Abstract
Background
Higher concentrations of AM19 and AM1c9, secondary metabolites of cyclosporine A (CsA), have been associated with nephrotoxicity in organ transplant patients. The risk of renal toxicity may depend upon the accumulation of CsA and its metabolites in the renal tissue. We evaluated the hypothesis that CYP3A5 genotype, and inferred enzyme expression, affects systemic CsA metabolite exposure and intra-renal CsA accumulation.
Methods
An oral dose of CsA was administered to 24 healthy volunteers who were selected based on their CYP3A5 genotype. CsA and its six main metabolites in whole blood and urine were measured by LC-MS. In vitro incubations of CsA, AM1, AM9 and AM1c with recombinant CYP3A4 and CYP3A5 were performed to evaluate the formation pathways of AM19 and AM1c9.
Results
The mean CsA oral clearance was similar between CYP3A5 expressors and nonexpressors. However, compared to CYP3A5 nonexpressors, the average blood AUC for AM19 and AM1c9 was 47.4% and 51.3% higher in CYP3A5 expressors (P = 0.040 and 0.011, respectively), corresponding to 30% higher AUCmetabolite/AUCCsA ratios for AM19 and AM1c9 in CYP3A5 expressors. The mean apparent urinary CsA clearance, based on a 48-hour collection, was 20.4% lower in CYP3A5 expressors compared to CYP3A5 nonexpressors (4.2 ± 1.0 and 5.3 ± 1.3 mL/min, respectively, P = 0.037), which is suggestive of CYP3A5-dependent intra-renal CsA metabolism.
Conclusions
At steady-state, intra-renal accumulation of CsA and its secondary metabolites should depend on the CYP3A5 genotype of the liver and kidneys. This may contribute to inter-patient variability in the risk of CsA-induced nephrotoxicity.
Keywords: Cyclosporine A, CYP3A5 genotype, secondary metabolites, chronic calcineurin inhibitor nephrotoxicity, intra-renal metabolism
INTRODUCTION
Introduction of the calcineurin inhibitor cyclosporine A (CsA) in human kidney transplantation in the late 1970s revolutionized transplantation medicine and dramatically increased graft and patient survival (1). However, its use is associated with significant adverse side effects, in particular, acute and chronic calcineurin inhibitor nephrotoxicity (CNIT) (2, 3). Therapeutic immunosuppressant strategies that include CsA call for targeting of trough drug concentration within the recommended therapeutic range. Nonetheless, many patients still experience acute and chronic nephrotoxicity (4, 5). It has been suggested that nephrotoxicity is not solely related to systemic exposure to CsA and that local concentrations of CsA and its metabolites in kidney tissue may be more causally related to the risk of CNIT (6).
CsA undergoes extensive biotransformation to more than 30 products. The major metabolic pathways involve initial hydroxylation/N-demethylation and further oxidation, sulfation, and cyclization (7). Formation of these metabolites is catalyzed principally by cytochromes P450 3A4 and 3A5 (CYP3A4 and CYP3A5), enzymes that are found mainly in the liver and the gastrointestinal tract. The expression of CYP3A5 is highly polymorphic and determined largely by single-nucleotide variations that distinguish the “active” CYP3A5*1 allele (inferred CYP3A5 expressor phenotype in individuals heterozygous or homozygous for CYP3A5*1) from the “inactive” CYP3A5*3, *6 or *7 alleles (inferred CYP3A5 nonexpressor phenotype) (8–12). The CYP3A5 polymorphism contributes to interindividual differences in the metabolic clearance of a number of drugs, including CsA. However, in the case of CsA, the in vitro intrinsic metabolic clearance calculated from total metabolite formation is approximately 2.3-fold higher for CYP3A4 than for CYP3A5 (13). Thus, CYP3A4 plays a more dominant role than CYP3A5 in the metabolism of CsA and the influence of the CYP3A5 polymorphism on the bioavailability and total systemic clearance of CsA is limited (14).
Although the contribution of CYP3A5 to CsA oral clearance is modest, it might contribute more significantly to inter-individual variation in CsA metabolite tissue exposure because of marked differences between the product selectivity of CYP3A4 and CYP3A5. The primary CsA metabolites, AM1, AM9 and AM4N, and several secondary and tertiary metabolites, AM1c, AM19 and AM1c9, can be detected in the blood and urine (15). CYP3A4 catalyzes the formation of all three primary metabolites, whereas only AM9 is produced to a significant degree by CYP3A5 (13). Moreover, human liver microsomes from CYP3A5 expressors exhibit higher AM9 formation rates than liver microsomes from CYP3A5 nonexpressors (13). In the kidney, because CYP3A5, and not CYP3A4, is expressed in the tubular epithelium, the rate of AM9, AM19 and AM1c9 formation by human kidney microsomes is strongly associated with detection of CYP3A5 protein and presence of the CYP3A5*1 allele (13). Thus, inter-individual variability in the systemic blood and renal concentration of CsA metabolites might be explained in part by differences in the expression and function of CYP3A5 in the major organs of drug elimination (16).
High blood and urinary concentrations of AM19 and AM1c9 have been associated with renal dysfunction in CsA treated patients (17–19), although the causality has not been shown. It is unclear if greater than average metabolite exposure is the cause or the result of impaired kidney function. The primary and secondary metabolites of CsA are equivalent or less toxic than CsA in cultured renal epithelial cells (20, 21). In contrast, AM19 and AM1c9 (but not CsA or its primary metabolites) have been shown to alter renal mesangial cell function by increasing endothelin release (22). Accordingly, the presence of CYP3A5 in the small intestine, liver and kidney may affect systemic and intra-renal concentrations of CsA and its putative nephrotoxic metabolites during drug therapy and, by inference, the risk of CNIT. To test this hypothesis, we measured and compared the concentrations of key CsA primary and secondary metabolites in blood and urine excretion among CYP3A5 expressors and nonexpressors. In addition, we evaluated the impact of CYP3A5 genotype on intra-renal CsA metabolism in vivo, using the apparent urinary CsA clearance as a surrogate marker of intra-renal drug clearance.
RESULTS
Demographic Characteristics of Healthy Volunteers
The demographic characteristics of 24 healthy volunteers who participated in this study are shown in SDC, Table 1. There were no significant differences between the CYP3A5 expressors and nonexpressors with respect to sex, weight, serum creatinine, creatinine clearance and estimated GFR (eGFR). However, the CYP3A5 expressors included more Blacks and on average were older than nonexpressors (30.8 ± 9.9 vs. 23.5 ± 3.5 yrs, P = 0.026).
Table 1.
Cyclosporine A blood pharmacokinetic parameters for study participants stratified by predicted CYP3A5 phenotype
| CYP3A5 | n | AUC0–48 (ng hr/mL) | AUC0–inf (ng hr/mL) | t1/2 (hr) | tmax (hr) | Cmax (ng/mL) | Clast (ng/mL) | CL/F (mL/min/kg) |
|---|---|---|---|---|---|---|---|---|
| Nonexpressors | 12 | 5287 ± 1432 | 5670 ± 1603 | 17.1 ± 4.1 | 1.5 ± 0.3 | 1161 ± 221 | 11.9 ± 4.5 | 15.7 ± 4.2 |
| Expressors | 12 | 5780 ± 1444 | 6098 ± 1509 | 17.8 ± 2.5 | 1.6 ± 0.5 | 1194 ± 319 | 12.6 ± 4.0 | 14.8 ± 4.8 |
| P value | 0.41 | 0.51 | 0.60 | 0.37 | 0.77 | 0.72 | 0.61 |
Data are presented as mean ± SD. AUC, area under the concentration–time curve; tmax, time to reach the maximum blood concentration; Cmax, maximum blood concentration; Clast, blood concentration at 48 hour after Cyclosporine A administration; CL/F, oral clearance.
Systemic Disposition of Cyclosporine A and Its Primary and Secondary Metabolites
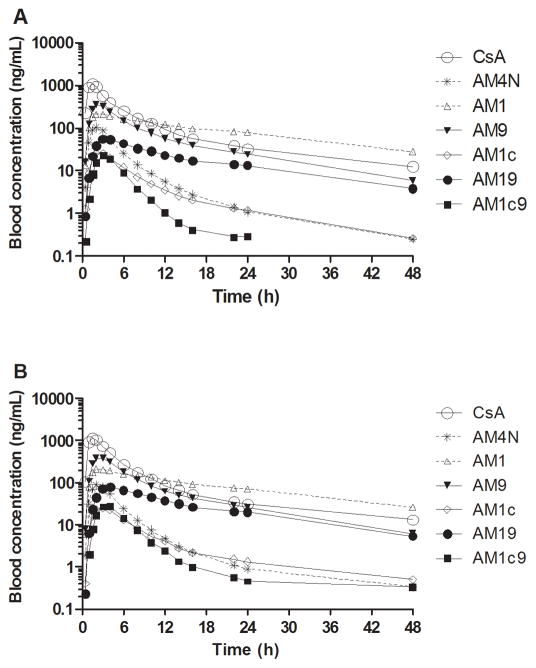
Mean blood CsA concentration-time profiles for the CYP3A5 expressors and nonexpressors who received a single 5 mg/kg dose of CsA are shown in Figure 1. CsA concentrations were similar, as reflected by comparable oral clearance (CL/F) for CYP3A5 expressors and nonexpressors (Table 1). Other blood pharmacokinetic parameters for the two groups were also comparable.
Figure 1.
Mean log blood concentration–time profiles of cyclosporine A (CsA) and its metabolites after 5 mg/kg oral CsA administration in (A) CYP3A5 nonexpressors (n = 12) and (B) CYP3A5 expressors (n = 12).
The mean blood concentration–time profiles of CsA metabolites after oral CsA administration are shown in Figure 1. The circulating blood CsA metabolite concentrations were lower than those of the parent drug. AM1, AM9 and AM19 were the major circulating metabolites. Comparing CYP3A5 expressors and nonexpressors, the average blood AUC for the primary CsA metabolites (AM1, AM9, AM4N, and AM1c) were similar (Table 2), as was the AUCmetabolite/AUCCsA ratio for primary CsA metabolites, an indirect measure of the respective metabolite formation clearances (Table 2).
Table 2.
AUC0–infinity and AUCmetabolite/AUCCsA(0-infinity) of Cyclosporine A and its metabolites for study participants stratified by predicted CYP3A5 phenotype.
| CYP3A5 Expressors (N=12) | CYP3A5 Nonexpressors (N=12) | P value | |
|---|---|---|---|
|
AUC0–infinity
| |||
| CsA | 6098 ± 1509 | 5670 ± 1603 | 0.51 |
| AM1 | 4711 ± 1509 | 4900 ± 2188 | 0.81 |
| AM9 | 3186 ± 766 | 2801 ± 712 | 0.22 |
| AM4N | 418 ± 118 | 456 ± 94 | 0.39 |
| AM1c | 197 ± 94 | 185 ± 79 | 0.74 |
| AM19 | 1360 ± 602 | 923 ± 343 | 0.040 |
| AM1c9 | 162 ± 62 | 107± 29 | 0.011 |
|
| |||
|
AUCm/AUCCsA
| |||
| AM1 | 0.76 ± 0.12 | 0.84 ± 0.23 | 0.29 |
| AM9 | 0.52 ± 0.06 | 0.49 ± 0.08 | 0.35 |
| AM4N | 0.07 ± 0.02 | 0.08 ± 0.02 | 0.16 |
| AM1c | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.82 |
| AM19 | 0.21 ± 0.05 | 0.16 ± 0.04 | 0.016 |
| AM1c9 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.025 |
Data are presented as mean ± SD. AUC, area under the concentration–time curve, expressed in units of ng hr/mL.
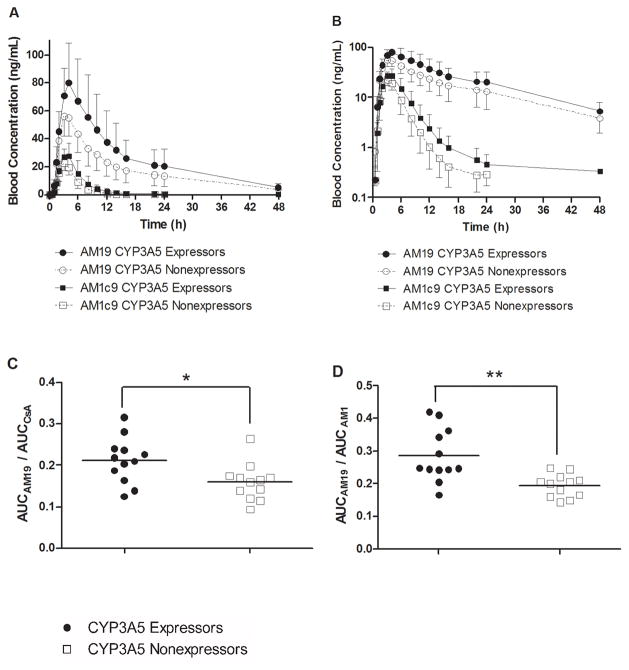
In contrast to results for CsA and its primary metabolites, the average blood AUC for the secondary metabolites AM19 and AM1c9 (Table 2) was 47.4% and 51.3% higher in CYP3A5 expressors compared to nonexpressors (P = 0.040 and 0.011, respectively). In accordance, the AUCmetabolite/AUCCsA ratio for AM19 and AM1c9 was 33.1% and 30.7% higher in CYP3A5 expressors compared to nonexpressors (P = 0.016 and 0.025), respectively (Table 2 and Figure 2C). Similarly, the AUCAM19/AUCAM1 (Figure 2D) and AUCAM1c9/AUCAM1c (not shown) ratio was 46.9% and 30.6% higher in CYP3A5 expressors compared to nonexpressors (P = 0.002 and 0.025), respectively.
Figure 2.
(A) Blood concentration–time profiles of AM19 and AM1c9 in CYP3A5 nonexpressors (n = 12) and CYP3A5 expressors (n = 12). (B) Blood concentration–time profiles of AM19 and AM1c9 displayed using a logarithmic Y-axis. Bars represent standard deviations. AUC ratios are shown for (C) AUCAM19/AUCCsA and (D) AUCAM19/AUCAM1 by predicted CYP3A5 phenotype. The solid line represents the mean ratios; * P < 0.05; ** P < 0.005.
Renal Excretion of CsA and Its Primary Metabolites
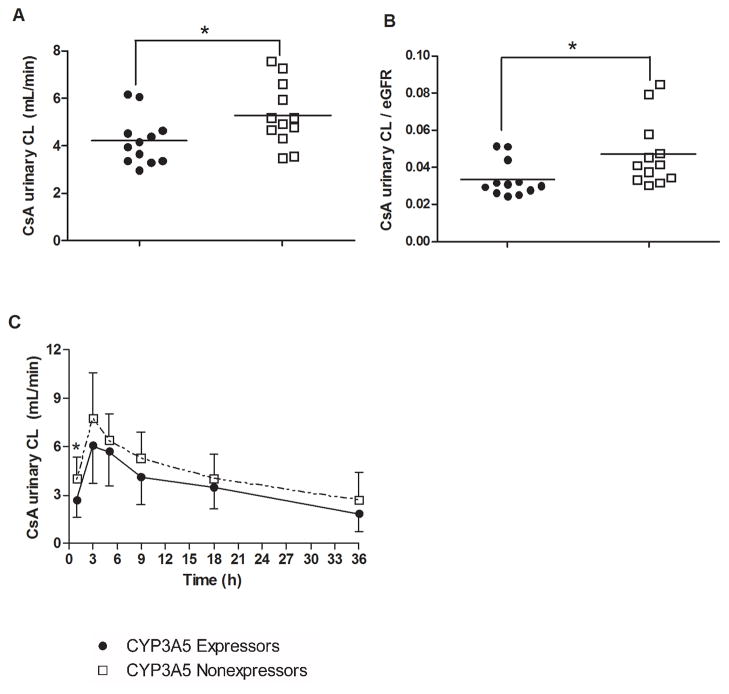
The total amount of intact CsA excreted in urine over 48 hours after oral administration was comparable between CYP3A5 expressors and nonexpressors (1445.9 ± 495.5 and 1677.0 ± 450.2 ng, respectively). However, the mean apparent urinary CsA clearance based on the 48-hour collection was 20.4% lower in CYP3A5 expressors compared to CYP3A5 nonexpressors (4.2 ± 1.0 and 5.3 ± 1.3 mL/min, respectively, P = 0.037) (Figure 3A). Similarly, the eGFR-normalized apparent urinary CsA clearance based on the 48-hour collection was 28.5% lower in CYP3A5 expressors compared to CYP3A5 nonexpressors (0.03 ± 0.01 and 0.05 ± 0.02, respectively, P = 0.035) (Figure 3B). Although the interindividual variability was large, CYP3A5 expressors exhibited increased intra-renal CsA metabolism compared to nonexpressors, as demonstrated by increased urinary CsA clearances over discrete urine collection time intervals (Figure 3C).
Figure 3.
(A) Apparent urinary CsA clearance and (B) eGFR normalized CsA urinary clearance based on a 48-hour urine collection; (C) the time-course of urinary CsA clearance calculated based on discrete urine collection intervals. The solid line represents the mean ratios; * P < 0.05.
The average cumulative amount of AM19 and AM1c9 excreted in urine was 48% and 50% higher in CYP3A5 expressors compared to nonexpressors (P = 0.077 and 0.069, respectively). This is in agreement with greater blood exposure for AM19 and AM1c9 in CYP3A5 expressors, compared to nonexpressors. For the other CsA metabolites, the average amount excreted in urine in the two predicted phenotype groups was comparable. Interestingly, there was no CYP3A5-dependent difference in the apparent urinary clearance (amount excreted/AUCblood) for all of the primary and secondary CsA metabolites measured.
Formation of AM19 and AM1c9 by CYP3A4 and CYP3A5 In Vitro
At a substrate concentration of 1 μM, CYP3A5 Supersomes converted AM1 to AM19 at a rate similar to that of CYP3A4 Supersomes (23.9 ± 5.3 vs. 28.5 ± 4.7 pmol/min/nmol, respectively). AM9 was converted to AM19 much more efficiently by CYP3A4 (11.3 ± 1.2 pmol/min/nmol) than by CYP3A5 (1.1 ± 0.3 pmol/min/nmol). The formation of AM1c9 from AM1c by CYP3A4 and CYP3A5 was also comparable (20.5 ± 5.5 vs. 13.0 ± 0.1 pmol/min/nmol, respectively). Similar results were found when 200 nM of AM1, AM9 and AM1c were incubated with CYP3A4 and CYP3A5 Supersomes for a shorter incubation of 30 min (data not shown).
DISCUSSION
Understanding the basis of interindividual differences in CsA clearance is an important step towards the goal of improving the safety and efficacy of immunotherapy. In the current study, we evaluated how CYP3A5 genetic variation (and the predicted enzyme expression phenotype) affected systemic and intra-renal CsA metabolism and exposure to its metabolites in blood.
Results showed that the mean oral CsA clearance for CYP3A5 expressors and nonexpressors was similar. This is in general agreement with some previous findings (23–26), but not with others (27, 28). The interindividual variability of CsA oral clearance was approximately 30% for both genotype groups. Thus, the interindividual variability of the CYP3A4 content may well have masked any effect of CYP3A5 expression. Because the AM9 pathway is only one of three primary CsA elimination routes and because CYP3A5 exhibits selective formation of only AM9 at an efficiency that is less than that of CYP3A4 (13), one would expect the total metabolic clearance to the primary metabolites to be influenced only modestly by the CYP3A5 genotype. In support of this prediction, both the AUCs and the AUCmetabolite/AUCCsA ratios for AM1, AM9, AM4N and AM1c were similar for the two CYP3A5 phenotype groups.
In contrast to what was seen for the primary CsA metabolites, the AUCs for both AM19 and AM1c9 were significantly higher in CYP3A5 expressors compared to nonexpressors. In addition, there were greater amounts of AM19 and AM1c9 excreted in the urine of CYP3A5 expressors compared to nonexpressors. Based on in vitro product formation rates and in vivo metabolite/parent AUC ratios, the predominant source of AM19 and AM1c9 appears to be through conversion of AM1 and AM1c to the secondary metabolites, reactions that can be catalyzed efficiently by both CYP3A4 and CYP3A5.
The above findings suggest that at steady-state, when CsA dose is adjusted to achieve a narrow therapeutic blood concentration range, there will be greater accumulation of AM19 and AM1c9 in the systemic blood of CYP3A5 expressors compared to nonexpressors. It has been previously suggested that the production and accumulation of the AM19 and AM1c9 secondary metabolites of CsA might contribute to drug-induced nephrotoxicity (17–19, 22). For example, Vollenbroeker et al. reported that AM19 and AM1c9 were the only CsA metabolites to show a positive correlation with the concentration of C-reactive protein and interleukin 6 (biomarkers of organ inflammation) measured in 202 blood specimens from kidney transplant recipients (17). Christians et al. found an inverse correlation between the steady-state blood concentration of AM1c9 and renal function in liver transplant patients during the early post-operative period (18). Likewise, Kempkes-Koch et al. found elevated urine AM19 levels in patients with histologically confirmed CsA nephrotoxicity late after renal transplantation (19). Elevated secondary metabolites of CsA in patients with impaired renal function could be the result, rather than the cause of CsA nephrotoxicity. Alternatively, individual variability in the formation and accumulation of secondary CsA metabolites in blood could contribute directly to differences in renal toxicity risk. With this in mind, formation of AM1c9 and AM19 may represent a toxification pathway.
Higher systemic levels of AM19 and AM1c9 in CYP3A5 expressors should enhance entry of these metabolites into the renal tubular cells either by secretion from the efferent arteriole or after reabsorption from the luminal side following glomerular filtration. This is turn, could influence nephrotoxicity risk. Results from combination therapy with ketoconazole and CsA support this hypothesis. In a prospective, randomized study, when systemic levels of CsA were maintained at a similar level compared with the control arms, renal function was significantly better in the ketoconazole co-treatment group compared to CsA treatment alone (29). Interestingly, in human liver microsomal incubations with CsA, ketoconazole inhibited the formation of secondary metabolites more than the formation of primary CsA metabolites (30), further suggesting that the secondary metabolites of CsA are contributory to CsA nephrotoxicity.
The relationship between CYP3A5 genotype and CsA nephrotoxicity has been studied by several research groups. Some investigators report a significant inverse association between CYP3A5 expression and renal function, as measured by serum creatinine or eGFR or clinically-evident CsA-related nephrotoxicity (31–33), whereas others found a positive association (34, 35). The impact of CYP3A5 expression on CsA nephrotoxicity is likely complicated by CYP3A5’s dual role in CsA clearance within the kidneys and in the systemic formation of active secondary metabolites. Moreover, in studies of kidney transplant recipients, the relationship between genotype and nephrotoxicity is complicated by the fact that the phenotype of the donor kidney may differ from the recipient’s intestinal and hepatic phenotype (36). The kidney transplant recipient’s CYP3A5 genotype and hepatic and intestinal CYP3A5 activity should determine the levels of CsA and its metabolites to which the transplanted kidney is exposed. At the same time, the donor’s renal CYP3A5 status would influence the amount of CsA and its metabolites formed locally in the renal tubular cells.
Results from the current study suggest that carriers of the CYP3A5*1 allele, and an inferred high CYP3A5 renal expression phenotype, exhibit greater renal CsA metabolism and a lower apparent urinary CsA clearance compared to those subjects lacking the active CYP3A5 allele. Such a relationship between renal metabolism and the apparent urinary clearance of unchanged drug was first reported by Sirianni et al., who showed that the urinary clearance of enalapril was increased due to inhibition of its esterolysis by paraoxon in isolated perfused rat kidneys (37). In our study, the mean apparent urinary CsA clearance was 20.4% lower in CYP3A5 expressors, compared to CYP3A5 nonexpressors, consistent with significant intra-renal CYP3A5-dependent CsA metabolism, presumably through AM9 formation (13). A semi-physiological model was developed to evaluate the effect of CYP3A5 polymorphism on intra-renal metabolism and tubulo-epithelial exposure to tacrolimus, another calcineurin inhibitor (38). In that case, the model fitting results supported the conclusion that reduced urinary tacrolimus clearance is due to increased intra-renal metabolism and decreased renal exposure to tacrolimus in metabolically competent cells, the tubular epithelia.
In individuals with significant renal CYP3A5 expression, one might expect higher intra-renal accumulation of AM19 and AM1c9, independent of an effect of intestinal and hepatic CYP3A5 genotype on systemic accumulation of the secondary metabolites. Such a difference might affect the risk of renal toxicity. However, the effect from a higher level of putatively nephrotoxic secondary metabolites might be counteracted by lower intra-renal levels of CsA. In addition, it is also important to consider the role of renal P-glycoprotein, which can transport CsA and in the renal tubular epithelium would act to reduce intracellular concentrations by active efflux activity. Polymorphisms in the ABCB1 gene, which putatively affect enzyme expression (14), have been associated with the risk of renal toxicity from CsA therapy (36, 39). High P-glycoprotein activity may independently influence intra-renal exposure to AM19 and AM1c9, if these metabolites are also substrates for active tubular efflux. This study was not designed to test the effect of ABCB1 gene variation on renal CsA clearance (would require a much larger number of subjects), however we did conduct genotyping for the transporter and found, as expected, there were no significant difference in key genotype or haplotype frequencies between CYP3A5 expressor and nonexpressor groups (SDC, Table 1). Thus, the CYP3A5 expressor association that was observed should not have been influenced by the ABCB1 genotype status.
In summary, we found that individuals expressing CYP3A5 exhibited enhanced formation of AM19 and AM1c9, secondary metabolites of CsA that have been associated with an increased risk of CsA-induced nephrotoxicity. Moreover, the same phenotype influenced the apparent urinary clearance of CsA, suggesting the presence of significant intra-renal CsA metabolism for individuals that carry the functional CYP3A5*1 allele. These findings point towards the need for careful evaluation of the impact of both recipient and donor CYP3A5 genotypes on renal function in organ transplant patients receiving chronic CsA immunotherapy.
MATERIALS AND METHODS
Clinical Protocol
This protocol was approved by the University of Washington Institutional Review Board. All study participants provided written informed consent and were selected based on their CYP3A5 genotype. Subjects (n=24) received a single oral dose of CsA (NEORAL® Soft Gelatin Capsules, Novartis, 5 mg/kg). None of the subjects had a significant medical history or abnormal clinical lab test results, and none had taken a known inhibitor, inducer, or activator of CYP3A4/5 (other than oral contraceptives) for at least 1 month preceding the start of and during the pharmacokinetic investigation, and all abstained from grapefruit products and alcohol one week prior to the start until the end of the study. Sequential blood samples (5 mL) were collected in EDTA tubes predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 14, 16, 22, 24, and 48 hr after oral drug administration of the CsA dose. Urine was collected in silanized glass containers over the following post-dose intervals: 0–2 hr, 2–4 hr, 4–6 hr, 6–12 hr, 12–24 hr and 24–48 hr. All samples were stored at −80 °C until analysis.
Genotyping
Buccal cell DNA was isolated using a DNeasy Blood & Tissue Kit or the Qiagen Gentra Puregene protocol (Qiagen, USA). Single-nucleotide polymorphisms in the CYP3A5 gene (*3, *6 and *7 alleles; rs776746, rs10264272 and rs41303343 respectively) and the ABCB1 gene (C3435T, C1236T and G2677T/A) were determined from a buccal swab tissue sample, using previously published methods (9, 40) or a validated Taqman® allelic discrimination assay from Applied Biosystems (Foster City, CA) (41).
Pharmacokinetic Analysis
Noncompartmental pharmacokinetic analysis was performed using WinNonlin (version 5.2, Pharsight, Mountain View, CA). Pharmacokinetic parameters were determined for CsA and metabolites. CLurinary was calculated as the amount of drug or metabolite excreted in urine divided by AUCblood for the drug or metabolite over the collection interval.
In Vitro Kinetic Protocol
To quantify rates of formation of secondary metabolites of CsA, the primary metabolites, AM1, AM9 and AM1c (1 μM) were incubated in duplicate with CYP3A4 and CYP3A5 Supersomes™ (1000 pmol/mL co-expressed with cytochrome b5). The reactions were initiated by addition of NADPH or buffer after a 5-min preincubation period and were terminated after 1 hr. Metabolites were extracted and quantified as described (SDC, Materials and Methods, Isolation and Mass Spectrometric Analysis of Cyclosporine Metabolites).
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation. Normality of the data was confirmed before statistical analysis. Statistical comparisons were conducted using an unpaired two-sided Student’s t-test by GraphPad Prism 5 (La Jolla, CA). A P value less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
Research support:
This work was supported in part by grants from the National Institutes of Health: R01 GM068871, U01 GM092676, P30 ES07033 and UL1 RR025014.
The authors would like to thank Ms. Christine Hoffer for her outstanding assistance in the conduct of the clinical study. The authors would also like to thank Taurence Senn for his help with the verification of CsA metabolite identities by Q-TOF and Dr. Edward Kelly for his assistance with CYP3A5 and ABCB1 genotyping.
Nonstandard abbreviations
- CsA
Cyclosporine A
- CYP3A4
cytochrome P450 3A4
- CYP3A5
cytochrome P450 3A5
- P-gp
P-glycoprotein
- SNP
single-nucleotide polymorphism
- CNI
calcineurin inhibitor
- CNIT
chronic calcineurin inhibitor nephrotoxicity
- LC-MS
liquid chromatography-mass spectrometry
- Cmax
maximum blood concentration
- Clast
blood concentration at 48 hour after Cyclosporine A administration
- F
systemic bioavailability
- CL/F
oral clearance of Cyclosporine A
- CLurinary
Cyclosporine A urinary clearance
- CrCL
renal creatinine clearance
- GFR
glomerular filtration rate
- eGFR
estimated glomerular filtration rate
Footnotes
Contributions:
Songmao Zheng, Participated in research design, Participated in writing the paper, Participated in the performance of the research, Participated in data analysis
Kenneth E. Thummel, Participated in research design, Participated in writing the paper, Participated in the performance of the research, Participated in data analysis
Danny D. Shen, Participated in research design, Participated in writing the paper, Participated in data analysis
Mary F. Hebert, Participated in research design, Participated in writing the paper, Participated in the performance of the research, Participated in data analysis
Connie L. Davis, Participated in research design, Participated in writing the paper, Participated in the performance of the research
Yoshihisa Shitara, Participated in research design, Participated in writing the paper, Participated in the performance of the research, Participated in data analysis
Yvonne S. Lin, Participated in writing the paper, Participated in the performance of the research, Participated in data analysis
Yasar Tasnif, Participated in writing the paper, Participated in the performance of the research
Justina C. Calamia, Participated in writing the paper, Participated in the performance of the research
Conflicts of Interest:
No conflict of interest
References
- 1.Calne RY, White DJ, Thiru S, et al. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet. 1978;2 (8104–5):1323. doi: 10.1016/s0140-6736(78)91970-0. [DOI] [PubMed] [Google Scholar]
- 2.Klintmalm GB, Iwatsuki S, Starzl TE. Nephrotoxicity of cyclosporin A in liver and kidney transplant patients. Lancet. 1981;1 (8218):470. doi: 10.1016/s0140-6736(81)91851-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myers BD, Ross J, Newton L, Luetscher J, Perlroth M. Cyclosporine-associated chronic nephropathy. N Engl J Med. 1984;311 (11):699. doi: 10.1056/NEJM198409133111103. [DOI] [PubMed] [Google Scholar]
- 4.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR. The natural history of chronic allograft nephropathy. N Engl J Med. 2003;349 (24):2326. doi: 10.1056/NEJMoa020009. [DOI] [PubMed] [Google Scholar]
- 5.Naesens M, Kuypers D, Sarwal MM. The bumpy road of genomic medicine in transplantation: lessons from studies on calcineurin inhibitor nephrotoxicity. Transplantation. 2012;93 (6):578. doi: 10.1097/TP.0b013e31824db954. [DOI] [PubMed] [Google Scholar]
- 6.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4 (2):481. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 7.Christians U, Sewing KF. Cyclosporin metabolism in transplant patients. Pharmacol Ther. 1993;57 (2–3):291. doi: 10.1016/0163-7258(93)90059-m. [DOI] [PubMed] [Google Scholar]
- 8.Lampen A, Christians U, Guengerich FP, et al. Metabolism of the immunosuppressant tacrolimus in the small intestine: cytochrome P450, drug interactions, and interindividual variability. Drug Metab Dispos. 1995;23 (12):1315. [PubMed] [Google Scholar]
- 9.Lin YS, Dowling AL, Quigley SD, et al. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol. 2002;62 (1):162. doi: 10.1124/mol.62.1.162. [DOI] [PubMed] [Google Scholar]
- 10.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27 (4):383. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 11.Haehner BD, Gorski JC, Vandenbranden M, et al. Bimodal distribution of renal cytochrome P450 3A activity in humans. Mol Pharmacol. 1996;50 (1):52. [PubMed] [Google Scholar]
- 12.Givens RC, Lin YS, Dowling AL, et al. CYP3A5 genotype predicts renal CYP3A activity and blood pressure in healthy adults. J Appl Physiol. 2003;95 (3):1297. doi: 10.1152/japplphysiol.00322.2003. [DOI] [PubMed] [Google Scholar]
- 13.Dai Y, Iwanaga K, Lin YS, et al. In vitro metabolism of cyclosporine A by human kidney CYP3A5. Biochem Pharmacol. 2004;68 (9):1889. doi: 10.1016/j.bcp.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 14.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I. Clin Pharmacokinet. 2010;49 (3):141. doi: 10.2165/11317350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 15.Lensmeyer GL, Wiebe DA, Carlson IH. Deposition of nine metabolites of cyclosporine in human tissues, bile, urine, and whole blood. Transplant Proc. 1988;20 (2 Suppl 2):614. [PubMed] [Google Scholar]
- 16.Hebert MF. Contributions of hepatic and intestinal metabolism and P-glycoprotein to cyclosporine and tacrolimus oral drug delivery. Adv Drug Deliv Rev. 1997;27 (2–3):201. doi: 10.1016/s0169-409x(97)00043-4. [DOI] [PubMed] [Google Scholar]
- 17.Vollenbroeker B, Koch JH, Fobker M, Suwelack B, Hohage H, Muller U. Determination of cyclosporine and its metabolites in blood via HPLC-MS and correlation to clinically important parameters. Transplant Proc. 2005;37 (4):1741. doi: 10.1016/j.transproceed.2005.03.149. [DOI] [PubMed] [Google Scholar]
- 18.Christians U, Kohlhaw K, Budniak J, et al. Ciclosporin metabolite pattern in blood and urine of liver graft recipients. I. Association of ciclosporin metabolites with nephrotoxicity. Eur J Clin Pharmacol. 1991;41 (4):285. doi: 10.1007/BF00314953. [DOI] [PubMed] [Google Scholar]
- 19.Kempkes-Koch M, Fobker M, Erren M, et al. Cyclosporine A metabolite AM19 as a potential biomarker in urine for CSA nephropathy. Transplant Proc. 2001;33 (3):2167. doi: 10.1016/s0041-1345(01)01929-7. [DOI] [PubMed] [Google Scholar]
- 20.Bowers LD. Studies of cyclosporine and metabolite toxicity in renal and hepatocyte culture systems. Transplant Proc. 1990;22 (3):1135. [PubMed] [Google Scholar]
- 21.Copeland KR, Thliveris JA, Yatscoff RW. Toxicity of cyclosporine metabolites. Ther Drug Monit. 1990;12 (6):525. doi: 10.1097/00007691-199011000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Copeland KR, Yatscoff RW. Comparison of the effects of cyclosporine and its metabolites on the release of prostacyclin and endothelin from mesangial cells. Transplantation. 1992;53 (3):640. doi: 10.1097/00007890-199203000-00028. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Y, Song M, Guan D, et al. Genetic polymorphisms of CYP3A5 genes and concentration of the cyclosporine and tacrolimus. Transplant Proc. 2005;37 (1):178. doi: 10.1016/j.transproceed.2005.01.077. [DOI] [PubMed] [Google Scholar]
- 24.Loh PT, Lou HX, Zhao Y, Chin YM, Vathsala A. Significant impact of gene polymorphisms on tacrolimus but not cyclosporine dosing in Asian renal transplant recipients. Transplant Proc. 2008;40 (5):1690. doi: 10.1016/j.transproceed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Hesselink DA, van Schaik RH, van der Heiden IP, et al. Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther. 2003;74 (3):245. doi: 10.1016/S0009-9236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- 26.Anglicheau D, Thervet E, Etienne I, et al. CYP3A5 and MDR1 genetic polymorphisms and cyclosporine pharmacokinetics after renal transplantation. Clin Pharmacol Ther. 2004;75 (5):422. doi: 10.1016/j.clpt.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Min DI, Ellingrod VL, Marsh S, McLeod H. CYP3A5 polymorphism and the ethnic differences in cyclosporine pharmacokinetics in healthy subjects. Ther Drug Monit. 2004;26 (5):524. doi: 10.1097/00007691-200410000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Haufroid V, Mourad M, Van Kerckhove V, et al. The effect of CYP3A5 and MDR1 (ABCB1) polymorphisms on cyclosporine and tacrolimus dose requirements and trough blood levels in stable renal transplant patients. Pharmacogenetics. 2004;14 (3):147. doi: 10.1097/00008571-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 29.el-Agroudy AE, Sobh MA, Hamdy AF, Ghoneim MA. A prospective, randomized study of coadministration of ketoconazole and cyclosporine a in kidney transplant recipients: ten-year follow-up. Transplantation. 2004;77 (9):1371. doi: 10.1097/01.tp.0000121133.84763.26. [DOI] [PubMed] [Google Scholar]
- 30.Omar G, Whiting PH, Hawksworth GM, Humphrey MJ, Burke MD. Ketoconazole and fluconazole inhibition of the metabolism of cyclosporin A by human liver in vitro. Ther Drug Monit. 1997;19 (4):436. doi: 10.1097/00007691-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Eng HS, Mohamed Z, Calne R, et al. The influence of CYP3A gene polymorphisms on cyclosporine dose requirement in renal allograft recipients. Kidney Int. 2006;69 (10):1858. doi: 10.1038/sj.ki.5000325. [DOI] [PubMed] [Google Scholar]
- 32.Klauke B, Wirth A, Zittermann A, et al. No association between single nucleotide polymorphisms and the development of nephrotoxicity after orthotopic heart transplantation. J Heart Lung Transplant. 2008;27 (7):741. doi: 10.1016/j.healun.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Hauser IA, Schaeffeler E, Gauer S, et al. ABCB1 genotype of the donor but not of the recipient is a major risk factor for cyclosporine-related nephrotoxicity after renal transplantation. J Am Soc Nephrol. 2005;16 (5):1501. doi: 10.1681/ASN.2004100882. [DOI] [PubMed] [Google Scholar]
- 34.Kreutz R, Bolbrinker J, van der Sman-de Beer F, et al. CYP3A5 genotype is associated with longer patient survival after kidney transplantation and long-term treatment with cyclosporine. Pharmacogenomics J. 2008;8 (6):416. doi: 10.1038/sj.tpj.6500488. [DOI] [PubMed] [Google Scholar]
- 35.de Denus S, Zakrzewski M, Barhdadi A, et al. Association between renal function and CYP3A5 genotype in heart transplant recipients treated with calcineurin inhibitors. J Heart Lung Transplant. 2011;30 (3):326. doi: 10.1016/j.healun.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Staatz CE, Goodman LK, Tett SE. Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II. Clin Pharmacokinet. 2010;49 (4):207. doi: 10.2165/11317550-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 37.Sirianni GL, Pang KS. Inhibition of esterolysis of enalapril by paraoxon increases the urinary clearance in isolated perfused rat kidney. Drug Metab Dispos. 1999;27 (8):931. [PubMed] [Google Scholar]
- 38.Zheng S, Tasnif Y, Hebert MF, et al. Measurement and Compartmental Modeling of the Effect of CYP3A5 Gene Variation on Systemic and Intrarenal Tacrolimus Disposition. Clin Pharmacol Ther. 2012 doi: 10.1038/clpt.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hebert MF, Dowling AL, Gierwatowski C, et al. Association between ABCB1 (multidrug resistance transporter) genotype and post-liver transplantation renal dysfunction in patients receiving calcineurin inhibitors. Pharmacogenetics. 2003;13 (11):661. doi: 10.1097/00008571-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 40.Asano T, Takahashi KA, Fujioka M, et al. ABCB1 C3435T and G2677T/A polymorphism decreased the risk for steroid-induced osteonecrosis of the femoral head after kidney transplantation. Pharmacogenetics. 2003;13 (11):675. doi: 10.1097/00008571-200311000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Hebert MF, Easterling TR, Kirby B, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84 (2):248. doi: 10.1038/clpt.2008.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.