Abstract
Dengue is the most important mosquito-borne viral disease. No specific treatment or vaccine is currently available; traditional vector control methods can rarely achieve adequate control. Recently, the RIDL (Release of Insect carrying Dominant Lethality) approach has been developed, based on the sterile insect technique, in which genetically engineered ‘sterile’ homozygous RIDL male insects are released to mate wild females; the offspring inherit a copy of the RIDL construct and die. A RIDL strain of the dengue mosquito, Aedes aegypti, OX513A, expresses a fluorescent marker gene for identification (DsRed2) and a protein (tTAV) that causes the offspring to die. We examined whether these proteins could adversely affect predators that may feed on the insect. Aedes aegypti is a peri-domestic mosquito that typically breeds in small, rain-water-filled containers and has no specific predators. Toxorhynchites larvae feed on small aquatic organisms and are easily reared in the laboratory where they can be fed exclusively on mosquito larvae. To evaluate the effect of a predator feeding on a diet of RIDL insects, OX513A Ae. aegypti larvae were fed to two different species of Toxorhynchites (Tx. splendens and Tx. amboinensis) and effects on life table parameters of all life stages were compared to being fed on wild type larvae. No significant negative effect was observed on any life table parameter studied; this outcome and the benign nature of the expressed proteins (tTAV and DsRed2) indicate that Ae. aegypti OX513A RIDL strain is unlikely to have any adverse effects on predators in the environment.
Introduction
Epidemic dengue fever and dengue haemorrhagic fever (DHF) have emerged as major global public health problems in recent decades. According to the World Health Organization (WHO) dengue epidemiology is rapidly worsening [1] with increased frequency of outbreaks and expansion into new geographical areas. This expansion has partly been driven by the rapid increase of the global range of Aedes aegypti in the last few decades. Ae. aegypti was eliminated from many areas of the world 40–50 years ago through the use of DDT but is now distributed more widely than it was before control began, and is now present in large urban areas where a greater number of people than in the past are at risk [2]. Failure to control the spread of Ae. aegypti has led to the re-emergence of the disease in many areas across the globe.
As for malaria there is no licensed vaccine for dengue, though several candidates are in various stages of trials. Unlike malaria, for dengue there are no specific therapeutic or prophylactic drugs. Control has therefore focused on the mosquito; however bed nets, widely used against malaria, are relatively ineffective for dengue as Ae. aegypti bites primarily in the day time [3]. Current control methods are therefore based primarily on breeding site elimination with larvicides or other methods, and some use of adulticides. These methods have not proven adequate to prevent epidemic dengue in any but the most favourable of circumstances [4], [5], [6]. More and better options for controlling Ae. aegypti are urgently required. The sterile insect technique (SIT) has been used for decades to control several insect pest species [7]. The technique mainly uses irradiation to sterilise the insects, however this appears to cause significant fitness effects on mosquitoes that prevent its widespread use for vector control [8], [9]. The release of insects with dominant lethality (RIDL) is a new method to control insects that replaces irradiation with the insertion of a conditional lethal gene [10], [11], [12]. The expression of the RIDL system is dependent on the absence of a suppressor (tetracycline) in the insects' diet. In the presence of tetracycline, expression is suppressed and the insects survive. The mechanism of sterility is the transmission to the progeny of a lethal transgene; equivalent to the transmission of radiation-induced dominant lethal mutations in classical SIT.
A line of Ae. aegypti (OX513A) has been developed that causes death of the mosquitoes at L4/pupae stage in the absence of tetracycline [13]. The protein tTAV is a codon optimised version of tTA for more efficient expression in insects [14] and is part of the positive feedback system in RIDL, developed from the well-known tet-off gene expression system [15], [16]. This system has been widely used in gene expression studies in mice [17], [18], [19], rats [20] and many different mammalian cell lines [21]. Only high level intra-cellular expression of tTA causes cell death, presumably via transcriptional squelching [15], [20], [22] and the levels that may be ingested by a predator eating mosquitoes would be predicted to have no potential adverse effects. The Ae. aegypti line also expresses a fluorescent marker protein DsRed2, for identification. DsRed2 is a member of the GFP superfamily of fluorescent proteins [23], [24]. DsRed2 has been used in a wide variety of transgenic organisms, including plants, insects and mice and is not expected to be harmful by ingestion [25], [26], [27], [28], [29].
As both tTA and DsRed2 are introduced proteins expressed in OX513A Ae. aegypti larvae, we asked the question if they could adversely affect potential predators that ingested the insect. Choice of a representative from the guild of potential predators in the invertebrate ecosystem is important as not all predator species can be tested in the laboratory [30], [31], consequently surrogate test species have to be used that are representative of potential non-target organisms in the field. An ideal surrogate test species would be amenable to testing under laboratory conditions, available, ecologically relevant, and sensitive to the substance under test, and in the case of oral exposure studies be capable of consuming significant quantities of test substance without gastric imbalance. Toxorhynchites is a predatory mosquito whose larvae feed on other aquatic invertebrates including mosquito larvae and has been used in attempts to control mosquitoes [32], [33], [34], [35]. Ae. aegypti tends to breed in small pools of water in and around human habitation as the females almost exclusively feed on humans [36]. These breeding sites are predominantly man made, plastic containers, water storage containers, discarded rubbish etc. fed from rain water, or human-filled [37], [38], [39]. These types of breeding sites do not contain many predators and to our knowledge there is no predator that exclusively feeds on Ae. aegypti larvae [40], [41]. However Toxorhynchites can be fed exclusively on Ae. aegypti larvae, is easily maintained in the laboratory, is a natural predator of Aedes species and therefore represented a credible test species from the guild of predators.
To test if Toxorhynchites was affected by feeding on OX513A larvae two different species, Tx. splendens and Tx. amboinensis, were fed on each of several types of Ae. aegypti larvae: wild type (WT), OX513A reared off tetracycline and OX513A reared on tetracycline. OX513A when reared off tetracycline expresses the tTAV protein at a higher level than when reared in the presence of tetracycline (on-tet); these two treatments therefore provide different doses of tTAV. Toxorhynchites life table parameters of larval development, survival, fecundity and size were compared between the different treatments.
Results
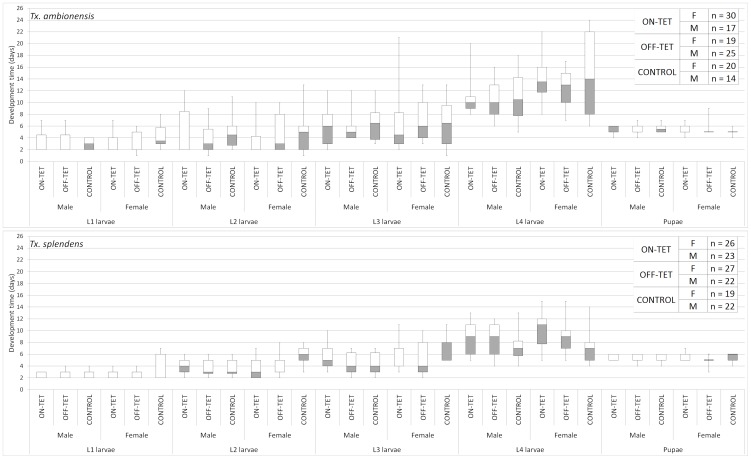
There was no significant difference between larval or pupal development time for any of the treatments or the two different species of Toxorhynchites (Figure 1 and Table S1). There was a significantly longer development time of L4 male and female larvae (identified from individuals that survived to adults) in the control fed group of Tx. amboinensis compared to Tx. splendens. However there was a trend for longer development of L4 larvae in Tx. amboinensis throughout the treatments suggesting that this species has a slightly longer development time for this stage. Larvae that did not survive to adulthood could not readily be identified as male or female. The survival of each life stage in the unclassified group was more variable due to some larvae remaining at a particular developmental stage for longer than normal before death. The reason for this delayed development is unknown but the proportion surviving to adults was not significantly different between treatment groups for Tx. splendens (χ2 = 4.0, d.f. = 2, p = 0.13). However for Tx. amboinensis the control treatment did have significantly less overall survival to adults than the OX513A on-tet or OX513A off-tet treatments (χ2 = 6.4, d.f. = 1, p<0.05, data not shown). This was due to one of the repeats of the control treatment having significantly lower survival than the other two repeats. The cause of this low survival is unknown and was not reflected in other treatments set up at the same time. Excluding the results of this repeat removed the significant difference in survival so we conclude that this result was due to one aberrant control treatment.
Figure 1. Box plot summary of development time (days) of different life stages.
Minimum and maximum development time are shown by vertical lines, the upper and lower quartiles are shown by the bottom and top of box respectively, the median is represented by horizontal line inside box; where the median value is the same as the upper and lower quartile the top of the gray or the bottom of the white box represents the median. Individuals for which sex could not be determined due to death prior to adult emergence were excluded from this analysis, these unclassified individuals represented at most 43% of each type and averaged 26.6% (see Table S1 for complete dataset. There was a significant difference in L4 larval development time between Tx. amboinensis and Tx. splendens.
In both Toxorhynchites species there were significantly more larvae consumed in the off tetracycline treatments; Tx. amboinensis (t = 9.2, p<0.001) and Tx. splendens (t = 8.3, p<0.001). However OX513A larvae when reared off tetracycline die at L4/pupal stage, to compensate more third instar (L3) larvae were used to provide the equivalent mass of fourth instar (L4) larvae that would have been used. This is reflected in the number of larvae consumed by L4 Toxorhynchites, on average L3 Ae. aegypti larvae are about one-third the weight of L4 larvae (in a parallel experiment, L3 larvae averaged 0.830 µg (+/−0.017ug) wet weight and L4 larvae 2.995 µg (+/−0.024)) and the number of L4 larvae consumed in the off-tet experiment was approximately 3–4 times on-tet and control experiments (data not shown). Therefore we attribute the variance in number of larvae consumed to the different feeding regimes used between the treatments.
Tx. amboinensis females reared on WT larvae consumed significantly more larvae than females fed on OX513A larvae reared on-tetracycline (t = −3.3, p<0.002). We don't know why this treatment consumed more larvae but there was no significant difference in any other parameters.
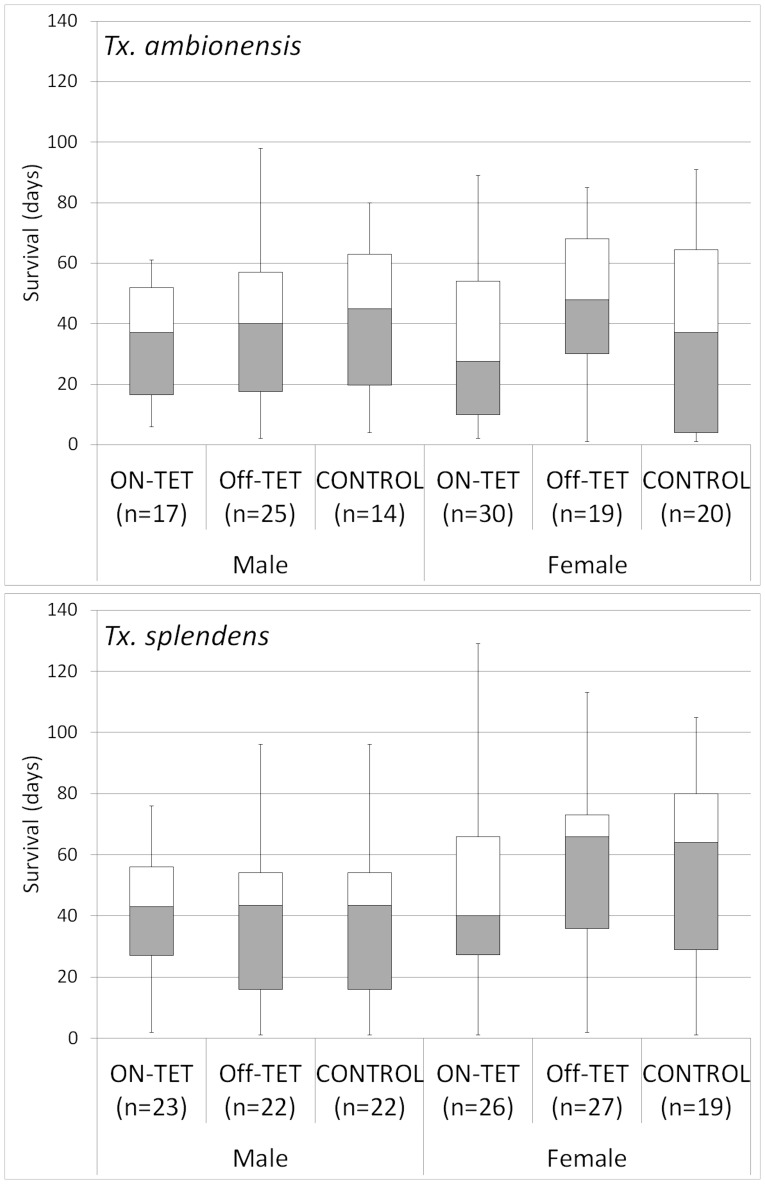
Adult survival is summarised in Figure 2. There was no significant difference in the survival of male (Tx. splendens χ2 = 1.0, d.f. = 2, p = 0.60 and Tx. amboinensis χ2 = 0.5, d.f. = 2, p = 0.76 ) and female (Tx. splendens χ2 = 2.6, d.f. = 2, p = 0.28 and Tx. amboinensis χ2 = 2.5 d.f. = 2, p = 0.29) adults across treatment groups for both species of Toxorhynchites.
Figure 2. Box plot of adult survival (days).
Minimum and maximum survival are shown by vertical lines, the upper and lower quartiles are shown by the bottom and top of box respectively, the median is represented by horizontal line inside the box. No significant difference was observed for adult survival between treatments or species.
The number of eggs laid per female for both Toxorhynchites species across all treatment groups did not significantly differ (see Table S1). However because of the large variation in egg production between individual females only relatively strong effects would likely have been detected by this assay.
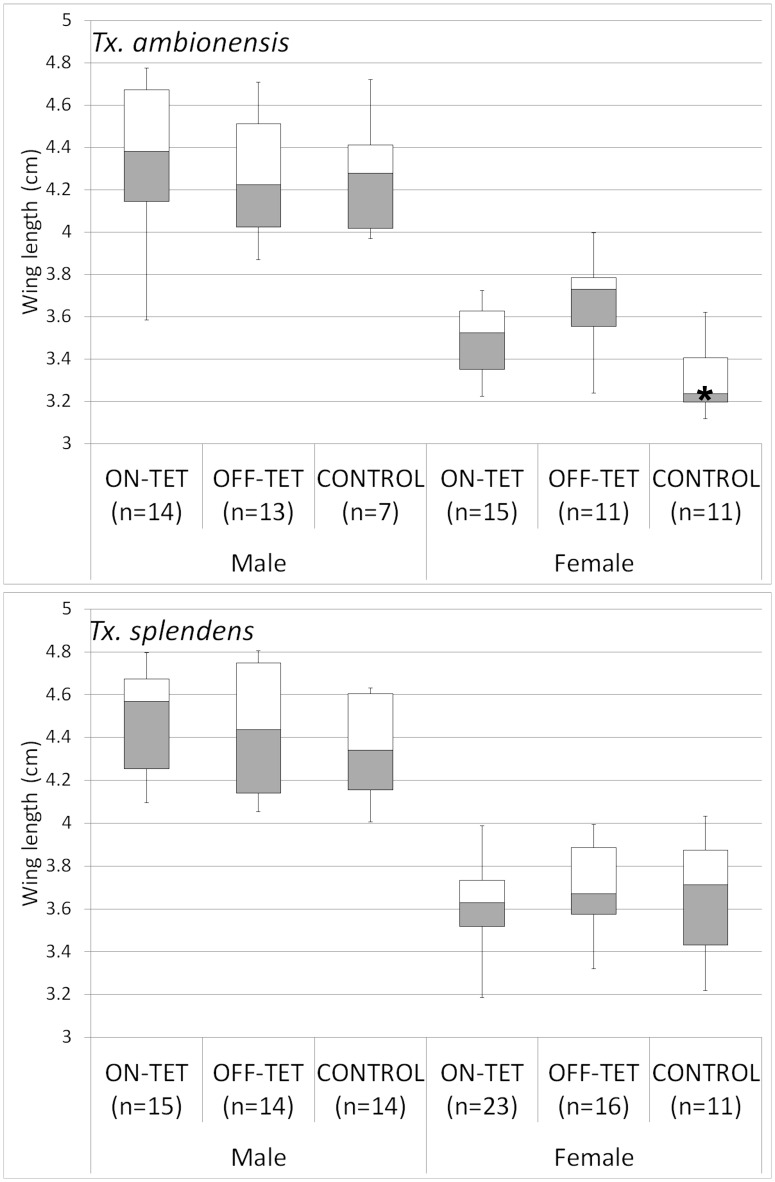
The size of the Toxorhynchites adults was determined from wing length measurements (Figure 3) and the only significantly different result was females from Tx. amboinensis control treatment were smaller than females from the off-tet treatment group (t = −3.1, p = 0.012). We are unsure why this group was significantly smaller but the difference is small and this is the same control group where one repeat had low survival. The females from this low survival group were smaller than usual however removing them from the analysis does not change the overall result; t = 2.06, p = 0.048.
Figure 3. Wing length results summarised in a box plot.
Wing lengths for each adult are the average of the left and right wing measurements. Minimum and maximum wing lengths are shown by vertical lines, the upper and lower quartiles are shown by the bottom and top of box respectively, the median is represented by the horizontal line inside the box. Females that were fed on WT larvae (Control) were significantly smaller than females that were fed on RIDL larvae reared off-tetracycline (OFF-TET), highlighted by asterisk. No other significant differences were observed.
We also examined Toxorhynchites fed on RIDL larvae for presence of the RIDL transgene by testing the adults by PCR. This test looked for unexpected persistence of the transgene, which might have indicated horizontal gene transfer (HGT), among other possibilities. A total of 121 adults gave DNA of sufficient quality to test, as judged by amplification of a control DNA fragment; none were positive for the transgene. On average each Toxorhynchites larvae consumed 431 RIDL larvae, thus there were over 52,000 events that had the potential for horizontal gene transfer; however none were detected in the adults tested. Comparative genomics and other considerations imply that HGT rates are expected to be extremely low, many orders of magnitude below the limits of sensitivity of this experiment [42], [43]. That we detected no such events is therefore not surprising but it does suggest that no unrecognised high-efficiency mechanism for DNA persistence or transfer exists in this case.
Discussion
In a control programme, RIDL male mosquitoes are released into the environment and subsequently mate with wild females. Those wild females that have mated a RIDL male may then lay eggs and the resulting larvae die before adulthood due to the lack of tetracycline in the environment. Predators that feed on these larvae or pupae are then exposed to the transgene and its products, e.g. encoded protein(s), raising the question of whether this exposure might have any potential adverse effects on such predators.
The positive feedback RIDL system is repressed by tetracycline, however off tetracycline there is increased expression of mRNA, up to 672 fold increase in homozygotes [14] and in OX513A death occurs in L4 larvae and pupae [13]. The aim of feeding Toxorhynchites on OX513A larvae was to investigate any effects of the transgene and/or the marker (DsRed2) on development. A significant advantage of using this predator was the ability to feed it exclusively on mosquito larvae (100% of diet) without expecting this restricted diet itself to have a negative effect on development or other parameters measured.
In separate experiments, OX513A larvae reared on-tet or off-tet were used, with equivalent wild type controls. This allows us to identify potential effects of high level expression of tTAV – produced in OX513A under off-tet conditions only – from other potential effects of the transgene, the only other obvious difference between the two treatments being the presence of tetracycline. In fact no negative effects were detected from feeding larvae reared either on or off-tet.
The transcriptional activator tTA has been used in several mammalian species and does not have any adverse effects unless expressed in large amounts and in various tissues [20]. Numerous experimental uses of tTA show that the effect of expression is cell-autonomous, i.e. only affects those cells in which the tTA protein is expressed. Dietary tTAV is not expected to have an effect due to considerations of the amount of biologically active protein potentially available and the lack of a mechanism for intact uptake of this protein to a relevant subcellular compartment. DsRed2 belongs to family of fluorescent proteins which are part of a group of proteins from the Anthozoa species. The protein family has been widely used in a variety of species, including plants, insects and mammals without adverse effects as well as subject to an evaluation by the FDA for food safety [29]. These factors lead to a lack of potential hazard from the ingestion by predators eating mosquito larvae or adults.
Furthermore, mosquitoes in aggregate are not a major diet component for vertebrates [40], [41], and Ae. aegypti is a relatively low-density species even in areas where it is epidemiologically important because of its anthropophagic nature. Each of these factors further indicates very low maximum exposure for predators and scavengers in the field relative to the 100% diet used in the experiments reported here.
Conclusion
Both Tx. splendens and Tx. amboinensis showed no adverse effects of being fed OX513A larvae either reared on tetracycline or off tetracycline compared to being fed non-transformed Ae. aegypti larvae. Although some significant variation was observed, partly due to species, no evidence was found that indicated the OX513A larvae had adverse effects on the development, fecundity and longevity of two species of Toxorhynchites larvae. No transfer of transgene DNA between the species was observed. These results show that Ae. aegypti OX513A RIDL strain is unlikely to have any adverse effects on predators in the environment.
Materials and Methods
Two different strains of Toxorhynchites were used, Tx. splendens originally isolated from Thailand and Tx. amboinensis originally isolated from Hawaii. Both of these species have been maintained at the Institute for Medical Research (IMR), Kuala Lumpar for 811 generations and 834 generations for Tx. splendens and Tx. amboinensis respectively. Both species were maintained at 25°C (+/−1°C) with 80% (+/−10%) humidity and fed on Ae. aegypti WT larvae. The Aedes aegypti transgenic strain used in this experiment was OX513A [13], produced in 2002 and subsequently made homozygous for the transgene. OX513A had been reared in the lab for more than 60 generations and maintained at 27oC (+/−1°C) and 80% (+/−10%) relative humidity. The wild type Ae. aegypti strain was isolated from Jinjang, Selangor, Malaysia in 1960. The OX513A insertion was originally in a Rockefeller strain background [13]. Prior to this study, that OX513A had been backcrossed into this Malaysian wild type strain background for 5 generation and then made homozygous for OX513A; ∼97% of its genome is expected to derive from the Malaysian wild type strain [44], [45].
Three treatments were used; Toxorhynchites fed on WT, OX513A reared on tetracycline (BioBasic Inc, 64-75-5) and OX513A reared without tetracycline. Tetracycline was added at a concentration of 30 µg/ml after hatching and not refreshed. Each treatment had twenty repeats each containing a single Toxorhynchites larva in a small circular plastic cup (7.5cm deep, 8.5cm diameter) half filled with tap water. Each treatment set was prepared simultaneously for both species of Toxorhynchites and three repeats were independently performed.
Ae. aegypti larval preparation for feeding Toxorhynchites; the eggs of Ae. aegypti for all treatments were hatched under vacuum and all larvae were reared at 1 larvae per ml and with equal amounts of food (Tetramin® flake fish food). The Ae. aegypti larvae were maintained at a level of 20 per Toxorhynchites larva by replenishing those that had been eaten daily. The larvae that were replenished were matched in developmental stage to the Toxorhynchites larvae. In the case of OX513A larvae that were reared off-tetracycline there were few L4 larvae available, many die at this stage and Toxorhynchites does not feed on dead larvae, so an equivalent mass of L3 larvae was added.
The duration of each developmental stage was recorded daily. The Toxorhynchites larvae from each treatment that survived to pupae were placed into separate cages (23cm X 23cm X 23cm). Females were provided with a plastic container filled with tap water for egg laying and 10% sucrose solution (including 1% vitamin B complex). Females were provided with 5–8 males from the stock colony. The number of eggs was recorded daily along with survival. After death the wing length was recorded [46].
PCR for presence of transgene in adult Toxorhynchites: Genomic DNA was extracted from single adult or late larval individuals using the GeneJET genomic DNA purification kit from Fermentas, according to the kit protocol. Genomic DNA was diluted 1 in 20 with water, and 1 µl of this dilution used in a 20 µl PCR reaction using Dreamtaq polymerase and buffer (Fermentas). Primers 38DrosF (ATGAGCAATTAGCATGAACGTT) and 48HspdiagR (GCAGATTGTTTAGCTTGTTCAGC) were used to amplify a fragment of the OX513 transgene (1233bp product). An OX513A RIDL Ae. aegypti control gDNA was included in each PCR reaction along with water negative control. In addition, all samples were amplified with primers 894AeMuAcF (CAGGGTGTGATGGTCGGTATGGG) and 895AeMuAcR (CCCAGGAAGGATGGCTGGAAGAG), which amplify endogenous muscle actin (660bp product), to check gDNA quality. For both primer sets, PCR conditions were: 94°C for 2 min's followed by 10 cycles of 94°C for 15s, 55°C (decreasing by 0.5°C per cycle) for 40s and 72°C for 1 min; followed by 25 cycles of 94°C for 15s, 50°C for 40s and 72°C for 1 min, with a final elongation step of 72°C for 7 min's.
Results were statistically analysed using STATA (version 12, College Station, TX, USA). All variables were assessed for normality. Experimental repeats were examined to determine if they could be combined for the final analysis. Differences in wing length across treatment group were compared using ANOVA and t-test. The non-parametric equivalents, Kruskal-Wallis and Mann-Whitney tests, were used to compare egg-counts and longevity across treatment groups. The proportion of individuals surviving to become adults was examined using Chi-squared test.
Supporting Information
Summary of results. The table shows the mean and (in brackets) standard deviation for each of the parameters measured, for Tx. spendens and Tx. amboinensis fed on WT (control), OX513A reared off tetracycline (OX513A OFF TET) and OX513A reared on tetracycline (OX513A ON TET). The results for females (F), males (M) and those individuals that did not survive to adults for identification of sex (U) are shown for each treatment. Because of the large variation in results from larvae and pupae that died (U) they have been excluded from statistical analysis; except for overall larval survival. Significantly different results discussed in the text are indicated by symbols; * and # for significantly different results within species and ¥ for between species.
(DOC)
Acknowledgments
We thank the Director-General of Health, Malaysia for permission to publish and Director, Institute for Medical Research, Kuala Lumpur for support and to staff of Medical Entomology Unit, IMR for technical assistance rendered. We also thank Pam Gray for proof reading the final draft of the manuscript.
Funding Statement
This project was financially supported by a Malaysian National Institutes of Health Research Grant (JPP-IMR-06-053); a Biotechnology and Biological Sciences Research Council training grant (BB/I015957/1); and a grant from the National Institutes of Health from the Ministry of Health, Malaysia (code: JPP-IMR 06-053). Oxitec Ltd. provided salary and other support for the research program of those authors employed by the company. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO/TDR (2009) Dengue. Guidelines for Diagnosis, Treatment, Prevention and Control - New Edition [Google Scholar]
- 2. Gubler DJ (2004) Cities spawn epidemic dengue viruses. Nature Med 10: 129–130. [DOI] [PubMed] [Google Scholar]
- 3. Yasuno M, Tonn RJ (1970) A study of biting habits of Aedes aegypti in Bangkok, Thailand. Bull World Health Organ 43: 319–325. [PMC free article] [PubMed] [Google Scholar]
- 4. Jirakanjanakit N, Rongnoparut P, Saengtharatip S, Chareonviriyaphap T, Duchon S, et al. (2007) Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J Econ Entomol 100: 545–550. [DOI] [PubMed] [Google Scholar]
- 5. Nam VS, Yen NT, Phong TV, Ninh TU, Mai LQ, et al. (2005) Elimination of dengue by community programs using Mesocyclops(Copepoda) against Aedes aegypti in central Vietnam. Am J Trop Med Hyg 72: 67–73. [PubMed] [Google Scholar]
- 6. Vu SN, Nguyen TY, Kay BH, Marten GG, Reid JW (1998) Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am J Trop Med Hyg 59: 657–660. [DOI] [PubMed] [Google Scholar]
- 7.Dyck V, Hendrichs J, Robinson A, editors (2005) Sterile Insect Technique: principles and practice in area-wide Integrated Pest Management. Dordrecht: Springer. 787 p.
- 8. Helinski ME, Parker AG, Knols BG (2006) Radiation-induced sterility for pupal and adult stages of the malaria mosquito Anopheles arabiensis . Malar J 5: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catteruccia F, Crisanti A, Wimmer EA (2009) Transgenic technologies to induce sterility. Malar J 8 Suppl 2 S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alphey L, Andreasen M (2002) Dominant lethality and insect population control. Mol Biochem Parasitol 121: 173–178. [DOI] [PubMed] [Google Scholar]
- 11. Alphey L, Nimmo D, O'Connell S, Alphey N (2008) Insect population suppression using engineered insects. Adv Exp Med Biol 627: 93–103. [DOI] [PubMed] [Google Scholar]
- 12. Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, et al. (2010) Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis 10: 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Phuc HK, Andreasen MH, Burton RS, Vass C, Epton MJ, et al. (2007) Late-acting dominant lethal genetic systems and mosquito control. BMC Biol 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gong P, Epton MJ, Fu G, Scaife S, Hiscox A, et al. (2005) A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nat Biotechnol 23: 453–456. [DOI] [PubMed] [Google Scholar]
- 15. Gossen M, Bujard H (1992) Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A 89: 5547–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas DD, Donnelly CA, Wood RJ, Alphey LS (2000) Insect population control using a dominant, repressible, lethal genetic system. Science 287: 2474–2476. [DOI] [PubMed] [Google Scholar]
- 17. Kistner A, Gossen M, Zimmermann F, Jerecic J, Ullmer C, et al. (1996) Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc Natl Acad Sci U S A 93: 10933–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furth PA, St Onge L, Boger H, Gruss P, Gossen M, et al. (1994) Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc Natl Acad Sci U S A 91: 9302–9306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schonig K, Bujard H (2003) Generating conditional mouse mutants via tetracycline-controlled gene expression. Methods Mol Biol 209: 69–104. [DOI] [PubMed] [Google Scholar]
- 20. Zhou H, Huang C, Yang M, Landel CP, Xia PY, et al. (2009) Developing tTA transgenic rats for inducible and reversible gene expression. Int J Biol Sci 5: 171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baron U, Bujard H (2000) Tet repressor-based system for regulated gene expression in eukaryotic cells: principles and advances. Methods Enzymol 327: 401–421. [DOI] [PubMed] [Google Scholar]
- 22. Gill G, Ptashne M (1988) Negative effect of the transcriptional activator GAL4. Nature 334: 721–724. [DOI] [PubMed] [Google Scholar]
- 23. Matz M, Fradkov A, Labas Y, Savitsky A, Zaraisky A, et al. (1999) Fluorescent proteins from nonbioluminescent Anthozoa species. Nat Biotechnol 17: 969–973. [DOI] [PubMed] [Google Scholar]
- 24. Shagin DA, Barsova EV, Yanushevich YG, Fradkov AF, Lukyanov KA, et al. (2004) GFP-like proteins as ubiquitous Metazoan superfamily: evolution of functional features and structural complexity. Mol Biol Evol 21: 841–850. [DOI] [PubMed] [Google Scholar]
- 25. Vintersten K, Monetti C, Gertsenstein M, Zhang P, Laszlo L, et al. (2004) Mouse in red: red fluorescent protein expression in mouse ES cells, embryos, and adult animals. Genesis 40: 241–246. [DOI] [PubMed] [Google Scholar]
- 26. Nishizawa K, Kita Y, Kitayama M, Ishimoto M (2006) A red fluorescent protein, DsRed2, as a visual reporter for transient expression and stable transformation in soybean. Plant Cell Reports 25: 1355–1361. [DOI] [PubMed] [Google Scholar]
- 27. Wenck A, Pugieux C, Turner M, Dunn M, Stacy C, et al. (2003) Reef-coral proteins as visual, non-destructive reporters for plant transformation. Plant Cell Reports 22: 244–251. [DOI] [PubMed] [Google Scholar]
- 28. Richards HA, Han CT, Hopkins RG, Failla ML, Ward WW, et al. (2003) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. J Nutr 133: 1909–1912. [DOI] [PubMed] [Google Scholar]
- 29.Pavely C, Fedorova M (2006) Early Food Safety Evaluation for a Red Fluorescent Protein:DsRed2. Pioneer Hi-Bred International.
- 30.Smrchek JC, Zeeman MG (1998) Assessing risks to ecological ecosystems from chemicals. In: P CP, editor. Handbook of Environmental Risk Assessment and Management: Blackwell. pp. 24–91. [Google Scholar]
- 31. Romeis J, Hellmich RL, Candolfi MP, Carstens K, De Schrijver A, et al. (2011) Recommendations for the design of laboratory studies on non-target arthropods for risk assessment of genetically engineered plants. Transgenic Res 20: 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nyamah MA, Sulaiman S, Omar B (2011) Field observation on the efficacy of Toxorhynchites splendens (Wiedemann) as a biocontrol agent against Aedes albopictus (Skuse) larvae in a cemetery. Trop Biomed 28: 312–319. [PubMed] [Google Scholar]
- 33. Dominic Amalraj D, Das PK (1998) Estimation of predation by the larvae of Toxorhynchites splendens on the aquatic stages of Aedes aegypti . Southeast Asian J Trop Med Public Health 29: 177–183. [PubMed] [Google Scholar]
- 34. Dominic Amalraj D, Sivagnaname N, Das PK (2005) Effect of food on immature development, consumption rate, and relative growth rate of Toxorhynchites splendens (Diptera: Culicidae), a predator of container breeding mosquitoes. Mem Inst Oswaldo Cruz 100: 893–902. [DOI] [PubMed] [Google Scholar]
- 35. Focks DA, Sackett SR, Dame DA, Bailey DL (1985) Effect of weekly releases of Toxorhynchites amboinensis (Doleschall) on Aedes aegypti (L.) (Diptera: Culicidae) in New Orleans, Louisiana. J Econ Entomol 78: 622–626. [DOI] [PubMed] [Google Scholar]
- 36. Jones JC, Pilitt DR (1973) Blood-feeding behavior of adult Aedes aegypti mosquitoes. Biol Bull 145: 127–139. [DOI] [PubMed] [Google Scholar]
- 37. Koenraadt CJM, Aldstadt J, Kijchalao U, Sithiprasasna R, Getis A, et al. (2008) Spatial and temporal patterns in pupal and adult production of the dengue vector Aedes aegypti in Kamphaeng Phet, Thailand. Am J Trop Med Hyg 79: 230–238. [PubMed] [Google Scholar]
- 38. Chadee DD, Huntley S, Focks DA, Chen AA (2009) Aedes aegypti in Jamaica, West Indies: container productivity profiles to inform control strategies. Trop Med Int Health 14: 220–227. [DOI] [PubMed] [Google Scholar]
- 39. Morrison AC, Gray K, Getis A, Astete H, Sihuincha M, et al. (2004) Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol 41: 1123–1142. [DOI] [PubMed] [Google Scholar]
- 40. Campos RE, Lounibos LP (2000) Life tables of Toxorhynchites rutilus (Diptera: Culicidae) in nature in southern Florida. J Med Entomol 37: 385–392. [DOI] [PubMed] [Google Scholar]
- 41. Blum S, Basedow T, Becker N (1997) Culicidae (Diptera) in the diet of predatory stages of Anurans (Amphibia) in humid biotopes of the Rhine Valley in Germany. J Vector Ecol 22: 23–29. [PubMed] [Google Scholar]
- 42. Bertolla F, Simonet P (1999) Horizontal gene transfers in the environment: natural transformation as a putative process for gene transfers between transgenic plants and microorganisms. Res Microbiol 150: 375–384. [DOI] [PubMed] [Google Scholar]
- 43. Nielsen KM (1998) Barriers to horizontal gene transfer by natural transformation in soil bacteria. APMIS Suppl 84 77–84. [DOI] [PubMed] [Google Scholar]
- 44. Lacroix R, McKemey AR, Raduan N, Kwee Wee L, Hong Ming W, et al. (2012) Open Field Release of Genetically Engineered Sterile Male Aedes aegypti in Malaysia. PLoS One 7: e42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee HL, Vasan S, Ahmad N, Idris I, Hanum N, et al. (2013) Mating compatibility and competitiveness of transgenic and wild type Aedes aegypti (L.) under contained semi-field conditions. Transgenic Research 22: 47–57. [DOI] [PubMed] [Google Scholar]
- 46.Harbach RE, Knight KL (1980) Taxonomist's Glossary of Mosquito Anatomy: Marlton, NJ, Plexus Publications.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of results. The table shows the mean and (in brackets) standard deviation for each of the parameters measured, for Tx. spendens and Tx. amboinensis fed on WT (control), OX513A reared off tetracycline (OX513A OFF TET) and OX513A reared on tetracycline (OX513A ON TET). The results for females (F), males (M) and those individuals that did not survive to adults for identification of sex (U) are shown for each treatment. Because of the large variation in results from larvae and pupae that died (U) they have been excluded from statistical analysis; except for overall larval survival. Significantly different results discussed in the text are indicated by symbols; * and # for significantly different results within species and ¥ for between species.
(DOC)