The ESI chamber was interfaced to an Agilent 6460 triple quadrupole mass spectrometer. The [M + H]+ ions were monitored for each compound.
The [M + H]+ ions were subjected to collision-induced dissociation using nitrogen as the collision gas. The capillary voltage was maintained at 4000 V. The MS-MS analyses were based on selected MRM transitions. Two mass transitions for 3-oxo-C12-HSL and C12-TA and one for each of the internal standards were monitored as shown in Table S-1.
Method Validation
For method validation, linearity of calibration curves, method reproducibility, limit of detection (LOD), lower limit of quantitation (LLOQ), intra- and inter-day precision and accuracy, extraction efficiencies in sputa, matrix effect, carryover effect and stability of 3-oxo-C12-HSL and C12-TA were analyzed. A calibration curve over the range of 5–1000 nM with eight concentration points (5, 10, 25, 50, 100, 250, 500, 1000 nM) was prepared. LOD was defined as the lowest concentration at which the signal-to-noise response ratio was at least three (S/N> 3). The lowest standard on the calibration curve was accepted as the LLOQ, when the analyte response at this concentration was at least ten times the blank response (S/N> 10).
Method accuracy was defined as the closeness of the measured mean value for a concentration, in a calibration curve, with its true concentration. It was determined at three different concentration levels, in the range of calibration curve, in a replicate analysis (n=3).
Method precision was measured by comparing the closeness of the measured values for a concentration, in a calibration curve, with each other. It was also determined at three different concentration levels, in the range of calibration curve, in a replicate analysis (n=3) and was expressed in percent relative standard deviation (%RSD).
Percent recoveries of both 3-oxo-C12-HSL and C12-TA in each sample were determined. Thus, a specific amount (500 nM) of their respective 13C labeled analogues (13C labeled 3-oxo-C12-HSL and C12-TA; internal standards) was added to each sputum sample followed by their extraction. Percent Recovery was the ratio of responses of internal standards in extracted sputum to un-extracted standards, times 100.
Matrix effects were determined by calculating the ratio of the responses for 3-oxo-C12-HSL and C12-TA both spiked at 250 nM in extracted sputum to the response of the same analyte in a pure standard solution without matrix, multiplied by 100. A value of >100% indicates ionization enhancement, and a value of <100% suppression.
Carryovers caused by residual analyte from a previously run high concentration standards or samples were also assessed. A wash step and a blank sample were run after the highest concentration calibration standard and between the real samples.
Stability of standard solutions of 3-oxo-C12-HSL and C12-TA after at least three freeze and thaw cycles was also determined. To assess, if there was any conversion of 3-oxo-C12-HSL to C12-TA due to sample handling and processing, a specific amount (500 nM) of 3-oxo-C12-HSL with 500 nM of internal standards was extracted. Finally, Guidance for industry, bioanalytical method validations was followed wherever possible.26
Sputa collection, preparation and analysis
A total of 47 sputa were obtained from 22 CF patients and 2 healthy volunteers. Approximately 1–5 mL of sputum was collected. These samples were provided by Prof. Michael G. Surette (Department of Microbiology and Infectious Diseases, University of Calgary), Prof. Douglas Conrad (Adult Cystic Fibrosis Program at the University of California, San Diego) and Prof. Joseph Zabner (Division of Pulmonary, Critical Care and Occupational Medicine at the University of Iowa). Patients gave informed consent and the protocols were approved by the respective Universities' Human Subjects Review Board.
There were 9 CF individuals with more than one sample; the total of these samples was 34. The patients' age ranged from 18 to 48 and samples represented different disease states of patients. These disease states were grouped in “stable/control” and “hospitalized”. Samples obtained during or immediately prior to hospitalization were considered “hospitalized” samples while all the other samples including samples from 2 non-CF patients and a sample taken on the day of discharge were considered “stable/control”. Thus, the samples in the “stable/control” group were from patients who were either considered clinically stable or were control samples. Samples in the “hospitalized” group represented the samples obtained from CF patients who were considered clinically sick, i.e. were experiencing a pulmonary exacerbation of their lung disease and were either hospitalized or receiving parenteral antibiotic therapy at home. The sum of samples from “hospitalized” and “stable/control” groups were 23 and 24, respectively. Additionally, there were six CF patients for whom we had samples from both their stable and hospitalized health states.
Sputum samples were first diluted with ~15 mL PBS buffer (pH 7.4) followed by an addition of internal standards stock solution. Specifically, 100μL of 500 nM internal standards stock solution was added to each diluted sputum. Sputum samples were then homogenized using sonication; for a total of 1 min and 30 s with pulse “ON” duration of 30 s and 1 min interval between the pulses. The resulting homogenized sputum samples were extracted thrice with at least an equal volume of acidified dichloromethane (0.1% formic acid). The organic layer was separated, collected and dried using anhydrous magnesium sulfate and then removed under reduced pressure. The residue was reconstituted in 100 μL of acidified methanol (0.1% formic acid).
The resuspended sputa extracts and standards for calibration were analyzed with LC-MS-MS. The concentrations of both compounds were first calculated using their respective calibration curves (peak area ratio against analyte concentration) obtained using their standard solutions with known concentrations. The actual amounts of 3-oxo-C12-HSL and C12-TA present in each sputum sample were then back-calculated using the percent recoveries measured with their respective 13C labeled analogues.
Statistical analysis
The distribution of 3-oxo-C12-HSL levels was assessed and found to be skewed; thus non parametric tests were used to determine differences between “hospitalized” and “stable/control” samples. The analysis was carried out using all the samples and not controlling for multiple samples for certain individuals. Analysis involved comparison of 3-oxo-C12-HSL levels in these groups using the Wilcoxon rank sum test. Additional statistical analysis is included in the supporting information.
RESULTS AND DISCUSSION
Given that both the parent molecule 3-oxo-C12-HSL and its corresponding rearranged product C12-TA are antibacterial agents; their production by P. aeruginosa might be a survival strategy in mixed microbial population environments as in the lung airways of CF individuals, ensuring successful establishment and prevention of infringement by competing bacteria. Indeed, 3-oxo-C12-HSL has been detected in P. aeruginosa in vitro biofilms,27 in various CF samples such as sputum,9,22,23 mucopurulent respiratory secretions,24 and lung tissues,25 and, we have detected C12-TA in P. aeruginosa cultures17. Sputum analysis presents the advantage of enabling noninvasive collection of samples and is reflective of the dominant organisms that are present in CF lungs.28 In the present study, we provide direct evidence that both 3-oxo-C12-HSL and C12-TA are produced in P. aeruginosa biofilms grown in a continuous flow system and for the first time in the lungs of CF patients.
Analysis of 3-oxo-C12-HSL and C12-TA in P. aeruginosa biofilms
For the analysis of P. aeruginosa biofilms grown in continuous flow systems in laboratory settings, a LC-MS-based method was developed. Retention times for standard mixtures of chemically synthesized 3-oxo-C12-HSL and C12-TA injected in a C8 column were obtained. The compounds were well separated with retention times of ~6.98 and ~7.95 min for 3-oxo-C12-HSL and C12-TA, respectively. A calibration curve was developed for 3-oxo-C12-HSL and it was found to be linear over 4 orders of magnitude (0.01–10 μM). For C12-TA, the calibration curve was linear in the range of 0.1–10 μM. The correlation coefficient values for both compounds were greater than 0.99.
The developed LC-MS method was applied to the detection of 3-oxo-C12-HSL and C12-TA in P. aeruginosa biofilm extracts. Gratifyingly, the LC retention times and MS ions corresponding to the QS compounds in standard solutions were also detected in the biofilm extracts (Figure S-2). The measured concentrations of 3-oxo-C12-HSL and C12-TA in the biofilm extracts (n=4) were 0.95 ± 0.68 μM and 1.45± 0.93 μM respectively. The peak areas corresponding to [M + H]+, [M + H + H2O]+ and [M + Na]+ were used toward the total peak area corresponding to 3-oxo-C12-HSL. We also observed that the measured concentrations in a 6 days old biofilm extract for both 3-oxo-C12-HSL (~0.68 μM) and C12-TA (~0.93 μM) were similar to the levels detected in 3 days old biofilms.
While we were successful in detecting the presence of both 3-oxo-C12-HSL and C12-TA in P. aeruginosa biofilms, we had expected an accumulation of 3-oxo-C12-HSL and C12-TA over days due to high cell density and limited diffusion caused by the exopolysaccharide matrix in biofilms but surprisingly did not observe such a trend which may be due to their efflux in flow cell systems. Additionally, the duration (3–6 days) of biofilm development in flow cells may not have been sufficient for a greater accumulation. Although, concentrations of 3-oxo-C12-HSL, up to ~600 μM, have been reported in 7–9 days old biofilms previously,27 it is critical to note that the values reported herein did not take into consideration the loss of compound incurred during sample preparation, matrix effects or biofilm volume. Our main objective was to demonstrate how biofilms formed by P. aeruginosa in flow cells could produce in tandem both C12-TA alongside with 3-oxo-C12-HSL.
LC-MS-MS method development and validation for detection and quantitation of 3-oxo-C12-HSL and C12-TA in CF sputa
Once the presence of 3-oxo-C12-HSL and C12-TA P. aeruginosa biofilms was demonstrated, our studies were extended to P. aeruginosa biofilms present in the lungs of CF individuals. We note that in contrast to simple in vitro biofilms, CF sputum can contain large polymers such as DNA, filamentous actin, lipids, proteoglycans, biofilms and inflammatory cells.29 Furthermore, we were concerned that the levels of 3-oxo-C12-HSL and C12-TA in CF sputa could be greatly diminished, which might affect the specificity and sensitivity of their detection using only LC-MS. Faced with these uncertainties, tandem mass spectrometry (MS-MS) was engaged as a means to monitor 3-oxo-C12-HSL and C12-TA. This method allows for monitoring of both precursor and fragmented product ions (selected reaction monitoring, SRM) characteristic of a given ionizable analyte or multiple analytes in parallel (multiple-reaction monitoring, MRM) in a sample, enabling identification with higher probability in multi-analyte matrices. Lastly, LC-MS-MS based approaches have been employed for the detection and quantitation of AHLs, however, these reports have focused simply upon bacterial cultures,30–34 or detection in human samples35.
LC separation and MS-MS detection
Standard mixtures of 3-oxo-C12-HSL and C12-TA, with a specific amount of their respective internal standards, were injected in a reverse phase C18 column to establish the respective retention times. Specifically, 3-oxo-C12-HSL and C12-TA in a set of standard solutions with 500 nM of their corresponding respective internal standards were well separated on a C18 column at 8.1 and 8.8 min, respectively (Table S-1). The retention times were highly reproducible over all subsequent runs, demonstrating precise conditions and reliability of the instrument.
The 3-oxo-C12-HSL, its corresponding tetramic acid, and their 13C labeled analogues were readily ionized in positive electrospray mode, forming [M + H]+ ions. The precursor/pseudomolecular ions ([M + H]+ ions) were selected in the first mass analyzer. Although the m/z of the precursor [M + H]+ ions for both compounds was the same, they be could be easily distinguished in HPLC-MS using their specific retention times.
In order to increase the selectivity of the method, two fragment ions (quantifier and qualifier ions) were monitored for both 3-oxo-C12-HSL and C12-TA in all standards and samples. For internal standards, only quantifier ion was monitored. In order to identify the quantifier and qualifier ions for 3-oxo-C12-HSL and C12-TA and the quantifier ion for the internal standards, a full scan MS-MS was obtained that allows for analysis of all the possible fragment ions derived from a precursor ion. Once the quantifier and qualifier ions were established, subsequent MS-MS analyses were based on the selected MRM transitions. Thus, precursor [M + H]+ ions and two of the most abundant MS-MS fragment ions for 3-oxo-C12-HSL and C12-TA and one for each of the internal standards were monitored. Mass spectrometric parameters such as MRM transitions and collision energy for 3-oxo-C12-HSL, C12-TA and their respective internal standards are listed in Table S-1. Consequently, the identification of 3-oxo-C12-HSL and C12-TA in a standard solution or a sample was based on their specific retention times and their respective precursor and fragment ions. A representative LC-MS-MS chromatogram and MRM based MS/MS spectra demonstrating the separation and detection of 3-oxo-C12-HSL and C12-TA are shown in Figure S-3.
Calibration and validation
Calibration curves were generated for 3-oxo-C12-HSL and C12-TA in mixed standard solutions over the concentration range 5–1000 nM using eight calibration points (5, 10, 25, 50, 100, 250, 500 and 1000 nM). All calibration and internal standard solutions were prepared in acidified methanol (0.1% formic acid). Stable-isotope forms of both analytes, specifically, 13C labeled analogues of 3-oxo-C12-HSL and C12-TA, were used as internal standards. Thus, 13C labeled 3-oxo-C12-HSL and C12-TA were used as internal standards and exhibited identical experimental conditions (such as extraction efficiency, retention time, ionization, MS and MS-MS fragmentation pattern) as for the unlabeled analogues and ensure method reliability and reproducibility. The concentrations of the internal standards were set in the middle of the curve at 500 nM; while, the ratios of LC-MS-MS peak areas of the analyte/internal standard (relative responses or response ratio) were calculated and the calibration curves were plotted as peak area ratio against analyte concentration using unweighted linear regression analysis. For both the compounds, the response was linear in the tested concentration range with corre-lation coefficient (r2) value greater than 0.99.
For the calibration curves of 3-oxo-C12-HSL and C12-TA, the deviations of the measured values from their true values for at least 6 out of 8 concentrations were observed to be within ±15% except at the LLOQ level, where the difference was higher than 15% but within 20%. The LOD was the lowest concentration in the calibration curve for both 3-oxo-C12-HSL and C12-TA with S/N> 3. The LLOQ concentration for 3-oxo-C12-HSL and C12-TA was 10 nM with S/N> 10, precision ≤20% and accuracy ±20%. The intra-day and inter-day accuracy and precision for 3-oxo-C12-HSL and C12-TA obtained at L, M, and H concentrations are listed in Table 2.
Table 2.
Concentrations of 3-oxo-C12-HSL and C12-TA measured in CF sputa using LC-MS-MS.
| Sample Number | Patient Number | Age | Disease State | Concentration (nM) | |
|---|---|---|---|---|---|
| 3-oxo-C12-HSL | C12-TA | ||||
| 1 | 12 | 20 | S | n.q. | n.q. |
| 2 | 5 | 22 | S | n. q. | - |
| 3 | 2 | 45 | S | 20.24 | 84.34 |
| 4 | 17 | 21 | S | 22.18 | 13.86 |
| 5 | 7 | 18 | S | 32.24 | - |
| 6 | 15 | 21 | S | 33.25 | - |
| 7 | 10 | 22 | S | 33.40 | - |
| 8 | 15 | 21 | S | 45.88 | |
| 9 | 9 | 26 | S | 49.36 | 224.41 |
| 10 | 20 | - | C | 56.66 | 290.35 |
| 11 | 6 | 48 | S | 61.66 | - |
| 12 | 21 | 44 | S | 66.26 | 13.38 |
| 13 | 11 | 36 | S | 78.62 | - |
| 14 | 3 | 34 | S | 78.99 | n.q. |
| 15 | 1 | 32 | S | 79.15 | - |
| 16 | 16 | 31 | S | 81.93 | - |
| 17 | 3 | 34 | S | 83.51 | - |
| 18 | 22 | 26 | C | 86.46 | 365.04 |
| 19 | 4 | 27 | S | 104.97 | - |
| 20 | 10 | 23 | S | 123.28 | - |
| 21 | 4 | 27 | S | 679.40 | - |
| 22 | 1 | 32 | S | 803.34 | - |
| 23 | 3 | 34 | S | 981.42 | 79.63 |
| 24 | 1 | 32 | S | 6833.20 | 899.85 |
| 1 | 11 | 37 | H | 41.49 | - |
| 2 | 7 | 19 | H | 44.05 | - |
| 3 | 4 | 26 | H | 45.98 | - |
| 4 | 10 | 23 | H | 49.20 | - |
| 5 | 7 | 19 | H | 56.58 | 407.03 |
| 6 | 10 | 23 | H | 92.96 | - |
| 7 | 9 | 26 | H | 136.98 | - |
| 8 | 13 | 25 | H | 163.26 | - |
| 9 | 10 | 23 | H | 182.84 | - |
| 10 | 9 | 26 | H | 214.01 | - |
| 11 | 9 | 26 | H | 232.30 | - |
| 12 | 15 | 21 | H | 242.95 | - |
| 13 | 4 | 26 | H | 250.88 | - |
| 14 | 11 | 37 | H | 253.58 | - |
| 15 | 4 | 26 | H | 305.16 | - |
| 16 | 9 | 26 | H | 422.03 | - |
| 17 | 18 | 41 | H | 633.14 | 12.80 |
| 18 | 14 | 32 | H | 772.95 | 39.78 |
| 19 | 14 | 32 | H | 781.88 | 75.25 |
| 20 | 14 | 32 | H | 1386.89 | - |
| 21 | 9 | 26 | H | 2241.75 | - |
| 22 | 19 | 26 | H | 3303.38 | 209.69 |
| 23 | 8 | 23 | H | 3820.79 | 49.11 |
S: Stable, C: Control, H: hospitalized,
“n.q.”: not quantifiable and “—”: not detected
The recoveries for both 3-oxo-C12-HSL and C12-TA were determined, in each sputum sample, using the 13C labeled analogues. It was observed that the percent recoveries for C12-TA (~ up to 25%) were in general much lower than those for 3-oxo-C12-HSL (~up to 60%) across all samples. Clues to tetramic acids poor recovery have been noted in the literature and contributing factors include its tautomeric equilibrium36 coupled with its metal-chelating ability17,21. Such low recoveries of C12-TA can further be expected in samples with complex biological matrices such as sputum, especially, with CF sputum since it contains excessive amounts of very thick mucus. Finally, even though the recoveries were lower for C12-TA in sputum samples, the actual amount of the tetramic acid present in these samples could be accurately calculated owing to the use of 13C labeled C12-TA.
The measured values of matrix effect for 3-oxo-C12-HSL and C12-TA were 126% and 104% respectively, indicating some matrix effect for 3-oxo-C12-HSL but minimal for C12-TA. To eliminate carryover effect, a “sawtooth” gradient wash step and a blank was run after the highest concentration standard (1000 nM) and after each sputum sample run. Carryover was considered insignificant if area in blank samples was ≤20% of peak area of analyte in LLOQ samples and ≤5% of mean peak area of internal standards in calibration curve standards.37,38 Both 3-oxo-C12-HSL and C12-TA, in standard pure and mixture solutions, were stable after repeated freeze and thaw cycles at least over a period of four months. It must also be pointed out that there was no observed conversion of 3-oxo-C12-HSL to C12-TA due to sample processing. Thus, the LC-MS-MS method was demonstrated to be sensitive, selective and accurate for the detection and quantification of 3-oxo-C12-HSL and C12-TA. Table 1 summarizes the validation results for this method.
Table 1.
Validation data: Calibration, intra- and inter-day precision and accuracy of method at three concentrations (L=50 nM, M=500 nM and H=1000 nM).
| Method Validation | 3-oxo-C12-HSL | C12-TA |
|---|---|---|
| Calibration range (nM) | 5–1000 | 5–1000 |
| Linearity (r2) | 0.9999 | 0.9937 |
| LLOQ (nM) | 10 | 10 |
| Concentration (nM) | Low | Medium | High | Low | Medium | High |
|---|---|---|---|---|---|---|
| 50 | 500 | 1000 | 50 | 500 | 1000 | |
| Intra-day (n=3) | ||||||
| Precision | 5.15 | 0.98 | 0.96 | 20.33 | 4.57 | 6.82 |
| Accuracy | 85.27 | 101.12 | 98.91 | 104.29 | 101.86 | 100.08 |
| Inter-day (n=3) | ||||||
| Precision | 3.28 | 3.11 | 0.7 | 3.12 | 12.47 | 4.09 |
| Accuracy | 102.33 | 99.23 | 100.28 | 103.4 | 91.37 | 101.94 |
Analysis of 3-oxo-C12-HSL and C12-TA in CF sputa
Having established that LC-MS-MS can successfully and reproducibly separate and identify 3-oxo-C12-HSL and C12-TA in standard solutions, we sought to probe the applicability of the method in the analysis of sputum samples.
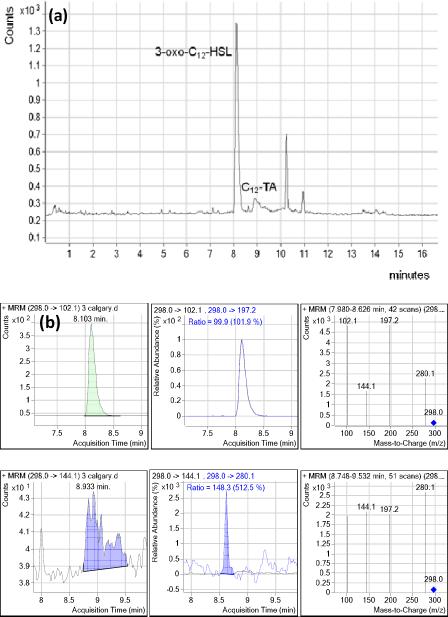
Sputum samples were obtained (see methods), and the LC retention times, MS and MS-MS fragment ions corresponding to 3-oxo-C12-HSL and C12-TA in standard solutions were confirmed in sputum samples. Figure 2 shows LC-MS-MS chromatogram and MRM based MS/MS spectra demonstrating the separation and detection of 3-oxo-C12-HSL and C12-TA in a sputum extract. In 47 sputa analyzed, 3-oxo-C12-HSL and C12-TA were detected and quantified in 45 and 14 of the samples, respectively (Table 2). The concentrations of 3-oxo-C12-HSL and C12-TA present in the samples ranged from 20 to > 1000 nM and 13 – 900 nM respectively. In addition, there were five sputum samples with 3-oxo-C12-HSL concentrations higher than 1000nM (up to ~6900 nM). Overall, 3-oxo-C12-HSL was detected in 45/47 of the analyzed sputa in the range of 0.02 to 7μM. Unfortunately, C12-TA was only detected in a smaller sample number, which might be due to the poor extraction efficiencies in sputum for this molecule.
Figure 2.
(a) LC-MS-MS chromatogram of a CF sputum extract. (b). MRM based MS-MS chromatograms and spectra of the sputum sample.
The concentrations of 3-oxo-C12-HSL and C12-TA found in CF sputa were in the lower range of the previously reported concentrations found to be bactericidal toward certain bacteria.17,18 The previously reported EC50 values for 3-oxo-C12-HSL and C12-TA antibacterial activities against various Gram-positive bacteria, which included a Staphylococcus aureus (co-colonizes with P. aeruginosa in CF lungs) strain were from 8–55 μM and 22 - >100 μM respectively.17 However, it can be envisioned that the effective local concentrations of 3-oxo-C12-HSL and C12-TA experienced by competing bacteria in vivo may be higher as the lung environment as well as the bacterial colonization density is unlikely to be homogeneous.
Furthermore, such levels in vivo may actually reflect a pertinent patho-physiological concentration. The reported concentration of 3-oxo-C12-HSL required for half-maximal activation of QS-regulated lasB gene in P. aeruginosa is ~1 μM,39 which is within the range of 3-oxo-C12-HSL levels found in sputa in the present study. Moreover, 3-oxo-C12-HSL in a concentration range of 0.1–100 μM has been demonstrated to modulate innate immune response in host cells by inhibiting lymphocyte proliferation and production of both tumor necrosis factor alpha and interleukin-12 by lipopolysaccharide-stimulated macrophages; ultimately resulting in the reduction of bacterial clearance from the host and promoting their persistence.40 It can be anticipated that that the effective concentrations of 3-oxo-C12-HSL encountered by host cells in vivo in the local vicinity of P. aeruginosa biofilms may be slightly higher in some scenarios. Therefore, it is plausible that these compounds may ward off microbial competitors, or confer a local competitive advantage to P. aeruginosain vivo and thus contribute to P. aeruginosa eventual dominance in the lung microbiome.
While our clinical sample set was limited, statistical analysis of distribution of 3-oxo-C12-HSL in “hospitalized” and “stable/control” groups of samples suggested that the concentrations of 3-oxo-C12-HSL in the “hospitalized” group of samples were higher with relatively lower concentrations in the samples from “stable/control” group (Table 3). Specifically, the 3-oxo-C12-HSL concentrations were elevated in samples obtained during hospitalization of the CF patients compared to the levels in samples obtained during their clinically stable periods. Furthermore, Figure 3 shows a profile of a patient with multiple samples collected during different clinical states. It was observed that the concentrations of 3-oxo-C12-HSL in all the samples that were collected during pulmonary exacerbations/ clinically sick or “hospitalized” states of the patient were higher when compared to the concentrations in samples from “stable” or remission periods. Of further note is the observed sudden drop in the concentration of 3-oxo-C12-HSL from “Hospital admission” to “Day 2 hospitalization”. While many theories for this could be put forth, we believe such an effect could simply be due to the arsenal of therapeutic agents41 administered to the patient on the day of hospitalization. Indeed aggressive treatments of pulmonary exacerbations in CF patients have been shown to reduce sputum bacterial burden, airway markers of inflammation/infection and consecutively improve overall pulmonary function.41–44 Lastly, additional research will be needed to fully confirm the observed correlation between elevated 3-oxo-C12-HSL concentrations and pulmonary exacerbations of “hospitalized” patients as the data were not normalized for P. aeruginosa counts in the sputum.
Table 3.
3-oxo-C12-HSL in “hospitalized” versus “stable/control” samples in an overall, unpaired non-parametric statistical analysis.
| Stable/Control | Hospitalized | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Median (IQR) (nM) | Range (nM) | n | Median (IQR) (nM) | Range (nM) | p* |
| 24 | 72 (33–96) | 0–6833 | 23 | 243 (93–773) | 41–3821 | 0.01 |
IQR; Interquartile Range
Wilcoxon rank sum test (not controlling for multiple samples from individuals)
Figure 3.
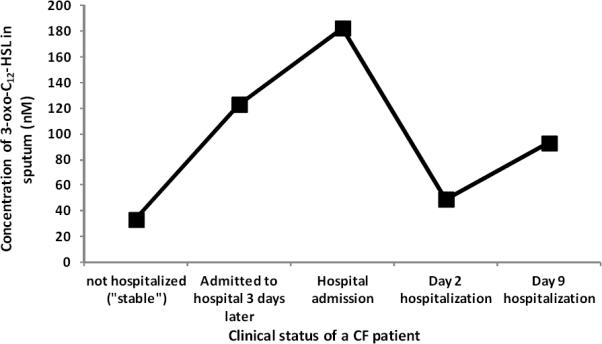
The concentration profile of 3-oxo-C12-HSL detected in sputa of a CF patient obtained during different clinical states.
CONCLUSIONS
In summary, this study is the first to validate the presence of C12-TA in sputum samples, while we have also been able to illustrate that 3-oxo-C12-HSL could reach concentrations in the micromolar (μM) range in clinical samples of CF patients; this reinforces the implication of a broader role for 3-oxo-C12-HSL. One of the obstacles in understanding roles of QS molecules in patient samples has been the inability to correlate sample concentrations with disease state. Noteworthy was the overall higher level of 3-oxo-C12-HSL determined in samples from “hospitalized” patients versus those from a “stable/control” group. Consequently, we envision that the presence, and relative changes in amounts of 3-oxo-C12-HSL in CF sputum could be developed as a biomarker of active P. aeruginosa pathogenesis, and in turn as an indicator of both exacerbation/disease state and therapy success in patients with CF.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by The Scripps Translational Science Institute (Grant NIH #UL1 RR025774 to K.D.J.) and The National Institutes of Health (AI080715 to G.F.K). We thank Professors Michael G. Surette (Department of Microbiology and Infectious Diseases, University of Calgary), and Joseph Zabner (Division of Pulmonary, Critical Care and Occupational Medicine at the University of Iowa) for kindly providing us with CF sputum samples. We especially thank all the patients and volunteers involved.
Footnotes
Supporting Information Synthetic procedures, characterization data of chemicals, LC-MS chromatogram of a P. aeruginosa biofilm extract and additional statistical analysis of data obtained with CF sputa. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).Lyczak JB, Cannon CL, Pier GB. Microbes and Infection. 2000;2:1051. doi: 10.1016/s1286-4579(00)01259-4. [DOI] [PubMed] [Google Scholar]
- (2).Goodman AL, Kulasekara B, Rietsch A, Boyd D, Smith RS, Lory S. Developmental cell. 2004;7:745. doi: 10.1016/j.devcel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- (3).Høiby N. Annual Review of Medicine. 1993;44:1. doi: 10.1146/annurev.me.44.020193.000245. [DOI] [PubMed] [Google Scholar]
- (4).Davis PB. Am. J. Respir. Crit. Care Med. 2006;173:475. doi: 10.1164/rccm.200505-840OE. [DOI] [PubMed] [Google Scholar]
- (5).Witt H. Nat Genet. 2011;43:508. doi: 10.1038/ng.844. [DOI] [PubMed] [Google Scholar]
- (6).Quinton PM. Physiology. 2007;22:212. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- (7).May RJ, Herrick NC, Thompson D. Archives of Disease in Childhood. 1972;47:908. doi: 10.1136/adc.47.256.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Rogers GB, Hart CA, Mason JR, Hughes M, Walshaw MJ, Bruce KD. Journal of Clinical Microbiology. 2003;41:3548. doi: 10.1128/JCM.41.8.3548-3558.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Nature. 2000;407:762. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- (10).Fuqua C, Greenberg EP. Nat Rev Mol Cell Biol. 2002;3:685. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- (11).Smith RS, Iglewski BH. The Journal of Clinical Investigation. 2003;112:1460. doi: 10.1172/JCI20364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Smith RS, Harris SG, Phipps R, Iglewski B. Journal of Bacteriology. 2002;184:1132. doi: 10.1128/jb.184.4.1132-1139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Davies DG, Parsek MR, Pearson JP, Iglewski BH, Costerton JW, Greenberg EP. Science. 1998;280:295. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- (14).Pesci EC, Pearson JP, Seed PC, Iglewski BH. Journal of Bacteriology. 1997;179:3127. doi: 10.1128/jb.179.10.3127-3132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Grauer DC, Lehmann M, Meijler MM, Janda KD, Ulevitch RJ. Science. 2008;321:259. doi: 10.1126/science.1156499. [DOI] [PubMed] [Google Scholar]
- (16).Shiner EK, Rumbaugh KP, Williams SC. FEMS Microbiology Reviews. 2005;29:935. doi: 10.1016/j.femsre.2005.03.001. [DOI] [PubMed] [Google Scholar]
- (17).Kaufmann GF, Sartorio R, Lee S-H, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:309. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Lowery CA, Park J, Gloeckner C, Meijler MM, Mueller RS, Boshoff HI, Ulrich RL, Barry CE, Bartlett DH, Kravchenko VV, Kaufmann GF, Janda KD. Journal of the American Chemical Society. 2009;131:14473. doi: 10.1021/ja9056079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Royles BJL. Chemical Reviews. 1995;95:1981. [Google Scholar]
- (20).Schobert R, Schlenk A. Bioorganic and Medicinal Chemistry. 2008;16:4203. doi: 10.1016/j.bmc.2008.02.069. [DOI] [PubMed] [Google Scholar]
- (21).Romano AA, Hahn T, Davis N, Lowery CA, Struss AK, Janda KD, Böttger LH, Matzanke BF, Carrano CJ. Journal of Inorganic Biochemistry. 2012;107:96. doi: 10.1016/j.jinorgbio.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Erickson DL, Endersby R, Kirkham A, Stuber K, Vollman DD, Rabin HR, Mitchell I, Storey DG. Infect. Immun. 2002;70:1783. doi: 10.1128/IAI.70.4.1783-1790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Middleton B, Rodgers HC, Camara M, Knox AJ, Williams P, Hardman A. FEMS microbiology letters. 2002;207:1. doi: 10.1111/j.1574-6968.2002.tb11019.x. [DOI] [PubMed] [Google Scholar]
- (24).Chambers CE, Visser MB, Schwab U, Sokol PA. FEMS Microbiology Letters. 2005;244:297. doi: 10.1016/j.femsle.2005.01.055. [DOI] [PubMed] [Google Scholar]
- (25).Favre-Bonté S, Pache J-C, Robert J, Blanc D, Pechère J-C, van Delden C. Microbial Pathogenesis. 2002;32:143. doi: 10.1006/mpat.2001.0487. [DOI] [PubMed] [Google Scholar]
- (26).U.S. Department of Health and Human Services. F. D. A.; 2001. [DOI] [PubMed] [Google Scholar]
- (27).Charlton TS, De Nys R, Netting A, Kumar N, Hentzer M, Givskov M, Kjelleberg S. Environmental Microbiology. 2000;2:530. doi: 10.1046/j.1462-2920.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- (28).Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Fligner CL, Singh PK. Proceedings of the National Academy of Sciences. 2012 doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Voynow JA, Rubin BK. CHEST Journal. 2009;135:505. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- (30).Morin D, Grasland B, Vallée-Réhel K, Dufau C, Haras D. Journal of Chromatography A. 2003;1002:79. doi: 10.1016/s0021-9673(03)00730-1. [DOI] [PubMed] [Google Scholar]
- (31).Ortori C, Dubern J-F, Chhabra S, Cámara M, Hardie K, Williams P, Barrett D. Analytical and Bioanalytical Chemistry. 2011;399:839. doi: 10.1007/s00216-010-4341-0. [DOI] [PubMed] [Google Scholar]
- (32).Gamage AM, Shui G, Wenk MR, Chua KL. Microbiology. 2011;157:1176. doi: 10.1099/mic.0.046540-0. [DOI] [PubMed] [Google Scholar]
- (33).Gould TA, Herman J, Krank J, Murphy RC, Churchill MEA. J. Bacteriol. 2006;188:773. doi: 10.1128/JB.188.2.773-783.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Lépine F, Déziel E. In: Quorum Sensing: Methods and Protocols. Rumbaugh KP, editor. Vol. 692. Humana Press; 2011. p. 61. [Google Scholar]
- (35).Kumari A, Pasini P, Daunert S. Analytical and Bioanalytical Chemistry. 2008;391:1619. doi: 10.1007/s00216-008-2002-3. [DOI] [PubMed] [Google Scholar]
- (36).Jeong Y-C, Moloney MG. The Journal of Organic Chemistry. 2011;76:1342. doi: 10.1021/jo102304y. [DOI] [PubMed] [Google Scholar]
- (37).Hughes N, Wong E, Fan J, Bajaj N. The AAPS Journal. 2007;9:E353. doi: 10.1208/aapsj0903042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kollipara S, Bende G, Agarwal N, Varshney B, Paliwal J. Chromatographia. 2011;73:201. [Google Scholar]
- (39).Pearson JP, Passador L, Iglewski BH, Greenberg EP. Proceedings of the National Academy of Sciences. 1995;92:1490. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Telford G, Wheeler D, Williams P, Tomkins PT, Appleby P, Sewell H, Stewart GSAB, Bycroft BW, Pritchard DI. Infection and Immunity. 1998;66:36. doi: 10.1128/iai.66.1.36-42.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Bell SC, Robinson PJ. Thorax. 2007;62:723. doi: 10.1136/thx.2006.060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Regelmann WE, Elliott GR, Warwick WJ, Clawson CC. American Journal of Respiratory and Critical Care Medicine. 1990;141:914. doi: 10.1164/ajrccm/141.4_Pt_1.914. [DOI] [PubMed] [Google Scholar]
- (43).Hoiby N. BMC Medicine. 2011;9:32. doi: 10.1186/1741-7015-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Macdonald NE. The Canadian journal of infectious diseases = Journal canadien des maladies infectieuses. 1997;8:335. doi: 10.1155/1997/617690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.