Abstract
Choosing to wait for a better outcome (delay choice) and sustaining the delay prior to that outcome (delay maintenance) are both prerequisites for successful self control in intertemporal choices. However, most existing experimental methods test these skills in isolation from each other, and no significant correlation has been observed in performance across these tasks. In this study we introduce a new paradigm, the hybrid delay task, which combines an initial delay choice with a subsequent delay maintenance stage. This allows testing how often choosing to wait is paired with the actual ability to do so. We tested 18 capuchin monkeys (Cebus apella) from two laboratories in various conditions, and we found that subjects frequently chose the delayed reward but then failed to wait for it, due to poor delay maintenance. However, performance improved with experience and different behavioral responses for error correction were evident. These findings have far reaching implications: if such a high error rate was observed also in other species (possibly including Homo sapiens), this may indicate that delay choice tasks that make use of salient, prepotent stimuli do not reliably assess generalized self control, insofar as choosing to wait does not entail always being able to do so.
Keywords: Intertemporal choice, Self control, Delay maintenance, Capuchins
1. INTRODUCTION
Intertemporal choices are ubiquitous in everyday life. Both human and non-human animals often have to decide between options available at different times, such as choosing whether to save money for the future rather than spending it immediately (Frederick, Loewenstein & O’Donoghue 2002) or waiting for a fruit to ripen rather than eating it unripe (Stevens & Stephens 2008). Exerting self control in intertemporal choices requires two distinct but intertwined capacities: being able to choose a better delayed option over a poorer one which is immediately available (delay choice), and being capable of sustaining the inhibition required to acquire the better option, instead of reversing one’s previous choice whenever faced by the temptation of doing so (delay maintenance). Dieting in humans provides a standard example: to successfully diet, one has first to prefer an improved physical health over the pleasure of eating at will, and then be steadfast in keeping this resolution when faced with an appealing but unhealthy food. Similarly, extracting a highly nutritive but hard to crack nut from its shell requires first choosing this long-term reward over more readily available but lesser ones, and then persevering in its pursuit when tempted or distracted by other appetitive stimuli.
Existing experimental protocols typically address either delay choice or delay maintenance in isolation from each other. Delay choice is measured in the most common forms of self control testing (called the intertemporal choice, temporal discounting, or delay choice test), where an organism is faced with one of two choices, to be made in the present: take a smaller (or less preferred) reward available sooner or wait for a larger (or more preferred) reward that will be available later. Once the choice is made it cannot be altered at any time during the trial. A large variety of species have been tested in this delay choice paradigm, including insects (Cheng, Peña, Porter & Irwin 2002), birds (Logue & Peña-Correal 1985; Chelonis, King, Logue & Tobin 1994; Mazur 2007), rodents (van Haaren, van Hest & van de Poll 1988; Tobin, Chelonis & Logue 1993; Green & Estle 2003), nonhuman primates (Tobin, Logue, Chelonis, Ackerman & May 1996; Stevens, Hallinan, & Hauser 2005; Addessi, Focaroli & Paglieri 2011; Stevens & Mühlhoff 2012), and humans (Green, Fry & Myerson 1994; Logue, Forzano & Ackerman 1996; Lawyer, Williams, Prihodova, Rollins & Lester 2010).
Delay maintenance is measured in another type of self control test (called the delay of gratification or delay maintenance task), in which an organism must maintain a course of action in the face of continual competition from the available, impulsive response. In this test situation, the immediate reward remains accessible throughout the trial, so that acquiring the delayed reward requires continuous inhibition of impulsive responses to the immediate option. Most studies of delay maintenance in humans focused on children (Toner & Smith 1977; Mischel, Shoda & Rodriguez 1989; but see Young, Webb & Jacobs 2011 for an example involving human adults), and these protocols have been modified for use with animals (Grosch & Neuringer 1981; Killeen, Smith & Hanson 1981; Beran, Savage-Rumbaugh, Pate & Rumbaugh 1999). In one of these tests (hereafter referred to as the accumulation task), a series of valuable items is progressively transferred within reach of the subjects at a fixed rate, and the process continues as long as they refrain from taking or touching the increasingly larger reward amount. However, once they take or touch the accumulated reward, no further items are added. So, if subjects want to obtain the maximum amount of reward available in a given session, they have to refrain from touching it until the transfer is completed. To date, species tested using this paradigm include chimpanzees (Pan troglodytes; Beran 2002; Beran & Evans 2006; 2009; Evans & Beran 2007a; Evans, Beran, Paglieri & Addessi, 2012), bonobos (Pan paniscus; Stevens, Rosati, Heilbronner & Mühlhoff 2011), orangutans (Pongo pygmaeus; Beran 2002), macaques (Macaca mulatta; Evans & Beran 2007b; Macaca tonkeana; Pelé, Micheletta, Uhlrich, Thierry & Dufour 2011), capuchin monkeys (Cebus apella; Anderson, Kuroshima & Fujita 2010; Pelé et al. 2011; Addessi, Paglieri, Beran, Evans, Macchitella, De Petrillo & Focaroli submitted; Evans et al. 2012), squirrel monkeys (Saimiri sciureus; Anderson et al. 2010), and African grey parrots (Psittacus erithacus; Vick, Bovet & Anderson 2010).
Crucially, delay choice tasks do not test for the ability to sustain the chosen delay because subjects cannot reverse their choice once they have made it; conversely, delay maintenance paradigms do not include any prior choice, since the overall amount of reward achievable is set by the experimenter and not chosen by the subject. Thus, we lack evidence on how well subjects can tolerate waiting for a delayed prize of their own choosing. To date, no study directly analyzed delay maintenance after a previous delay choice, to check whether a response that indicates preference for a delayed reward is consistently followed by the capacity to maintain such delay in the face of temptation.
Indeed, existing evidence suggests a lack of correlation between delay choice performance and delay maintenance skills in experimental situations. When both delay maintenance and delay choice tasks were administered to the same group of preschool children, there was no consistent correlation between performance in these two tasks across genders (Toner, Holstein & Heterington 1977), and a recent meta-analysis of 282 multi-method samples to examine the convergent validity of self control measures in adult subjects found only a moderate degree of convergence among different types of delay task, including both delay choice and delay maintenance (Duckworth & Kern 2011). Similarly, in non-human primates, the same species can perform relatively well in delay choice tasks but rather poorly in delay maintenance tasks. For instance, capuchin monkeys (Cebus apella) often make what appear to be self-control choices in a delay choice task (Addessi et al. 2011) but not in a delay maintenance task (Anderson et al. 2010); conversely, long-tailed macaques (Macaca fascicularis) perform more poorly in a delay choice task (Amici, Aureli & Call 2008; Tobin, Logue, Chelonis, Ackerman & May 1996), but performed reasonably well in a delay maintenance task (Pelé, Dufour, Micheletta & Thierry 2010). When we tested the same population of capuchins used for this study in both delay choice and delay maintenance tasks, their performance failed to correlate across tasks in three conditions out of four (for further details, see Addessi et al. submitted).
However, the absence of a consistent correlation between these kinds of tests should be interpreted with caution, for two reasons: first, only two studies to date (Toner et al. 1977, with children; Addessi et al. submitted, with capuchins) tested the same population in both delay choice and delay maintenance tasks, whereas all other evidence is based on comparing different studies performed on different samples; second, delay choice and delay maintenance were always tested separately, using distinct tasks. This leaves open the possibility that, even if in general the ability to sustain a delay does not correlate with the propensity to opt for a delayed reward, subjects would still be capable of tolerating a delay of their own choosing, that is, after they had previously chosen to wait. In other words, we do not know whether choosing to wait versus being forced to wait makes a difference in the subject's ability to sustain the ensuing delay. If it does, then standard delay choice tasks would arguably provide a good estimate of delay tolerance, even if they do not control for subsequent delay maintenance performance.
This is a relevant concern because the delay choice task has two limits that could result in an inflated estimate of subjects’ self control. First, there is reason to doubt in some versions of the task that preferring the larger delayed option is an indication of self control at the time of choice. This is because, in versions of the task in which the choice options are represented by prepotent rewards such as food items, the larger delayed option could also be the target of an impulsive response based purely on larger quantity or better quality. However, versions of the delay choice task that involve choice options represented by arbitrary icons or objects (e.g., levers in an operant chamber) are free of this criticism. Second, direct inhibition of the prepotent response is not needed during the waiting time, since the chosen reward is not accessible to the subjects until the appropriate time – as opposed to what happens in delay maintenance tasks. In other words, in the delay choice task that involves visible prepotent rewards, a failure to inhibit the impulsive response “go for more” could be mistaken for a far-sighted preference for the delayed option, and even such preference could not be indicative of an effective capacity to sustain the corresponding delay, if given the chance to reverse one’s previous choice.
In light of these considerations, it is important to address two issues:
How many choices of the delayed option are in fact motivated by a failed attempt to inhibit the prepotent response towards the larger reward?
When subjects opt for the larger delayed reward, how often would they be able to actually wait to get it, if they were given the chance to reverse their choice before receiving the reward?
To empirically investigate both issues, we designed a new protocol, henceforth referred to as the hybrid delay task. In this task, the subject is presented with a choice between a smaller option available immediately (SS, sooner and smaller), and a larger one available only after a certain delay (LL, larger and later). If the subject chooses SS, this is immediately delivered to him/her; if, on the contrary, the subject chooses LL, then the experimenter starts accumulating the corresponding reward in front of the subject at a fixed rate. Accumulation stops, however, as soon as the subject starts eating the reward items; so, to achieve the whole delayed reward, the subject must choose the LL option but also refrain from taking and eating the accumulating contents of that choice option for the whole duration of the accumulation process.
This procedure combines delay choice and delay maintenance, and it allows one to measure how many times a choice for the larger delayed reward is in fact combined with the behavioral capacity to sustain such delay, and how often instead the subject makes errors in the overall performance, by choosing LL but then failing to accumulate at least the same amount of reward offered immediately with SS. Such instances can be unambiguously classified as errors, because the subject could have obtained an equal or larger reward immediately, instead of suffering a certain delay to get a smaller outcome: this violates intake rate maximization, using a fixed trial duration protocol (as we did in this study, see General Procedure), and also implies a negative discount rate for gains, in stark contrast with all existing evidence on delay discounting.
Being able to identify clearly irrational choices constitutes a further novelty of this procedure, with respect to standard delay choice and delay maintenance tasks. Once identified, errors in the hybrid task can be diagnosed in two different ways: (i) the LL choice reflects a failure to inhibit a prepotent response towards the larger option (choice failure, see point 1 above), or (ii) the LL choice indicates a preference for the delayed option, but subjects do not have enough inhibitory control to implement that plan when given access to the reward during delay (maintenance failure, see point 2 above). To disambiguate between these two possible explanations, we introduced a symbolic version of the task, in which the choice stimuli were replaced by abstract stimuli that subjects had learned to associate with the corresponding amounts of food. Because symbolic stimuli eliminate the perceptual saliency of the larger quantity, they should also reduce prepotent responses to the larger prize (see Boysen & Berntson 1995, for a discussion of how symbolic stimuli might release animals from prepotent responses in tasks requiring inhibition). Therefore, a reduced number of errors in the symbolic condition would suggest that such errors were in fact caused, at least partially, by an inhibitory failure at the time of choice; on the contrary, a lack of improvement in the symbolic condition would indicate that inhibitory control is failing in the delay maintenance phase of the task, where subjects do not manage to accumulate as many items as they would like to.
Based on the existing evidence reviewed above, we predicted that:
Subjects would make many errors, showing that delay choice tasks involving food rewards are likely to overestimate self control in intertemporal choices in this species;
The distribution of these errors across food and symbolic conditions would reveal their root cause – either a prepotent response in the choice phase or poor delay maintenance in the accumulation phase.
In addition, we were also interested to see whether capuchin monkeys could master the rather intricate contingency of this task, and whether and how they would try to correct their errors over repeated sessions. Previous studies on delay commitment by Rachlin and colleagues (e.g., Rachlin & Green 1972; Rachlin 1995) have shown that, under certain conditions, both pigeons and humans are capable of countering their propensity to opt for a smaller sooner prize by advanced commitment to a course of action that avoids that choice and guarantees the larger later reward. A further advantage of the hybrid delay task is that it allows the subject to implement two distinct behavioral changes for error correction: either reducing the number of LL reward responses in the choice phase, given one’s inability to reap the rewards of such a plan (give-up solution), or improving performance in the accumulation phase, while continuing to choose the LL reward with the same frequency in the choice phase (improvement solution). This offers insight, not only into the limits of self control in a given species or sample of subjects, but also on how such limits might be overcome with experience.
2. MATERIALS AND METHODS
2.1 Subjects
At the Primate Center of the ISTC-CNR, in Rome, we tested 10 adult capuchins: 5 males and 5 females, mean age 17 years, range 9–29. Each group was housed in indoor–outdoor compartments, and individuals were tested in an indoor testing compartment. Monkeys were not food deprived for testing. The main meal took place in the afternoon when fresh fruits, vegetables, eggs, monkey chow, and cheese porridge were provided. Water was available ad libitum. All monkeys had a large amount of experience with several different cognitive tasks. Of particular relevance were experiments on intertemporal choice (Addessi et al. 2011; Addessi et al. in preparation) and delay of gratification (Addessi et al., submitted). This study complied with protocols approved by the Italian Health Ministry. All procedures were performed in full accordance with the European law on humane care and use of laboratory animals and conformed to the “Guidelines for the use of animals in research.”
At the Language Research Center of Georgia State University, in Atlanta, we tested 8 capuchins: 5 males and 3 females, mean age 11 years, range 5–20 years. Monkeys were group housed with indoor-outdoor access, but were separated into individual enclosures for testing. Monkeys had 24-hour access to water and were fed manufactured chow and various fruits and vegetables daily between 1600 and 1800 hours. Like the Rome monkeys, prior to the current study, Atlanta monkeys had participated in experiments on intertemporal choice (unpublished data) and delay of gratification (Evans et al. 2012). This study complied with protocols approved by the Georgia State University IACUC. All procedures were performed in full accordance with the USDA Animal Welfare Act and conformed to the “Guidelines for the use of laboratory animals”.
2.2 Apparatus
In Rome and in Atlanta, as shown in Figure 1, the apparatus consisted of a rolling cart (52.5 × 68.5 × 85 cm high) with a sliding shelf (34 × 48 cm) placed in front of the testing compartment and of a vertical Plexiglas panel (56.6 × 74 cm) inserted in place of one of three vertical mesh-walls of the testing compartment.
Figure 1.
Apparatus for the hybrid delay task
In the intertemporal choice phase, according to the condition (see below) either two food options were placed in two small dishes on the sliding shelf or two symbolic stimuli were placed directly on the sliding shelf, 15 cm apart. Subjects could choose one of the two options by inserting their finger in one of two poke-holes (diameter: 2 cm) of the vertical Plexiglas panel, located 9 cm from each side of the panel and 26 cm from the bottom of the panel. In order to train the subjects to indicate the preferred option by inserting the finger through the poke-holes, in a preliminary phase they were presented with 10-trial sessions in which they were required to choose between two non-test foods of different value (e.g., one raisin vs. one small piece of salad) until they reliably chose the preferred option in 9 of 10 trials in one session.
In the accumulation phase (see below), the experimenter placed or accumulated the food items (see below) in a Plexiglas pan (25 × 6.5 cm) attached on the experimenter’s side of the testing compartment at 14.5 cm from the bottom of the panel. The experimenter could either lock or unlock the pan by sliding a deadbolt; when it was unlocked, the subject could pull the pan, causing it to rotate towards the inside of the testing compartment and thus allowing the subject to grasp the rewards.
2.3 General procedure
The study consisted of four conditions: (1) Food with a 3-s accumulation rate for the LL reward choice (Food 3 s); (2) Food with a 6-s accumulation rate for the LL reward choice (Food 6 s); (3A) Symbolic stimuli with a 3-s accumulation rate for the LL reward choice (Symbolic 3 s); (3B) Food with a 3-s accumulation rate after symbolic stimuli (Post-symbolic food 3 s). All 18 subjects were tested in Condition 1, and then were assigned to the other experimental conditions depending on their performance in previous conditions: Table 1 summarizes which subjects participated in each condition. See below for a detailed description of the procedure for each condition.
Table 1.
Subjects tested in each condition
| Subject | Lab | 1: Food 3 s 10 sessions, N = 18 |
2: Food 6 s 10 sessions, N = 5 |
3A: Symbolic 3 s 5 sessions, N = 10 |
3B: Post-symbolic Food 3 s 5 sessions, N = 9 |
|---|---|---|---|---|---|
| Carlotta | Rome | x | x | x | |
| Drella | Atlanta | x | |||
| Gabe | Atlanta | x | |||
| Gal | Rome | x | x | ||
| Griffin | Atlanta | x | x | ||
| Liam | Atlanta | x | x | x | |
| Lily | Atlanta | x | x | ||
| Logan | Atlanta | x | |||
| Nala | Atlanta | x | x | ||
| Paprica | Rome | x | x | x | |
| Pedro | Rome | x | x | x | |
| Pippi | Rome | x | x | x | |
| Robin Hood | Rome | x | x | ||
| Robinia | Rome | x | x | x | |
| Robot | Rome | x | x | ||
| Sandokan | Rome | x | x | x | |
| Saroma | Rome | x | x | x | |
| Wren | Atlanta | x | x | x |
Each trial consisted of two phases: the intertemporal choice phase and the accumulation phase. In the intertemporal choice phase, the subject received a choice either between two food quantities (4 vs. 12 identical food items) or between two symbolic stimuli (see Condition 3A for details) that represented 4 and 12 food items. The side with the larger option was counterbalanced across trials. The smaller food quantity was immediately available, whereas the larger food quantity could be entirely obtained only if the monkey waited for the experimenter to place each food item, one-at-a-time, into the Plexiglas pan during the accumulation phase (see below). Previous studies on numerical judgments in capuchin monkeys (e.g., Addessi, Crescimbene & Visalberghi 2008; Beran, Evans, Leighty, Harris & Rice 2008; Evans, Beran, Harris & Rice 2009; vanMarle, Aw, McCrink & Santos 2006) demonstrated that the quantities used in this study are well within the discriminatory capacity of this species; moreover, the present experiment used many of the monkeys tested in those studies.
In the intertemporal choice phase, the experimenter (i) unlocked the Plexiglas pan by sliding the deadbolt, (ii) rolled the cart up to the test compartment so that the rolling cart touched the bottom of the vertical Plexiglas panel and was centered in front of the subject, (iii) pulled the sliding shelf all the way to the back of the rolling cart and, according to the condition (see below), either baited two small dishes with food items or placed two symbolic stimuli on the sliding shelf, taking care to ensure that the dishes or the stimuli lined up with the poke-holes on the Plexigals panel, (iv) slid the shelf towards the subject, (v) noted the subject’s selection (the side pointed to first) and started the 120-s trial-timer (see below), and (vi) removed the unselected option. If the subject did not make a selection within 10 seconds, the experimenter pulled back the shelf, waited three seconds, and then pushed the shelf forward again. If the subject required three or more opportunities to make a selection on two consecutive trials, then the session was aborted.
After the subject chose one of the two options, the accumulation phase started. If the subject chose the smaller option, in the accumulation phase the experimenter immediately placed all the chosen food items (or the amount of food corresponding to the chosen symbolic stimulus) into the Plexiglas pan, and the monkey could immediately eat that food. If, in contrast, the subject chose the larger option, in the accumulation phase the experimenter began transferring food items, one at a time, after each time interval (depending on the condition, see below) into the unlocked Plexiglas pan until the subject started eating them. No more items were presented once a monkey ate any of the accumulated items. The experimenter was instructed not to make eye contact with the animals and keep still during testing, to minimize chances of inadvertently cuing the subjects; moreover, potential cuing was also difficult to define, as there was no expectation of whether the monkeys should choose the SS or LL reward, or whether they should be able to wait through the accumulation phase.
To avoid intake rate issues, we used a fixed-trial duration. The trial was timed from the choice point and, regardless of which option the monkey selected, the experimenter waited to begin the next trial until 120 seconds elapsed. Thus, there were three different possible behaviors: (i) the monkey selected the smaller option, received all four food items immediately, and then waited until 120 s elapsed and the next choice was presented, (ii) the monkey selected the larger option and waited for some portion of the food items to accumulate before eating them, then waited for the remainder of the 120 s interval before the next choice was presented, and (iii) the monkey selected the larger option and waited for all 12 food items to accumulate, then used the remaining time to eat the accumulated rewards.
In each condition, we scored which option the monkey chose in the intertemporal choice phase and, after choices of the larger option, how many food items accumulated before the monkey began eating those items. The study was conducted between January and July of 2011.
2.4 Condition 1. Food with a 3-s accumulation rate (Food 3 s)
Condition 1, Food 3 s, consisted of 10 sessions of eight trials each, including two initial forced trials (in which the subject was presented with only one option, either the small one or the large one), followed by six binary choice trials (in which the subject was presented with 4 vs. 12 food items: in Rome we used pieces of peanut weighing on average 0.2 grams; in Atlanta we used 45 mg banana-flavored, grain-based pellets – Bio-Serv, Frenchtown, NJ, USA). In a forced choice trial involving the large option, the food was accumulated at a 3 s rate in the locked Plexiglas pan, which was unlocked only after transferring all 12 food items: this allowed the subjects to experience the accumulation rate characteristic of this condition and that they could obtain more food if they waited for it to accumulate. In a forced choice trial involving the small option, the food was transferred into the locked pan all at once before allowing the subject to take it. In the binary choice trials, if the subject chose the smaller option, the experimenter would place it in the unlocked Plexiglas pan all at once, and if the subject chose the larger option, the experimenter would transfer it into the unlocked Plexiglas pan at a rate of 1 item every 3 s until a food item was eaten by the monkey.
Subjects were considered successful in this condition if they had a mean error rate lower than 30% in the last 5 sessions. An error was defined as the choice of the larger delayed quantity (i.e., 12 food items) in the intertemporal choice phase followed by the accumulation of less items than those offered with the smaller immediate option (i.e., 3 items or less) in the accumulation phase. The error rate was calculated for each session as the proportion of errors over the number of trials in which the larger option was selected. In contrast, trials where the subject either accumulated 4–12 items of the larger reward, or opted for the smaller immediate prize, were both considered correct.
2.5 Condition 2. Food with a 6-s accumulation rate (Food 6 s)
Only the subjects who were successful in Food 3 s (see above) were tested in Condition 2, Food 6 s. The procedure was the same as in Food 3 s, with the only exception that in the accumulation phase the experimenter accumulated the food items at a 6 s rate. This allowed us to examine longer delay intervals to determine what effect that might have on either the delay choice component or the delay maintenance component of the task.
2.6 Condition 3A. Symbolic stimuli with a 3-s accumulation rate (Symbolic 3 s)
The monkeys that failed in Food 3 s (i.e., that had a mean error rate higher than 30% in the last five sessions) were tested in Condition 3A, Symbolic 3 s. The procedure was the same as in Food 3 s, with the only exception that in the delay choice phase the monkey was presented with choices between two symbolic stimuli, representing 4 and 12 food items, respectively. As stimuli, we used two square cards of identical dimensions (8 cm × 8 cm), including one with a pattern of two by two black-and-white checkers and one with a dense speckled pattern of gray dots on a white background. The assignment of the two types of symbolic stimuli to the two food quantities was counterbalanced across monkeys. After the monkey’s choice in the intertemporal choice phase, the experimenter removed the unselected stimulus. If the monkey chose the stimulus corresponding to the smaller food amount, the experimenter placed 4 food items, all at once, into the Plexiglas pan. If the monkey chose the stimulus corresponding to the larger food amount, the experimenter began placing the 12 food items into the pan at a rate of one every 3 s.1
Before proceeding to Symbolic 3 s, we had to train monkeys to choose between the symbolic stimuli by inserting the finger through the poke-holes. In a preliminary phase they were presented with forced-choice trials in a minimum of four 8-trial sessions (4 trials for each stimulus type). First, we placed the corresponding amount of food behind each stimulus, in view of the monkey, and we presented sessions like this until the monkey reliably and quickly pointed to the 12-item stimulus in every trial of 2 consecutive sessions. Second, we presented the stimuli without baiting them with food amounts, and we assessed the same performance criterion as above. This allowed us to confirm that monkeys understood which symbolic stimulus led to which amount of food. Three animals in Atlanta failed to pass the criterion in the training phase and were therefore excluded from Symbolic 3 s.
Symbolic 3 s consisted of 5 sessions of eight trials each, beginning with two forced trials (in which the monkey was presented with only one type of stimulus, either the one corresponding to the small food option or the one corresponding to the large food option), and then six binary choice trials (in which the monkey was presented with choices between the two stimuli). Trial presentations were the same as those given with food items in Food 3 s.
2.7 Condition 3B. Food with a 3-s accumulation rate after symbolic stimuli (Post-symbolic food 3 s)
All monkeys that were tested in Symbolic 3 s were subsequently tested in Condition 3B, Post-symbolic food 3 s.2 The procedure was exactly the same as in Food 3 s, with the only exception that 5 sessions were run. The goal of this condition was to determine if the experience with symbolic stimuli would improve performance with actual food items for monkeys that originally did not show efficient responding in Food 3 s, or if whatever change in performance observed in Symbolic 3 s would be contingent on the presence of symbolic stimuli and thus disappear in this experiment.
3. RESULTS
Comparing performance between individuals from our two populations (Rome and Atlanta) revealed interesting differences in delay tolerance, and yet several basic patterns were observed in both samples, providing evidence of their generality. Although Atlanta capuchins chose the delayed reward more often than Rome capuchins in most conditions, and also demonstrated a better performance in the accumulation phase (see below), the key results followed the same trends across both populations. Both Rome and Atlanta capuchins made a substantial percentage of errors in all conditions, and both groups showed the capacity to flexibly correct such errors over time. The fact that these findings were replicated in two populations with obvious differences in delay attitude provides a strong indication of their generality.
Table 2 summarizes mean values and SEs for error rate, percentage of choices for the delayed option in the delay choice phase, and number of items accumulated in the delay maintenance phase. All 18 subjects took part in the first condition: of these, the 5 successful subjects were moved to the Food 6 s condition (F3s → F6s); of the remaining 13, only 9 were moved to the Symbolic 3 s condition and later on to the Post-symbolic Food 3 s condition (F3s → S3s → PSF3s), whereas the other 4 did not perform any other condition, either because they failed to learn the symbolic association between stimuli and food rewards (3 subjects), or due to experimenter error (1 subject). Table 2 is organized according to this sampling of subjects, so that row values can be meaningfully compared across conditions.
Table 2.
Aggregate results: % of errors, % of choices for LL option in delay choice phase, number of items accumulated in the delay maintenance phase. Means ± SE for all subjects tested in each experiment, sampled according to the conditions performed by each subject for ease of comparison.
| Sample | 1: Food 3 s 10 sessions |
2: Food 6 s 10 sessions |
3A: Symbolic 3 s 5 sessions |
3B: Post-symbolic Food 3 s 5 sessions |
|
|---|---|---|---|---|---|
| % error | All (N=18) | 61.4 ± 7.1 | - | - | - |
| F3s → F6s (N=5) | 32.7 ± 11.4 | 28.3 ± 8.2 | - | - | |
| F3s → S3s → FPS3s (N=9) | 78.0 ± 8.2 | - | 40.1 ± 12.2 | 25.7 ± 11.4 | |
| % LL choices | All (N=18) | 59.3 ± 2.4 | - | - | - |
| F3s → F6s (N=5) | 62.3 ± 5.7 | 41.3 ± 12.2 | - | - | |
| F3s → S3s → FPS3s (N=9) | 55.2 ± 2.3 | - | 59.3 ± 7.3 | 56.7 ± 6.1 | |
| accumulated items | All (N=18) | 3.6 ± 0.5 | - | - | - |
| F3s → F6s (N=5) | 5.9 ± 1.3 | 4.8 ± 1.2 | - | - | |
| F3s → S3s → FPS3s (N=9) | 2.4 ± 0.5 | - | 4.9 ± 0.8 | 6.7 ± 1.2 |
3.1 Percentage of errors
As shown in Table 2, capuchins tended to err frequently in this task: frequency of error was especially high in Condition Food 3s (over 60% for the whole sample), and, even when they performed most successfully (Post-symbolic Food 3 s), capuchins on average still made an error in one fourth of their choices for the delayed option. Looking at accumulated items, in most conditions capuchins managed to obtain slightly more than what would have been available by always opting for the SS option (4 food items). However, on average they still remained far below the maximum amount available in each trial of this task (12 food items).
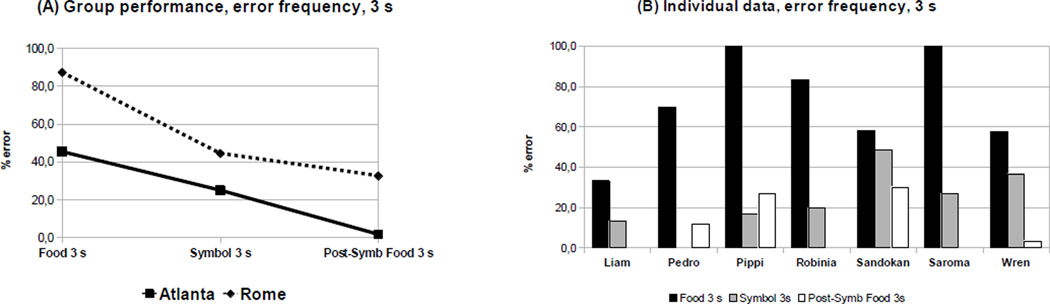
Looking at the 9 individuals who took part in all conditions with a 3 s rate of accumulation, a mixed-model ANOVA with condition (Food 3 s, Symbolic 3 s, and Post-symbolic food 3 s) as within-subject factor and laboratory (Rome and Atlanta) as between-subject factor showed a clear pattern of improvement for the percentage of errors, which decreased across conditions (F(2,14) = 8.85, p = 0.003; Figure 2A). Pairwise comparisons (Tukey HSD test) revealed that subjects made fewer errors in Symbolic 3 s and Post-symbolic Food 3 s than in Food 3 s. This pattern becomes even more obvious looking at individual data: Figure 2B shows the decrease in frequency of error for 7 individuals (out of 9) across all 3 s conditions. Even though Atlanta monkeys qualitatively tended to make fewer mistakes than those in Rome, there was no main effect of the laboratory on percentage of errors (F(1,7) = 2.20, p = 0.18).
Figure 2.
Decrease in error frequency in 3 s conditions: group performance (A) and individual data (B)
Regardless of laboratory, the introduction of a slower accumulation rate did not impact the percentage of error for the 5 individuals who reached the criterion in the first condition (Food 3 s) and were therefore tested in Food 6 s. A mixed-model ANOVA with condition (Food 3 s and Food 6 s) as within-subject factor and laboratory (Rome and Atlanta) as between-subject factor showed that the percentage of error and the overall amount of food items obtained did not significantly differ between conditions (F(1,3) = 0.07, p = 0.81), and that there was only a marginally significant effect of laboratory (F(1,3) = 7.41, p = 0.07).
3.2 Delay Choice and Delay Maintenance Performance
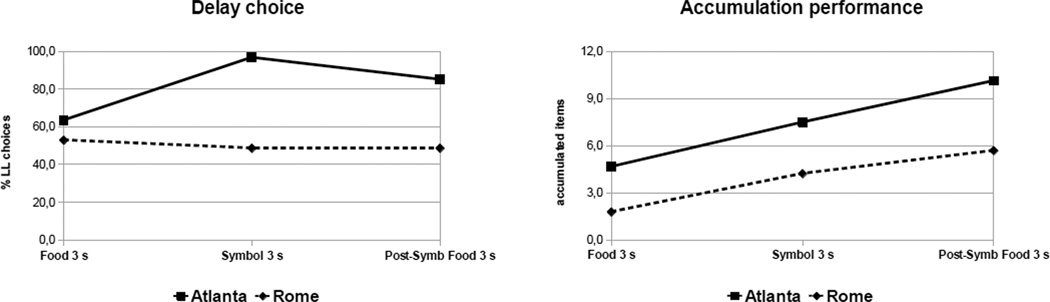
When considering the 9 individuals who took part in all conditions with a 3 s rate of accumulation, a mixed-model ANOVA with condition (Food 3 s, Symbolic 3 s, and Post-symbolic food 3 s) as within-subject factor and laboratory (Rome and Atlanta) as between-subject factor showed that for the percentage of LL choices there was a significant interaction between laboratory and condition (F(2,14) = 10.74, p = 0.001). Specifically, Atlanta capuchins chose the LL option significantly more than Rome capuchins in the Symbolic Food 3 s (p < 0.001) and in the Post-symbolic Food 3 s conditions (p < 0.001). A similar analysis of the number of accumulated items showed a main effect of condition (F(2,14) = 10.90, p = 0.001) and laboratory (F(1,7) = 7.37, p = 0.03), with no interaction. Atlanta capuchins accumulated more food items than Rome capuchins in all conditions.
Recalling that monkeys in 3 s conditions did improve performance with experience (i.e., that their percentage of errors decreased over time; see above), these findings explain how they did it: most capuchins improved their performance in the delay maintenance phase of the hybrid task, without significant changes in delay choice, thereby making fewer errors and obtaining a larger quantitative payoff. As shown in Figure 3, only Atlanta capuchins increased the percentage of LL choices in Symbolic 3 s and Post-symbolic Food 3 s, in comparison with Food 3 s, whereas both groups showed an increase in accumulation performance across conditions. This suggests that Atlanta capuchins not only managed to improve their performance in the task by improving their delay maintenance performance, but also came to choose more often the LL option. This is superior to the mere enhancement of delay maintenance noted before.
Figure 3.
Mean performance of both populations across three conditions (Food 3 s, Symbolic 3 s, Post-Symbolic Food 3 s) for delay choice (left panel) and accumulation (right panel)
The 5 individuals who moved to Food 6 s after being successful in Food 3 s reduced their propensity to opt for the larger reward only marginally (F(1,3) = 7.36, p = 0.07), whereas no significant effect was observed on accumulation performance (F(1,3) = 0.72, p = 0.46). There was also a marginally significant main effect of laboratory on the percentage of LL choices (F(1,3) = 9.43, p = 0.05) but no significant interaction between laboratory and condition (F(1,3) = 1.98, p = 0.25).
3.3 Change in Performance across Sessions
Finally, as shown in Table 3, we looked at how behavior changed across sessions within each condition, for both populations.3 Error correction, measured by a decrease in error frequency, was present in both groups but was more marked for Rome capuchins than for Atlanta capuchins. The same is true for the gradual increase in accumulation performance. This is not surprising: since individuals in Atlanta were performing better than those in Rome from the onset, the latter had a greater potential for improvement, which is reflected by these trends across sessions. Another similarity between populations concerns the reaction to a slower accumulation rate: both groups reduced across sessions their percentage of LL choices in Food 6 s, suggesting a process of adaptation to the new test conditions.
Table 3.
Changes in performance across sessions in each condition for both groups (within-subject regressions)
| Food 3 s | Food 6 s | Symbolic 3 s + Post-symbolic Food 3 s |
||
|---|---|---|---|---|
| % error | Rome | Decrease | Decrease | Decrease |
| t100 = −5.90, p < 0.001 | t30 = −2.22, p = 0.04 | t70 = −2.30, p = 0.03 | ||
| Atlanta | NS | NS | Decrease | |
| t80 = 0.07, p = 0.95 | t20 = 0.02, p = 0.98 | t20 = −3.64, p = 0.002 | ||
| % LL choices | Rome | NS | Decrease | NS |
| t100 = −1.80, p = 0.08 | t30 = −3.45, p = 0.002 | t70 = 0.10, p = 0.92 | ||
| Atlanta | NS | Decrease | NS | |
| t80 = 1.82, p = 0.07 | t20 = −2.14, p = 0.05 | t20 = −1.77, p = 0.09 | ||
| accumulated items | Rome | Increase | NS | Increase |
| t100 = 4.45, p < 0.001 | t30 = −1.35, p = 0.19 | t70 = 2.64, p = 0.01 | ||
| Atlanta | NS | NS | Increase | |
| t80 = 1.05, p = 0.30 | t20 = −0.85, p = 0.41 | t20 = 4.31, p < 0.001 | ||
4. DISCUSSION
The main finding of our study is that capuchin monkeys, as predicted, made many errors in the hybrid delay task, frequently failing to accumulate enough items to justify their choice of the larger delayed option. This was true for both the populations we tested, in spite of significant differences in their intertemporal behavior. In three out of four conditions, individual error rates were high also for Atlanta capuchins that showed on average a stronger propensity to opt for delayed rewards and better delay maintenance skills than Rome capuchins. Regardless of baseline delay tolerance in a population, for capuchin monkeys choosing to wait does not entail the ability to do so, if given the opportunity to terminate trials by taking less food than was possible to obtain. This shows that delay choice might tell us whether a subject would like to wait for a certain prize, but not whether s/he may be actually capable of doing so, if faced by tempting alternatives – as we often are. This should serve as a cautionary tale for the literature on intertemporal choice in humans and non-human animals: isolated delay choice tasks should not be interpreted as measuring delay tolerance, but rather as indicating only willingness to attempt to delay gratification. Whether or not this finding generalizes beyond capuchins remains to be seen and requires testing other species in the hybrid task. However, the high incidence of errors does not seem to depend on a general inability of capuchins to improve their objective levels of performance (as measured by food items obtained) in the task, given that performance often improved substantially over time, and 9 subjects out of 18 managed to reduce their error rate over the last five sessions below 15% (with a 3 s rate: Griffin, Liam, Lily, Robin Hood, Robinia, Robot, Saroma, Wren; with a 6 s rate: Gal, Griffin).
Crucially, choosing the LL reward but then failing to accumulate at least the amount of rewards represented by the SS reward cannot be interpreted as mere inconsistency in temporal preferences due to non-exponential delay discounting, as it is sometimes discussed in the literature on intertemporal choice (e.g., Frederick et al. 2002), because here no reason for a preference shift is apparent. Hyperbolic and quasi-hyperbolic discount models predict that preferences between SS and LL rewards might reverse when the former becomes temporally proximate. However, in the hybrid task the temporal proximity of the smaller option does not change between choice and accumulation: the SS offered at the onset is just as proximate as the fraction of LL reward available at any time during the delay maintenance phase. Hence, no discount model with minimally stable discount rates can explain why a subject would renounce an immediate prize SS for the possibility of accumulating LL, and then forsake this possibility for an even smaller immediate prize, that is, any fraction of LL reward lower than SS. For instance, why should a rational agent refuse 4 food items now for the prospect of getting 12 food items in 36 s, and then 6 s later accept only 2 food items over the possibility of getting all 36 items after another 30 s? Any discount rate compatible with opting for LL in the former choice is incompatible with preferring SS in the latter, and vice versa (for further discussion, see the Supplementary Materials). Lacking any reason to assume rapid shifts in discount rates across these choices, the most parsimonious interpretation is to regard similar patterns as genuine mistakes.
Our results also suggest that errors in the hybrid delay task are mostly due to an inability to sustain the delay associated with the larger option once the subject is given the possibility of grabbing the food rewards during the waiting time (maintenance failure). This is consistent with the fact that capuchins tended to improve accumulation performance over time when the inter-item interval was shorter (3 s), and this often resulted in a reduction of error rate (the only exception was in Food 3 s, where Atlanta capuchins who did not reach the criterion neither improved in accumulation nor reduced their error rate). In contrast, we found less evidence of choice failure, that is, errors resulting from failed inhibition of a prepotent response for the larger quantity while choosing between the two options. If that was entirely the case, introducing symbolic stimuli in Symbolic 3 s should have facilitated inhibition of the prepotent response, thereby resulting in a reduction of error rate due to a drop in the number of LL choices: instead, symbolic stimuli did not affect delay choice behavior, and error reduction was continuous across all 3 s conditions and paralleled by an improvement in accumulation performance. This is not sufficient to exclude that the larger delayed option could still be the target of an impulsive response based purely on quantity, but it does show that maintenance failures were more frequent and more relevant than choice failures in determining error rate in our sample.
Capuchins often changed their behavior and thereby corrected their error rates. When the rate of accumulation was faster (3 s), animals tended to keep constant their choice behavior and improved instead their delay maintenance performance, thereby both reducing the error rate and improving the total quantitative payoff. Some of them even reacted to the fact that better accumulation skills justified opting for the delayed reward more often, and in fact these individuals (Wren and Liam) ended up with the best quantitative performance of the whole sample in their last condition (measured as the overall amount of food items obtained over a whole session). But the pattern was different with a slower accumulation rate (6 s), which made waiting for the larger reward much more demanding and possibly less appealing for monkeys. In the latter case, no improvement in accumulation was observed, whereas 4 out of 5 subjects (Griffin, Lily, Gal, and Robot) stopped choosing the LL option, as indicated by the decrease in the percentage of LL choices observed in Food 6 s. This also effectively reduced the error rate, since opting for the smaller immediate option was, by definition, not a mistake, but just a statement of preferences.4
The fact that capuchins successfully employed both means for error correction is impressive, especially considering that monkeys stopped trying to get the larger reward only when the accumulation rate became too slow to make the delayed option preferable or achievable. It is also worth noting that these error correction methods differ in terms of potential gains and associated risks. Not trying to get the larger reward is a safe approach, because once SS is chosen it is immediately and invariably obtained. On the other hand, when applied consistently, this method at best avoids losing food, with respect to the amount offered immediately. In contrast, improving one’s accumulation skills to try to obtain all the rewards offered by the LL option can potentially ensure a substantial gain (in this study, up to three times the amount of the SS reward), but can also result in a net loss if delay maintenance fails. It is, then, an open issue whether engaging one approach or the other may depend also on one’s feelings towards risk, in addition to awareness of one’s skills and tolerance for waiting.
5. CONCLUSIONS
The capacity to delay gratification and tolerate waiting times is rightly considered an important component of self control. It is paramount to have reliable measures of self control, and delay choice tasks have been frequently used for that purpose. In this paper we presented a novel methodology to study self control, the hybrid delay task, which combines an initial delay choice with a subsequent delay maintenance phase, to check whether a preference for a delayed outcome is stably followed by the ability to sustain the associated delay. Our results with a sample of 18 capuchin monkeys show that, in a significant proportion of choices, this is not the case. This suggests that delay choice and delay maintenance may actually rely on two separate regulatory processes: e.g., temporal discounting and sustained inhibition, respectively. Moreover, performance in different versions of the hybrid delay task allowed us to partially diagnose the main reason behind such repeated failure (poor accumulation skills), and to describe two different error correction behaviors successfully employed by several capuchins: either improving accumulation performance (with or without changing delay choice behavior), in conditions with a faster accumulation rate, or reducing the number of choices for the larger delayed option, in conditions with a slower accumulation rate.
These findings indicate that the hybrid delay task complements nicely existing methodologies to study self-control in intertemporal choices. Similarly to the studies on delay commitment pioneered by Rachlin and colleagues (Rachlin & Green 1972; Rachlin 1995), this new experimental paradigm generates data not only on the limits of self control, but also on what causes such limits and how individuals might adjust to cope with them. With respect to capuchins, we observed that, for most animals, a marked tendency to make mistakes was compensated by behavioral changes with increased experience, and they showed different behavioral changes depending on the rate of accumulation for the larger delayed reward. This constitutes a clear sign of behavioral flexibility, and demonstrates that understanding the contingencies of the task is well within the capabilities of this species.
Testing other species on the hybrid delay task will give us new insights into animals’ delay tolerance skills and their evolutionary patterns, and will serve to verify whether our findings with capuchins also generalize to other species, and to what extent. Because sustaining delay in the presence of tempting stimuli is presumably more arduous than simply postponing the acquisition of a larger reward to a later time, we expect to observe high error rates in the hybrid delay task also in other species, humans included. As Mischel and Ayduk succinctly put it, “the highly valued goals of the self too often turn into failed good intentions” (2002, p. 113).
Supplementary Material
Highlights.
Choosing to wait is often not followed by the actual ability to do so.
We introduced a new experimental method combining delay choice and delay maintenance.
We tested 18 capuchin monkeys (Cebus apella) with this new method.
Capuchins make many errors in this task but can flexibly correct them.
Delay choice must be paired with other inhibition tasks, to measure self-control.
ACKNOWLEDGMENTS
The work of FP, EA, VF, and VT was supported by an ISTC-CNR intramural grant. The research efforts of MJB, JM, JB, and TAE were supported by grants HD38051 and HD060563from the National Institute of Child Health and Human Development. The authors thank Betty Chan for assistance with data collection, Valentina Truppa and Gabriele Schino for statistical advice, and two anonymous reviewers for helpful comments on a previous version of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Due to experimenter error, one of the subjects in Atlanta was tested instead with an accumulation rate of 2 s for the larger delayed reward in Conditions 3A and 3B. Since this procedural difference did not seem to impact his test behavior, we retained his data for analysis.
The only exception to this was that one Atlanta monkey was unintentionally tested in Symbolic 3 s for 10 sessions and not tested in Post-symbolic food 3 s; thus, only her first 5 sessions in Symbolic 3 s were used for data analysis.
Only for the purpose of this analysis, we pooled together the data of conditions 3A and 3B (Symbolic 3 s and Post-symbolic Food 3 s), in order to have a sufficient number of sessions (10) to perform a meaningful regression analysis. This choice is also justified by the absence of differences in behavior between these two conditions, as discussed above.
This flexibility is here understood as a purely behavioral notion, without implying anything on the degree of awareness and/or control animals might have on the different behaviors that, in different contexts, resulted in error correction. Still, it is suggestive that such behaviors were well adapted to the contingencies of each condition: improving accumulation performance when the rate was not too onerous vs. stopping to choose the delayed reward when such rate became too slow. Future studies will be needed to ascertain whether this indicates a sophisticated understanding of task contingencies, resulting in deliberate strategic thinking, or just an associative response to the different features of the task.
REFERENCES
- Addessi E, Crescimbene L, Visalberghi E. Food and token quantity discrimination in capuchin monkeys (Cebus apella) Animal Cognition. 2008;11:275–282. doi: 10.1007/s10071-007-0111-6. [DOI] [PubMed] [Google Scholar]
- Addessi E, Paglieri F, Focaroli V. The ecological rationality of delay tolerance: Insights from capuchin monkeys. Cognition. 2011;119:142–147. doi: 10.1016/j.cognition.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Addessi E, Paglieri F, Beran M, Evans T, Macchitella L, De Petrillo F, Focaroli V. Delay choice vs. delay maintenance: Different measures of delayed gratification in capuchin monkeys. Journal of Comparative Psychology. doi: 10.1037/a0031869. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amici F, Aureli F, Call J. Fission-fusion dynamics, behavioral flexibility, and inhibitory control in primates. Current Biology. 2008;18:1415–1419. doi: 10.1016/j.cub.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Kuroshima H, Fujita K. Delay of gratification in capuchin monkeys (Cebus apella) and squirrel monkeys (Saimiri sciureus) Journal of Comparative Psychology. 2010;124:205–210. doi: 10.1037/a0018240. [DOI] [PubMed] [Google Scholar]
- Beran MJ. Maintenance of self-imposed delay of gratification by four chimpanzees (Pan troglodytes) and an orangutan (Pongo pygmaeus) Journal of General Psychology. 2002;129:49–66. doi: 10.1080/00221300209602032. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Maintenance of delay of gratification by four chimpanzees (Pan troglodytes): The effects of delayed reward visibility, experimenter presence, and extended delay intervals. Behavioural Processes. 2006;73:315–324. doi: 10.1016/j.beproc.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Evans TA. Delay of gratification by chimpanzees (Pan troglodytes) in working and waiting situations. Behavioural Processes. 2009;80:177–181. doi: 10.1016/j.beproc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beran MJ, Evans TA, Leighty KA, Harris EH, Rice D. Summation and quantity judgments of sequentially presented sets by capuchin monkeys (Cebus apella) American Journal of Primatology, 2008;70:191–194. doi: 10.1002/ajp.20474. [DOI] [PubMed] [Google Scholar]
- Beran MJ, Pate JL, Rumbaugh DM. Delay of gratification in chimpanzees (Pan troglodytes) Developmental Psychobiology, 1999;34:119–127. doi: 10.1002/(sici)1098-2302(199903)34:2<119::aid-dev5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Boysen S, Berntson G. Responses to quantity: perceptual versus cognitive mechanisms in chimpanzees (Pan troglodytes) Journal of Experimental Psychology, Animal Behavior Processes, 1995;21:82–86. doi: 10.1037//0097-7403.21.1.82. [DOI] [PubMed] [Google Scholar]
- Chelonis JJ, King G, Logue AW, Tobin H. The effect of variable delays on self control. Journal of the Experimental Analysis of Behavior. 1994;62:33–43. doi: 10.1901/jeab.1994.62-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Peña J, Porter M, Irwin J. Self-control in honeybees. Psychonomic Bulletin & Review. 2002;9:259–263. doi: 10.3758/bf03196280. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Kern ML. A meta-analysis of the convergent validity of self control measures. Journal of Research in Personality. 2011;45:259–268. doi: 10.1016/j.jrp.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Chimpanzees use self-distraction to cope with impulsivity. Biology Letters. 2007a;3:599–602. doi: 10.1098/rsbl.2007.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TA, Beran MJ. Delay of gratification and delay maintenance in rhesus macaques (Macaca mulatta) Journal of General Psychology. 2007b;134:199–216. doi: 10.3200/GENP.134.2.199-216. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Harris EH, Rice D. Quantity judgments of sequentially presented food items by capuchin monkeys (Cebus apella) Animal Cognition. 2009;12:97–105. doi: 10.1007/s10071-008-0174-z. [DOI] [PubMed] [Google Scholar]
- Evans TA, Beran MJ, Paglieri F, Addessi E. Delaying gratification for food and tokens in capuchin monkeys (Cebus apella) and chimpanzees (Pan troglodytes): When quantity is salient, symbolic stimuli do not improve performance. Animal Cognition. 2012;15:539–548. doi: 10.1007/s10071-012-0482-1. [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O’Donoghue T. Time discounting and time preference: A critical review. Journal of Economic Literature. 2002;40:351–401. [Google Scholar]
- Green L, Estle SJ. Preference reversals with food and water reinforcers in rats. Journal of the Experimental Analysis of Behavior. 2003;79:233–242. doi: 10.1901/jeab.2003.79-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Fry AF, Myerson J. Discounting of delayed rewards: A life-span comparison. Psychological Science. 1994;5:33–36. [Google Scholar]
- Grosch J, Neuringer A. Self-control in pigeons under the Mischel paradigm. Journal of the Experimental Analysis of Behavior. 1981;35:3–21. doi: 10.1901/jeab.1981.35-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR, Smith JP, Hanson SJ. Central place foraging in Rattus norvegicus . Animal Behaviour. 1981;29:64–70. [Google Scholar]
- Lawyer SR, Williams SA, Prihodova T, Rollins JD, Lester AC. Probability and delay discounting of hypothetical sexual outcomes. Behavioural Processes. 2010;84:687–692. doi: 10.1016/j.beproc.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Logue AW, Peña-Correal TE. The effect of food deprivation on self control. Behavioural Processes. 1985;10:355–368. doi: 10.1016/0376-6357(85)90036-1. [DOI] [PubMed] [Google Scholar]
- Logue AW, Forzano LB, Ackerman KT. Self-control in children: Age, preference for reinforcer amount and delay, and language ability. Learning and Motivation. 1996;27:260–277. [Google Scholar]
- Mazur JE. Species differences between rats and pigeons in choices with probabilistic and delayed reinforcers. Behavioural Processes. 2007;75:220–224. doi: 10.1016/j.beproc.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O. Self-regulation in a cognitive-affective personality system: attentional control in the service of the self. Self and Identity. 2002;1:113–120. [Google Scholar]
- Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244:933–938. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- Pelé M, Dufour V, Micheletta J, Thierry B. Long-tailed macaques display unexpected waiting abilities in exchange tasks. Animal Cognition. 2010;13:263–271. doi: 10.1007/s10071-009-0264-6. [DOI] [PubMed] [Google Scholar]
- Pelé M, Micheletta J, Uhlrich P, Thierry B, Dufour V. Delay maintenance in tonkean macaques (Macaca tonkean) and brown capuchin monkeys (Cebus apella) International Journal of Primatology. 2011;32:149–166. [Google Scholar]
- Rachlin H. Self-control: Beyond commitment. Behavioral and Brain Sciences. 1995;18:109–159. [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self control. Journal of the Experimental Analysis of Behavior. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati AG, Stevens JR, Hare B, Hauser MD. The evolutionary origins of human patience: Temporal preferences in chimpanzees, bonobos, and human adults. Current Biology. 2007;17:1663–1668. doi: 10.1016/j.cub.2007.08.033. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Mühlhoff N. Intertemporal choice in lemurs. Behavioural Processes. 2012;89:121–127. doi: 10.1016/j.beproc.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Stephens DW. Patience. Current Biology, 2008;18:R11–R12. doi: 10.1016/j.cub.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Rosati AG, Heilbronner SR, Mühlhoff N. Waiting for grapes: Expectancy and delayed gratification in bonobos. International Journal of Comparative Psychology, 2011;24:99–111. [Google Scholar]
- Tobin H, Chelonis JJ, Logue AW. Choice in self control paradigms using rats. The Psychological Record. 1993;43:441–453. [Google Scholar]
- Tobin H, Logue AW, Chelonis JJ, Ackerman KT, May JG. Self-control in the monkey Macaca Fascicularis . Animal Learning & Behavior. 1996;24:168–174. [Google Scholar]
- Toner IJ, Smith RA. Age and overt verbalization in delay-maintenance behavior in children. Journal of Experimental Child Psychology. 1977;24:123–128. [Google Scholar]
- Toner IJ, Holstein RB, Heterington EM. Reflection-impulsivity and self-control in preschool children. Child Development. 1977;48:239–245. [Google Scholar]
- van Haaren F, van Hest A, van de Poll NE. Self-control in male and female rats. Journal of the Experimental Analysis of Behavior. 1988;49:201–211. doi: 10.1901/jeab.1988.49-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanMarle K, Aw J, McCrink K, Santos L. How capuchin monkeys (Cebus apella) quantify objects and substances. Journal of Comparative Psychology. 2006;120:416–426. doi: 10.1037/0735-7036.120.4.416. [DOI] [PubMed] [Google Scholar]
- Vick S-J, Bovet D, Anderson J. How do African grey parrots (Psittacus erithacus) perform on a delay of gratification task? Animal Cognition. 2010;13:351–358. doi: 10.1007/s10071-009-0284-2. [DOI] [PubMed] [Google Scholar]
- Young M, Webb T, Jacobs E. Deciding when to “cash in” when outcomes are continuously improving: An escalating interest task. Behavioural Processes. 2011;88:101–110. doi: 10.1016/j.beproc.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.