Abstract
Background
Although total knee arthroplasty reduces pain and improves function, patients continue to walk with asymmetrical movement patterns, that may affect muscle activation and joint loading patterns. The purpose of this study was to evaluate the specific biomechanical abnormalities that persist after total knee arthroplasty and examine the neuromuscular mechanisms that may contribute to these asymmetries.
Methods
Dynamic joint stiffness at the hip, knee and ankle, as well as co-contraction at the knee and ankle, were compared between the operated and non-operated limbs of 32 subjects who underwent total knee arthroplasty and 21 subjects without lower extremity impairment. Dynamic joint stiffness was calculated as the slope of the line of joint moment plotted as a function of joint angle.
Findings
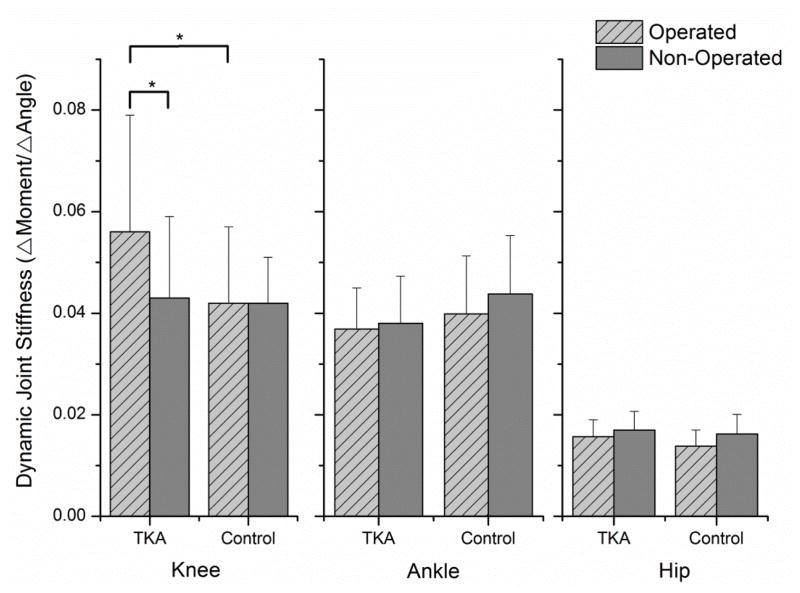
Subjects after total knee arthroplasty demonstrated higher dynamic joint stiffness in the operated knee compared to the non-operated knee (0.056 (0.023) Nm/kg/m/deg vs. 0.043 (0.016) Nm/kg/m/deg, P=0.003) and the knees from a control group without lower extremity pathology (controls: 0.042(0.015) Nm/kg/m/deg, P =0.017). No differences were found between limbs or groups for dynamic joint stiffness at the hip or ankle. There was no relationship between dynamic joint stiffness at the knee and ankle and the amount of co-contraction between antagonistic muscles at those joints.
Interpretation
Patients after total knee arthroplasty walk with less knee joint excursion and greater knee stiffness, although no differences were found between groups for stiffness at the hip or ankle. Mechanisms other than co-contraction are likely the underlying cause of the altered knee mechanics. These findings are clinically relevant because the goal should be to create interventions to reduce these abnormalities and increase function.
Keywords: Total Knee Arthroplasty, Knee Osteoarthritis, Dynamic Joint Stiffness, Co-contraction, Knee Biomechanics
1. Introduction
Osteoarthritis (OA) is a degenerative disease that most often occurs in the knee and causes substantial pain, decreased range of motion and reduced functional performance (Jacobs et al. 2009). Although OA is classically described as degeneration of the articular cartilage within the joint, knee OA is also associated with muscle weakness (Becker et al. 2004), joint instability (Fitzgerald et al. 2004), loss of proprioception (Hassan et al. 2002), altered muscle coordination patterns (Zeni et al. 2009) and abnormal kinetics and kinematics in the affected and adjacent joints (Zeni & Higginson 2011; Zeni & Higginson 2009; Briem & Snyder-Mackler 2009). Knee replacements are the most common surgical treatment for end-stage OA. Although patients typically report reduced pain and improved functional performance after surgery (Petterson et al. 2009), biomechanical asymmetries (Farquhar et al. 2008) and muscle weakness (Valtonen et al. 2009) are not concomitantly resolved.
Asymmetrical movement patterns adopted in the presence of the pain and weakness associated with OA persist one year after total knee arthroplasty (TKA) (Farquhar et al. 2008). These gait patterns are characterized by reduced stance time, reduced knee joint excursions and reduced use of the quadriceps to attenuate the rate of force development during loading response (Yoshida et al. 2008). These gait patterns are typically associated with the ‘stiff-legged’ or ‘quadriceps avoidance’ gait patterns that are seen in patients with OA who present with increased joint laxity and increased co-contraction of antagonistic muscles (Schmitt & Rudolph 2007; Rudolph et al. 2007). In a study of patients with knee OA, Fitzgerald et al. found that 63% of their subjects reported knee instability (the knee buckling or ‘giving way’) and that 44% of these subjects reported that it affected their ability to function (Fitzgerald et al. 2004). This perception of joint instability persists after TKA, which can have negative implications for overall function and joint health (Lo et al. 2010; Barsoum et al. 2011). In patients with OA, increased antagonistic muscle activity may be used to increase the stability of the joint (Zeni et al. 2009), some studies suggest that patients after TKA may use a similar strategy (Benedetti et al. 2003). Although this increase in co-contraction may help alleviate the perception of instability, it can also increase the compressive load experienced by the lower extremity (Lu et al. 1997). These gait patterns may be especially detrimental to the prosthesis as higher rates of force development and higher peak forces may play a role in the breakdown of the prosthetic components.
Although gait asymmetries and biomechanical alterations exist after TKA, the mechanisms underlying these abnormalities are not well understood and the timecourse of their development and resolution is not known. Therefore the purpose of this study was to evaluate the specific biomechanical abnormalities that persist in the knee, ankle, and hip after TKA and examine the mechanisms that contribute to these persistent asymmetries. We hypothesized that subjects 6 months after TKA would demonstrate greater stiffness in the operated knee and this would be related to greater co-contraction of the muscles surrounding the joint.
2. Methods
2.1 Subjects
A total of 53 subjects participated in this cross-sectional study. Thirty-two subjects 6 months after unilateral TKA for osteoarthritis and 21 healthy adults with no reported knee pain were included (Table 1). Subjects were excluded if they had a self-reported pain greater than or equal to 4 out of 10 in the non-operated limb, neurological or vascular problems that interfered with their ability to perform the ascribed tasks, diabetes that impaired lower extremity sensation, or were currently receiving treatment for cancer. All subjects in the TKA group underwent rehabilitation at one of our community clinics where the standard of care is a progressive rehabilitation paradigm that focuses on normalizing strength, range of motion and functional ability. Modalities are used to control pain and swelling. This work was approved by the appropriate institutional review board, and all subjects signed an informed consent prior to participation.
Table 1.
Subject Characteristics, mean (SD)
| TKA | Control | P-Value | |
|---|---|---|---|
|
|
|||
| Sex (M/F) | 20/12 | 12/11 | |
| Age (years) | 69.9 (7.9) | 62.7 (6.6) | |
| Knee Outcome Survey (%) | 87 (9) | 99 (1) | <0.001* |
| Walking Speed (m/s) | 1.26 (0.17) | 1.44 (0.15) | <0.001* |
| Height (m) | 1.72 (0.10) | 1.73 (0.11) | 0.818 |
| Weight (kg) | 92.7 (21.4) | 85.8 (12.5) | 0.177 |
| Body Mass Index (kg/m2) | 31.3 (6.1) | 29.0 (4.8) | 0.147 |
| Extension ROM Op. Limb (°) | 0 (5) | −1 (3) | 0.427 |
| Flexion ROM Non-Op. Limb (°) | 119 (8) | 134 (6) | <0.001* |
| MVIC Op. Limb (N/BMI) | 18.3 (6.6) | 30.0 (10.4) | <0.001* |
| MVIC Non-Op. Limb (N/BMI) | 21.9 (8.8) | 27.9 (8.8) | 0.015* |
2.2 Motion Analysis
Joint kinematics and kinetics during gait were measured using an 8-camera motion capture system (VICON, Oxford Metrics Ltd., London, UK) synchronized with two force plates (Bertec Corporation, Worthington, OH, USA). Spherical retro-reflective markers were placed bilaterally on iliac crest, greater trochanter, lateral femoral condyle, lateral malleolus, head of the 5th metatarsal, and 2 markers on the heel. Rigid thermoplastic shells with 4 markers were secured on the lower leg and thigh bilaterally. The pelvic motion was tracked using a rigid thermoplastic shell with 3 markers. Motion data was collected at 120 Hz and analog data from the force plate was sampled at 1080 Hz. Subjects walked at a self-selected pace. Five walking trials were collected and the average of these trials was used in the analysis. Marker trajectories were low pass filtered at 6 Hz, and force platforms data were filtered at 40 Hz using a second-order phase-corrected butterworth filter. Joint angles were calculated using Euler X-Y-Z sequence corresponding to flexion/extension, abduction/adduction, and then rotation sequences. Joint moments were calculated using 3D inverse dynamics and were normalized to subject height and weight using Visual 3D software (C-motion, Germantown, MD, USA). The time points between consecutive heel strikes were normalized to 101 points. Heel strike events were determined when the vertical ground reaction force crossed a threshold of 20 N. Joint excursions and peak joint moments were calculated for the operated and non-operated limbs for all subjects. “Operated” limbs in the control subjects were randomly selected between left and right limbs, and matched to the percentage left and right knee replacements in our TKA groups.
2.3 Dynamic Joint Stiffness
To evaluate the biomechanical stiffness of the limb during gait, we calculated dynamic joint stiffness (DJS) of each limb and joint. Dynamic joint stiffness was defined as the change in moment (M) divided by the change in angle (θ):
| (2.1) |
The joint moment was plotted against the knee angle and a linear fit of the slope was determined to be the joint stiffness. Knee stiffness was calculated during weight acceptance, which was determined to be the linear region in which the average external knee flexion moment started to increase and ended with peak knee flexion (Zeni and Higginson, 2009). Ankle stiffness was calculated during stance in the linear region that began with maximum plantarflexion and ended at maximum dorsiflexion. Hip stiffness was calculated during stance from minimum to maximum hip flexion. These phases encompass important components of the stance phase: weight acceptance for the knee and the end of weight acceptance to push-off for the hip and ankle. Excursions and joint moments for the hip, knee and ankle were also analyzed during the same periods of time used to calculate hip, knee and ankle stiffness, respectively.
2.4 Electromyography
Electromyographic (EMG) data was collected bilaterally for each subject using active surface electrodes (Motion Lab Systems, Baton Rouge, LA, USA). The skin was cleaned with alcohol prior to electrode placement. Electrodes were placed on the following eight muscles on each limb: gluteus medius, lateral hamstring, vastus lateralis, vastus medialis, tibialis anterior, soleus, and the medial and lateral heads of the gastrocnemius. Prior to walking, maximum volitional isometric contractions (MVIC) were performed to determine the maximum levels of voluntary contraction. For knee extension (vastus lateralis), the subject sat with his or her leg flexed to 75 degrees and performed an isometric contraction with a leg cuff that was secured to the table with metal chains. For hamstring testing, the subjects stood supported at a table and were asked to flex their knee while the investigator applied opposing force to resist knee flexion. MVICs for different muscle groups were recorded in separate trials, with each trial containing 1–2 seconds in which the muscle is not active for normalization to resting levels.
The EMG signal was pre-amplified at the skin and sampled at a rate of 1080 Hz. Visual 3D software (C-motion, Germantown, MD, USA) was used to filter the signals using a low pass filter at 350 Hz. A linear envelope was created on the absolute value of the raw EMG signal using a phase-corrected low-pass Butterworth filter with a cutoff of 20 Hz. Data were normalized to the maximum signal obtained during MVIC. All EMG data were visually inspected prior to analysis. EMG signals that were not usable were excluded from the analysis. EMG data were excluded if the signal was excessively noisy, demonstrated excessive motion artifact (large periods of low frequency signal), or the signal was clipped during the dynamic trials.
2.5 Co-Contraction
Antagonist muscle co-contraction index (CCI) was calculated using the following equation developed by Rudolph et al. (2001).
| (2.2) |
EMGS is the signal from the least active muscle while EMGL is the signal from the more active muscle. The vastus lateralis and the lateral hamstring were used to calculate co-contraction at the knee, and the tibialis anterior and the medial head of the gastrocnemius were used to calculate co-contraction at the ankle. Co-contraction was determined during the same time periods as joint stiffness for the respective joints. Peak co-contraction was determined to be the maximum value during these time periods. Average co-contraction was calculated as the sum of the area under the co-contraction curve during these time periods. Because antagonistic EMG data was not available from the muscles that control the hip in the sagittal plane, co-contraction was not calculated for this joint.
2.7 Clinical metrics
Subject sex, age, height and weight were recorded. Weight was taken on the same clinical scale that is calibrated on an annual basis. Body mass index (BMI) was calculated for all individuals. The Knee Outcome Score – Activities of Daily Living Subscale (KOS-ADLS) was use to assess patient reported functional ability. This test is scored as a percentage out of 100. Active knee extension and flexion were measured in a supine position using a long-arm goniometer. Quadriceps strength was calculated as the peak force output during an isometric quadriceps contraction as the subject was seated with the hip flexed at 90 degrees and knee flexed to 75 degrees on a dynamometer (Kin-Com, Chattex Corp, Harrison, TN). Quadriceps strength was normalized to BMI (N/BMI).
2.6 Statistical Analysis
Independent t-tests were performed to evaluate differences between groups in subject characteristics. Separate 2×2 ANOVAs were run to compare joint stiffness between limbs and groups for each joint. In the event of a significant interaction effect, follow-up t-tests were used to determine differences between limbs in each group and between groups for each limb. If there was a significant interaction effect, we also performed follow-up t-tests to compare joint excursion and peak moments between limbs and groups to quantify the biomechanical alterations that contributed to differences in joint stiffness. Separate ANOVAs were run for each joint: knee, ankle, and hip. Because we hypothesized that co-contraction would be positively correlated to the amount of dynamic joint stiffness, separate Pearson’s correlation coefficients were calculated for stiffness and co-contraction at both the knee and ankle for operated limb of the TKA. To determine the effect of walking speed, knee range of motion and quadriceps strength on DJS at the knee, we performed regression analyses with these variables as independent variables and DJS of the operated limb as the dependent variable. Linear regressions were performed for each group separately and the with the sample as a whole. P -values equal to or less than 0.05 were considered significantly different for all analyses.
3. Results
3.1 Subject characteristics
The TKA groups scored significantly lower on the Knee Outcome Survey – Activities of Daily Living Subscale (Table 1). The TKA group walked significantly slower, were significantly weaker on the operated and non-operated limbs and had less active knee flexion on the operated limb. There were no significant differences between groups for height, weight, BMI, or active knee extension range of motion.
3.2 Stiffness, Peak Moments, and Excursion
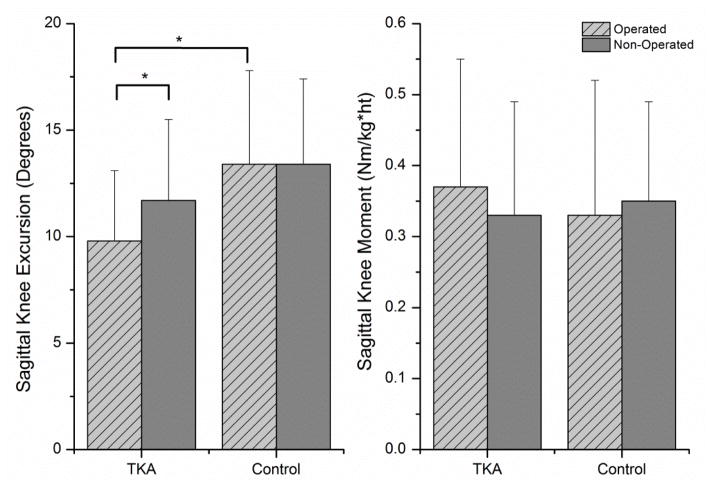
There was a significant group x limb interaction effect for dynamic joint stiffness at the knee (P =0.018). Follow-up paired t-tests revealed the operated limb of the subjects in the TKA group had a higher dynamic joint stiffness than the non-operated limb (0.056(0.023) Nm/kg/m/deg vs. 0.043(0.016) Nm/kg/m/deg; P=0.003). There was no difference between limbs in the control group (operated: 0.042(0.015) Nm/kg/m/deg vs. non-operated: 0.042(0.009) Nm/kg/m/deg; P =0.916). There was also a significant difference between groups for the operated limb (P =0.017), but not for the non-operated limb (P =0.848) (Figure 1). To determine the underlying biomechanical alterations that resulted in differing joint stiffness, paired t-tests were used to evaluate inter-limb differences of joint moment and excursion in each group. The operated limb of subjects in the TKA group demonstrated significantly less knee excursion than the non-operated limb (9.8(3.8) degrees vs. 11.7(3.8) degrees; P =0.023), but no difference in peak sagittal plane knee moment between limbs (operated: 0.37(0.18) Nm/kg/m vs. non-operated: 0.33(0.16) Nm/kg/m; P =0.230). There were no significant differences in sagittal plane knee excursion (operated: 13.4(4.4) degrees vs. non-operated: 13.4(4.0) degrees; P =0.968) or peak sagittal plane knee moment (operated: 0.33(0.19) Nm/kg/m vs. non-operated: 0.35(0.14) Nm/kg/m; P =0.630) between limbs in the control group (Figure 2). The only difference between groups was knee excursion on the operated knee, with subjects in the TKA group having an average of 3.6 less degrees of knee excursion during loading response (P =0.003).
Figure 1.
Dynamic joint stiffness in the knee, ankle, and hip. (*P<0.05)
Figure 2.
Sagittal plane knee joint excursion and knee joint moment. (*P<0.05)
There were no significant limb (P =0.071) or limb x group interaction effects for dynamic joint stiffness at the ankle (P =0.299) and there was no significant limb x group interaction effect at the hip (P =0.370) (Figure 1). There was a significant main effect of limb (P =0.004) for dynamic joint stiffness at the hip, although the magnitude of difference was small with the groups collapsed (operated: 0.015(0.003) Nm/kg/m/deg vs. non-operated: 0.017(0.004) Nm/kg/m/deg). Follow-up testing revealed no significant difference between limbs for sagittal plane hip joint excursion (operated: 39.7(6.2) degrees vs. non-operated 39.4(6.4) degrees; P =0.638) and no significant difference in peak sagittal plane hip moments, although the difference approached significant levels (operated: 0.476(0.118) Nm/kg/m vs. non-operated 0.522(0.199) Nm/kg/m; P =0.053).
3.3 Co-contraction
Twenty-eight of the 32 subjects in the TKA group and 18 of the 22 subjects in the control group had EMG data at the knee. There were no significant differences between groups for peak (P =0.317) or average (P =0.535) co-contraction at the knee (Table 2). There was no significant correlation between knee stiffness and either peak (r=0.268, P =0.076) or average co-contraction (r=0.201, P =0.187) in the TKA group.
Table 2.
Co-contraction at the Knee and Ankle, mean (SD).
| TKA | Control | P-value | |
|---|---|---|---|
| Peak Knee (%MVIC) | 54.0 (23.7) | 46.2 (28.1) | 0.317 |
| Average Knee (sum) | 607.0 (286.3) | 542.7 (411.9) | 0.535 |
| Peak Ankle (%MVIC) | 30.6 (12.2) | 17.2 (8.6) | <0.001 |
| Average Ankle (sum) | 714.8 (250.7) | 363.1 (180.9) | <0.001 |
Thirty subjects in the TKA group and 17 subjects in the Control group had EMG data for the ankle. The subjects in the TKA group demonstrated significantly greater peak co-contraction (P <0.001) and average co-contraction (P <0.001) at the ankle during the period at which ankle stiffness was calculated (Table 2). However, neither peak co-contraction (r=−0.259, P =0.082) nor average co-contraction (r=−0.151, P =0.315) was significantly correlated with ankle joint stiffness in the TKA group.
3.4 Regression Analyses
When the TKA and control groups were combined, there was no significant relationship between walking speed or active knee range of motion on DJS (Table 3), but there was a significant relationship between quadriceps strength and DJS. As quadriceps strength increased, there was a decrease in DJS. When the groups were analyzed separately, there was no relationship between any variable and DJS, except active knee extension range of motion and DJS in the control group. As active knee extension range of motion increased, there was a decrease in DJS. In particular, those subjects with hyperextension tended to show lower stiffness values at the knee during gait.
Table 3.
Relationship between clinical metrics and stiffness (R2 values; * p<0.05)
| TKA | Control | Combined (TKA+Control) | |
|---|---|---|---|
|
|
|||
| Walking Speed | 0.026 | 0.001 | 0.002 |
| Extension ROM Op. Limb (°) | 0.019 | 0.384* | 0.003 |
| Flexion ROM Non-Op. Limb (°) | 0.002 | 0.000 | 0.059 |
| MVIC Op. Limb (N/BMI) | 0.052 | 0.032 | 0.099* |
| MVIC Op. Limb (N) | 0.023 | 0.052 | 0.118* |
4. Discussion
Our results support our hypothesis that subjects after TKA have greater dynamic joint stiffness in the operated knee compared to the non-operated knee. This stiffness was also significantly greater than an age-matched control group without knee pathology. This increase in joint stiffness was isolated to the knee joint and we did not see any significant change in joint stiffness at the ankle or hip. Although increased joint stiffness is often attributed to increased co-contraction, we did not find any significant relationship between the peak or average co-contraction and joint stiffness at the knee and ankle; however, increased DJS was related to weaker knee extensors.
Less joint excursion and higher joint moments are the two biomechanical factors that contribute to increased joint stiffness. Our subjects after TKA demonstrated significantly reduced joint excursions on the operated knee compared to both their non-operated knee and knee excursions in the control group. There were no differences in knee joint moments between groups or limbs, suggesting that the increase in dynamic joint stiffness was a direct result of changes in the kinematic movement patterns. These findings agree with previous work that have identified reduced peak knee flexion and reduced knee excursion during the weight acceptance phase of gait after TKA (Ishii et al. 1998; Smith et al. 2006; Smith et al. 2004; Benedetti et al. 2003).
Subjects prior to TKA also demonstrate significant reductions in knee joint excursion (Zeni & Higginson 2009) and it is suggested that this may be attributed to pain and weakness in that limb (Henriksen et al. 2010). Although pain is largely resolved 6 months after TKA, our subjects demonstrated persistent muscle weakness in the operated limb, which is consistent with other reports (Valtonen et al. 2009; Stevens-Lapsley et al. 2010). This unilateral strength loss is related to asymmetrical movement strategies and reduced knee joint excursion during dynamic tasks (Mizner & Snyder-Mackler 2005), which may be an underlying reason for the increased dynamic joint stiffness in our postoperative sample. This theory was supported by the significant, albeit weak, relationship between quadriceps weakness and increased DJS in our sample. It is also feasible that these stiff-legged movement patterns are learned motor strategies that were adopted before surgery in the presence of weakness and pain, but persist despite the resolution of these impairments. This theory is supported by a longitudinal study that found decreased contribution from the knee to total support moment in patients with knee OA both before and after TKA (Mandeville et al. 2007). However, we cannot make conclusive causative statements from our results due to the cross-sectional design of the current study.
All subjects in our sample underwent a rehabilitation protocol that was heavily focused on progressive strengthening, but no patients specifically received training to normalize movement patterns or enhance movement symmetry. Reducing joint stiffness in the operated knee should be an important focus of post-operative care. With a reduction in knee joint excursion, the quadriceps do not function in a normal shock-absorbing manner. Reduced joint excursion is related to increased axial loading rates, which may have negative consequences for the prosthesis. Although the relationship between loading rate and prosthetic wear in largely unknown, greater loading rates have been shown to be related to expedited damage within a joint (Ewers et al. 2001) and mechanical wear of the prosthesis (Blunn et al. 1991). Additionally, these movement strategies may perpetuate a pattern of disuse in the non-operated limb that promotes weakness and range of motion deficits after TKA.
Patients demonstrate greater antagonistic muscle activity, or co-contraction, at the knee prior to TKA (Zeni et al. 2009) in response to greater joint instability (Yakhdani et al. 2010). A similar muscle activation pattern exists after surgery with greater average activity of the rectus femoris, medial and lateral hamstrings and tibialis anterior; and this increased muscle activity is coupled with less knee flexion excursion during stance (Benedetti et al. 2003). Therefore, we anticipated subjects after TKA would demonstrate greater co-contraction at the knee and that would be related to greater joint stiffness. This hypothesis was not supported by our findings; there was no difference between groups in the magnitude of co-contraction at the knee and co-contraction was not related to joint stiffness. Therefore, it is possible that other biomechanical factors lead to a greater joint stiffness. Increased quadriceps activity without a concomitant increase in hamstring activity may be a mechanism by which subjects increase joint stiffness and reduce joint excursion during walking. It is also possible that dynamic joint stiffness does not fully account for changes in the mechanical properties of the joint after TKA that may be related to joint stiffness. However, it is also feasible that our co-contraction metric does not completely explain the motor strategies that would directly affect joint motion in response to external forces.
Although we did find greater ankle co-contraction in the operated limb of subjects after TKA compared to the control group, this was not related to joint stiffness. This discrepancy may be attributable to differences in the normalization values for each group. Although both groups were normalized to an MVIC, the electrical activity of that MVIC may differ between groups. If this were the case, higher individual EMG signals from the TKA group may represent a greater muscular ‘effort’ on an individual basis and may not be indicative of greater muscular force that would affect kinetic and kinematic patterns.
Although this work found asymmetries and abnormalities in post-operative movement patterns, several methodological limitations should be noted. First, this was a cross-sectional sample, so we cannot determine how pre-operative status affected postoperative outcomes or evaluate the effect of the surgical procedure on DJS. Because we did not constrain or collect the duration and intensity of post-operative rehabilitation, we cannot evaluate the effect of rehabilitation on DJS in these subjects. Errors during motion capture associated with soft tissue artifact are also possible; however, both groups were moving at relatively low speeds and although significantly different, these speeds did not differ by a large magnitude. Errors resulting from inherent variability in three-dimensional motion analysis methods were reduced by only examining sagittal plane excursions, which have the lower error than motion in the transverse plane (McGinley et al. 2009), standardizing data collection protocols and using experienced, reliable investigators. For the EMG analysis, we chose to assess the vastus lateralis because it is capable of generating the greatest force, and subsequent largest extension moment, at the knee joint (Delp et al. 1990). However, it is possible that the medial and lateral vasti demonstrate different activation patterns, which could ultimately influence co-contraction values. Additionally, all subjects in this study underwent the same surgical procedure (unilateral posterior-stabilized TKA) by one of two surgeons, which limits the variability in the surgical procedure affecting outcomes. However, we acknowledge that soft tissue balancing or other intra- or peri-operative factors that may contribute to DJS.
5. Conclusions
Although increased medial joint compression is associated with radiographic evidence of cartilage deterioration (Miyazaki et al. 2002), the variables assessed in this study have yet to be linked to prosthetic wear and future disability. However, our findings support the theory that movement patterns 6 months after TKA are not symmetrical or normal and the increased DJS of the operated limb may be attributed to weakness of the quadriceps. Our results also demonstrate that DJS is not related to the antagonistic muscle activity. Rehabilitation protocols after joint replacement should focus on improving knee joint excursion during dynamic activities and addressing the strength impairments that may contribute to asymmetries and reduced joint motion during walking. Future work is needed to explore biomechanical variables other than altered antagonistic muscle activity that may be responsible for a reduction in knee joint excursion in subjects after TKA and whether these variables are volitional or inherent in the post-surgical outcome. Increased friction at the prosthetic joint surface interface, change in tension of the soft tissue in the knee joint and increased quadriceps activity may account for the increase in joint stiffness after TKA, but these possibilities require future analysis.
Supplementary Material
Acknowledgments
This study was performed under the following grants: P20 RR16458 and P20-RR16458-S1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barsoum W, Lee H, Murray T, Colbrunn R, Klika K, Butler S, et al. Robotic testing of proximal tibio-fibular joint kinematics for measuring instability following total knee arthroplasty. J Orthop Res. 2011;29(1):47–52. doi: 10.1002/jor.21207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker R, Berth A, Nehring M, Awiszus F. Neuromuscular quadriceps dysfunction prior to osteoarthritis of the knee. J Orthop Res. 2004;22(4):768–773. doi: 10.1016/j.orthres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Benedetti M, Catani F, Bilotta T, Marcacci M, Mariani E, Giannini S. Muscle activation pattern and gait biomechanics after total knee replacement. Clin Biomech. 2003;18(9):871–876. doi: 10.1016/s0268-0033(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Blunn G, Walker P, Joshi A, Hardinge K. The dominance of cyclic sliding in producing wear in total knee replacements. Clin Orthop Relat Res. 1991;273:253–260. [PubMed] [Google Scholar]
- Briem K, Snyder-Mackler L. Proximal gait adaptations in medial knee OA. J Orthop Res. 2009;27(1):78–83. doi: 10.1002/jor.20718. [DOI] [PubMed] [Google Scholar]
- Delp S, Loan J, Hoy M, Zajac F, Topp E, Rosen J. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37(8):757–767. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- Ewers B, Dvoracek-Driksna D, Orth M, Haut R. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19(5):779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- Farquhar S, Reisman D, Snyder-Mackler L. Persistence of altered movement patterns during a sit-to-stand task 1 year following unilateral total knee arthroplasty. Phys Ther. 2008;88(5):567–579. doi: 10.2522/ptj.20070045. [DOI] [PubMed] [Google Scholar]
- Fitzgerald G, Piva S, Irrgang J. Reports of joint instability in knee osteoarthritis: its prevalence and relationship to physical function. Arthritis Rheum. 2004;51(6):941–946. doi: 10.1002/art.20825. [DOI] [PubMed] [Google Scholar]
- Hassan B, Doherty S, Mockett S, Doherty M. Effect of pain reduction on postural sway, proprioception, and quadriceps strength in subjects with knee osteoarthritis. Ann Rheum Dis. 2002;61(5):422–428. doi: 10.1136/ard.61.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen M, Graven-Nielsen T, Aaboe J, Andriacchi T, Bliddal H. Gait changes in patients with knee osteoarthritis are replicated by experimental knee pain. Arthritis Care Res. 2010;62(4):501–509. doi: 10.1002/acr.20033. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Terajima K, Koga Y, Takahashi H, Bechtold J, Gustilo R. Gait analysis after total knee arthroplasty. Comparison of posterior cruciate retention and substitution. J Orthop Sci. 1998;3(6):310–7. doi: 10.1007/s007760050058. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Andersson G, Bell J, Weinstein S, Dormans J, Gnatz S, et al. The burden of musculoskeletal diseases in the United States - Executive Summary. Bone and Joint Decade 2009 [Google Scholar]
- Lo J, Müller O, Dilger T, Wülker N, Wünschel M. Translational and rotational knee joint stability in anterior and posterior cruciate-retaining knee arthroplasty. Knee. 2010;18(6):491–5. doi: 10.1016/j.knee.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Lu T, Taylor S, O’Connor J, Walker P. Influence of muscle activity on the forces in the femur: an in vivo study. J Biomech. 1997;30(11–12):1101–1106. doi: 10.1016/s0021-9290(97)00090-0. [DOI] [PubMed] [Google Scholar]
- Mandeville D, Osternig L, Chou L. The effect of total knee replacement on dynamic support of the body during walking and stair ascent. Clin Biomech. 22(7):787–794. doi: 10.1016/j.clinbiomech.2007.04.002. [DOI] [PubMed] [Google Scholar]
- McGinley J, Baker R, Wolfe R, Morris M. The reliability of three-dimensional kinematic gait measurements: a systematic review. Gait Posture. 29(3):360–369. doi: 10.1016/j.gaitpost.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Wada M, Kawahara H, Sato M, Baba H, Shimada S. Dynamic load at the baseline can predict radiographic disease progression in medial compartment knee osteoarthritis. Ann Rheum Dis. 2002;61(7):617–622. doi: 10.1136/ard.61.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizner R, Snyder-Mackler L. Altered loading during walking and sit-to-stand is affected by quadriceps weakness after total knee arthroplasty. J Orthop Res. 2005;23(5):1083–1090. doi: 10.1016/j.orthres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Petterson S, Mizner R, Stevens J, Raisis L, Bodenstab A, Newcomb W, et al. Improved function from progressive strengthening interventions after total knee arthroplasty: a randomized clinical trial with an imbedded prospective cohort. Arthritis Rheum. 2009;61(2):174–183. doi: 10.1002/art.24167. [DOI] [PubMed] [Google Scholar]
- Rudolph K, Schmitt L, Lewek M. Age related changes in strength, joint laxity, and walking patterns: are they related to knee osteoarthritis? Phys Ther. 2007;87(11):1422–1432. doi: 10.2522/ptj.20060137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt L, Rudolph K. Influences on knee movement strategies during walking in persons with medial knee osteoarthritis. Arth Rheum. 2007;57(6):1018–1026. doi: 10.1002/art.22889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Lloyd D, Wood D. Pre-surgery knee joint loading patterns during walking predict the presence and severity of anterior knee pain after total knee arthroplasty. J Orthop Res. 2004;22(2):260–266. doi: 10.1016/S0736-0266(03)00184-0. [DOI] [PubMed] [Google Scholar]
- Smith A, Lloyd D, Wood D. A kinematic and kinetic analysis of walking after total knee arthroplasty with and without patellar resurfacing. Clin Biomech. 2006;21(4):379–386. doi: 10.1016/j.clinbiomech.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley J, Balter J, Kohrt W, Eckhoff D. Quadriceps and hamstrings muscle dysfunction after total knee arthroplasty. Clin Orthop Relat Res. 2010;468(9):2460–2468. doi: 10.1007/s11999-009-1219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtonen A, Pöyhönen T, Heinonen A, Sipilä S. Muscle deficits persist after unilateral knee replacement and have implications for rehabilitation. Phys Ther. 2009;89(10):1072–1079. doi: 10.2522/ptj.20070295. [DOI] [PubMed] [Google Scholar]
- Yakhdani HRF, et al. Stability and variability of knee kinematics during gait in knee osteoarthritis before and after replacement surgery. Clin Biomech. 2010;25(3):230–236. doi: 10.1016/j.clinbiomech.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Mizner R, Ramsey D, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech. 2008;23(3):320–328. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni J, Higginson J. Differences in gait parameters between healthy subjects and persons with moderate and severe knee osteoarthritis: a result of altered walking speed? Clin Biomech. 2009;24(4):372–378. doi: 10.1016/j.clinbiomech.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni J, Higginson J. Knee osteoarthritis affects the distribution of joint moments during gait. Knee. 2011;18(3):156–159. doi: 10.1016/j.knee.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni J, Rudolph K, Higginson J. Alterations in quadriceps and hamstrings coordination in persons with medial compartment knee osteoarthritis. J Electromyogr Kinesiol. 2009;20(1):148–154. doi: 10.1016/j.jelekin.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.