Abstract
Facial cues contain important information for guiding social interactions, but not all humans are equally expert at face processing. A number of factors, both genetic and environmental, contribute to differences in face processing ability. For example, both heritable individual differences in temperament and exposure to childhood maltreatment are associated with alterations in face processing ability and social function. Understanding the neural correlates of alterations in face processing can provide insights into how genetic and environmental risk factors impair social functioning. We examined the association between childhood maltreatment and blood-oxygenation-level-dependent (BOLD) signal in a group of young adults with an inhibited temperament. We hypothesized that childhood maltreatment exposure would correlate positively with BOLD signal in regions subserving face processing and novelty detection during viewing of novel compared to familiar faces. Degree of exposure to childhood maltreatment was positively correlated with BOLD signal in the bilateral fusiform gyri and the left hippocampus. These findings suggest that young adults with an inhibited temperament and a history of maltreatment may be particularly vulnerable to neural alterations. These differences could be related to a heightened sensitivity to potential threat—for example, from new people, and may contribute to both the altered social functioning and increased incidence of anxiety disorders in these individuals.
Keywords: face processing, fusiform gyrus, hippocampus, fMRI, inhibited temperament, anxiety
1. Introduction
The ability to interact with others and interpret social information is critical for humans. Facial cues are an important source of information used in social interactions. Consistent with the importance of understanding facial cues, facial processing skills emerge early in infancy (for review, see Gauthier and Nelson 2001) and are refined throughout development. However, not all humans are equally expert at facial processing; variation in face processing ability may be due to a variety of factors, including genetic and environmental factors. For example, inhibited (or shy) temperament is a heritable (Robinson et al., 1992) and biologically based trait (Kagan et al., 1987; Goldsmith et al., 2000; Fox et al., 2005) that influences face processing ability. Shy children make more errors in identifying faces (Brunet et al., 2010), and both eye-tracking (Brunet et al., 2009) and ERP studies (Jetha et al., 2012) suggest altered sensitivity to emotional faces. Environmental insults, such as childhood maltreatment, also impact face processing. For example, adults who were maltreated as children show behavioral alterations in face processing, such as enhanced sensitivity to emotional faces, and attentional biases to threatening faces (Pollak et al., 2000; Pollak and Kistler, 2002; Gibb, Schofield, and Coles, 2009). Given that faces provide critical social cues, genetic or environmental factors that impact face processing may cause impairments in social functioning.
Identifying neural correlates of altered face processing can provide critical insights into pathophysiological risk factors related to impaired social functioning. Face processing is supported by a neural network composed of sensory regions—such as the fusiform face area (FFA)—and limbic regions—such as the amygdala and hippocampus. The FFA is a secondary visual cortical region that shows increased activation in response to viewing face stimuli (Grill-Spector et al., 2004). In humans, the FFA response to faces may represent its broader role in visual expertise (Gauthier and Tarr, 2002). In the medial temporal lobe, the amygdala and hippocampus are limbic regions critically involved in face processing. The amygdala is involved in processing of emotional faces (Fusar-Poli et al., 2009), modulation of face recognition (Gobbini and Haxby, 2007), and evaluation of potential threat, such as the trustworthiness of a face (Adolphs et al., 1998; Winston et al., 2002). The hippocampus modulates visual processing (Vuilleumier et al., 2004) by facilitating contextual interpretation of social stimuli such as faces, particularly face recognition and familiarity (Bengner and Malina, 2008).
Previous studies have shown that individuals with an inhibited temperament show alterations during face processing in the amygdala (Schwartz et al., 2003; Perez-Edgar et al., 2007, Blackford et al., 2011) and hippocampus (Blackford et al., 2012). Given the behavioral effects of childhood maltreatment on face processing ability, one might also expect for childhood maltreatment to be associated with neural differences during face processing; however, only a few studies have directly investigated this topic. Functional neuroimaging studies examining the neural correlates of trauma—either traumatic event histories or post-traumatic stress disorder—report altered amygdala activation to emotional stimuli in general (for review, see Lanius et al., 2006). When viewing threatening faces, adults with a history of childhood maltreatment show increased amygdala activation (Maheu et al., 2010; Dannlowski et al., 2012) and hippocampal activation (Maheu et al., 2010). When viewing sad faces, depressed adults with a history of childhood maltreatment demonstrate increased amygdala activation (Grant et al., 2011). These studies provide preliminary evidence for an association between childhood maltreatment and neural responses during emotional face processing; however, the ability to discriminate novel from familiar faces is also a critical component of face processing ability.
Quickly determining whether a face is new or familiar serves multiple functions, including allocating attentional resources to determine whether a new person is safe or potentially threatening, and facilitating positive social interactions with familiar people. Novel faces may be particularly salient because novel faces represent an unknown—ambiguous and potentially threatening—social stimulus (Masten et al., 2008; Jovanovic et al., 2009). The key brain regions involved in face processing are also involved in novel face detection. For example, both the amygdala and hippocampus are involved in the detection of novel stimuli (Yamaguchi et al., 2004; Rutishauser et al., 2006; Blackford et al., 2010), including novel faces (Rossion et al., 2003; Wright et al., 2003; Pedreira et al., 2010). The FFA is also sensitive to slight differences in faces (Halgren et al., 2000), suggesting that a sensory-limbic network facilitates novel face processing. The effects of childhood maltreatment on the neural substrates of novel face processing remain unknown.
Both inhibited temperament and childhood maltreatment are associated with behavioral deficits in face and emotion recognition, as well as alterations in the neural network processing of faces. Furthermore, a trait related to inhibited temperament—early difficult temperament—has been associated with an increased incidence of childhood maltreatment (Cohen and Brooks, 1987). Therefore, in a population that is already genetically predisposed to altered face processing, exposure to childhood maltreatment may compound existing neural alterations. To test this hypothesis, we examined the effects of childhood maltreatment on neural responses to novel faces in a sensory-limbic network in a group of inhibited young adults.
2. Methods
2.1 Participants
Eighteen young adults with an inhibited temperament were included in this study. Participants, recruited from the Nashville community, were ages 19 to 30 at the time of the study and had a mean age of 23 years (±2.91 years); 67 percent were female. Only one participant was left-handed. Thirteen participants were Caucasian, three were African American, one participant was Hispanic, and one was Asian. Written informed consent was obtained from participants prior to enrollment in the study.
Data from these participants were previously reported (Blackford et al, 2011). The present study is a novel analysis to test the effect of childhood maltreatment on novel face processing in the inhibited temperament group. While our primary question of interest was focused on the effects of maltreatment in the genetically more vulnerable inhibited group, we performed a post-hoc analysis with the uninhibited group (n = 15) to determine specificity of the observed effects. However, the uninhibited group has relatively low rates of maltreatment (mean = 6.40, SD =7.87) which limits the ability to detect significant relationships between maltreatment and brain function.
2.2 Temperament Measures
Temperament was assessed using two self-report instruments (Reznick et al., 1992): the Retrospective Self-Report of Inhibition (RSRI; child) and the Adult Self-Report of Inhibition (ASRI; adult). The RSRI is a 30-item assessment of behaviors during childhood and is scored on a likert scale ranging from 1 to 5 (1 = uninhibited, 5 = inhibited). Example questions include: “Did you enjoy meeting new children your age?” and “Were you scared of the dark?” The ASRI is a 31-item assessment of current behaviors and is also scored on a 1 to 5 likert scale. Example questions include: “Do you feel comfortable speaking in front of a large group of people?” and “Do open-air high places bother you?” Both measures have demonstrated reliability and validity in a non-selected sample (Reznick et al., 1992) and excellent reliability in this sample (Cronbach’s alpha = .84 and .82, respectively). Participants were determined to have inhibited temperament if they scored in the top 15% on both the RSRI and the ASRI scores based on normative population data (Reznick et al., 1992). The mean and standard deviations for RSRI and ASRI scores were 3.17 (SD =0.50) and 3.14 (SD = 0.38).
2.3 Childhood Maltreatment Measures
To assess severity of childhood maltreatment, participants were also administered the Childhood Trauma Questionnaire (CTQ; Bernstein et al., 2003). This questionnaire assesses five types of maltreatment including emotional abuse, emotional neglect, physical abuse, physical neglect and sexual abuse. Participants rate items on the CTQ using a 5-point scale ranging from “never true” to “very often true.” The CTQ has demonstrated reliability and validity (Scher et al., 2001; Paivio and Cramer, 2004) and reliability in this sample was high (Cronbach’s alpha = .92). The five childhood maltreatment subtypes scores are summed for a Total CTQ score, which ranges from 25 to 125. To better reflect the frequency of maltreatment in our sample, we subtracted 25 from all CTQ total scores to create a range from 0-100, with a score of 0 reflecting no reported maltreatment. To determine reporting of significant maltreatment, we used thresholds suggested by Walker et al. (1999).
2.4 Psychiatric Assessment
Participants were administered the Structured Clinical Interview for DSM-IV (SCID; Spitzer et al. 1992) by a trained interviewer. As expected, six participants met criteria for at least one type of anxiety disorder (several participants met criteria for more than one disorder): Social Anxiety Disorder (n = 5), Generalized Anxiety Disorder (n = 3), Specific Phobia (n = 1) and Anxiety Not Otherwise Specified (NOS; n = 2). Two participants also met criteria for comorbid Dysthymia and one participant met criteria for only Dysthymia.
2. 5 fMRI Task
Participants completed a previously validated fMRI task involving passive viewing of both novel and recently familiarized faces with neutral expressions (Blackford et al, 2011; Schwartz et al., 2003). Stimuli were black and white images of human faces selected from two standard sets of emotional expressions (Lundqvist et al., 1998; Gur et al., 2001). All images were edited to ensure uniform face size, as well as eye and nose position. All extraneous features (e.g. shirt collars, hair) were removed. Stimuli were randomly selected for the novel or familiar group and the two groups were balanced across gender and stimulus set. Participants were told while in the scanner, “In this study a face will appear in the middle of the screen. Your job is to stay focused on the screen and look at each face. The faces will flash quickly.”
During a pre-test phase, participants were familiarized to a set of six faces presented during four blocks for a total of eight presentations of each face. During the test phase, participants were randomly presented familiar faces and novel faces during four separate runs. Within each run participants viewed 12 distinct novel and 12 familiar faces (each of the 6 familiar faces presented twice) for a total of 48 novel and 48 familiar face presentations. Each novel face was only presented once across all runs.
Each face stimulus was presented for 0.5 seconds. The inter-stimulus interval consisted of a gray circle to maintain visual orientation, followed by a brief white circle as a cue for the upcoming face. Inter-stimulus intervals were jittered, with a 12 second average duration. Each run was approximately 300 seconds. To ensure that participants did attend to the stimuli and to assess encoding of the familiar faces, participants were given a brief recognition memory test after the scan. Participants were not told that there would be a recognition task after the scan. Two previous studies have shown that recognition memory is similar for inhibited and uninhibited participants (Blackford et al., 2009, Blackford et al., 2011). In this sample, recognition memory was good (familiar: M = 95%, SD = 7%; novel: M = 69%, SD = 17%) and was not significantly correlated with childhood maltreatment (both ps > .20).
Participants wore earplugs and headphones to reduce the MRI scanner sounds. Participants who required correction for 20/20 vision either wore contact lenses or MR compatible glasses while in the scanner.
2.6 fMRI Data Collection and Analysis
Functional and structural images were collected on a 3 Tesla magnet (Philips Healthcare, Inc., Best, The Netherlands). Functional echo planar images (EPI) were acquired using a sequence optimized for the amygdala and hippocampus: 2s TR, 22ms TE; 90° flip angle; 1.8 SENSE, 240 mm FOV; 3×3 mm in plane resolution using an 80×80 matrix (reconstructed to 128×128), and higher order shimming to limit susceptibility artifacts. Each volume contained 36 2.5mm (0.25 gap) axial oblique slices (titled 15° anterior higher than posterior relative to the intercommissural plane), which provided complete anterior–posterior coverage and inferior–superior coverage from the bottom of the temporal lobe to the top of the cingulate gyrus. High-resolution T1-weighted anatomical images were collected using the following parameters: 256mm FOV, 170 slices, 1-mm slice thickness, and 0-mm gap.
MRI data were pre-processed using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/) and Matlab (Version 7.1, The MathWorks, Inc, Natick, MA, USA). Data were slice time corrected, realigned to the first slice, resampled to 3×3×3mm voxels, spatially normalized into standard stereotactic space (MNI EPI template), and high pass filtered (128s). Data were smoothed with a 6-mm FWHM Gaussian kernel to account for individual differences in brain anatomy.
Data were modeled using the first-level general linear model function in SPM5. The two stimulus types (novel and familiar faces) were used as regressors and a novel – familiar contrast was created. To test for the effects of childhood maltreatment on signal change differences between novel and familiar faces, a correlation analysis was performed between CTQ total scores and the novel-familiar face contrast in SPM5. Given our apriori interest in cortico-limbic regions subserving visual processing, the analyses were restricted to three bilateral regions of interest (ROIs): the amygdala, the hippocampus and the fusiform gyrus. Each of these ROIs was defined using the AAL templates from the WFU pickatlas (Version 2.4; Maldjian et al., 2003). Cluster-based thresholding was used to control for Type I error. Based on simulations performed with AlphaSim (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), a family-wise error rate of α=<0.05 is achieved with the following cluster sizes: 11 voxels for the amygdala, 18 voxels for the hippocampus, and 29 voxels for the fusiform gyrus. To examine the specificity of the effect, correlations between the CTQ total score and the familiar-novel face contrast were also performed. Finally, an exploratory whole brain analysis was used to determine whether additional correlations between fMRI blood-oxygenation-level-dependent (BOLD) signal and CTQ total scores were present in other brain areas. A more conservative p-value was used because there were no apriori hypotheses (p < 0.005 cluster size =25).For significant clusters identified in the main analysis, percent signal change values were extracted using MarsBar (Brett et al., 2002). To confirm the SPM results, post hoc correlation analyses were performed using SAS (Version 9.1, SAS Institute Inc., Cary, NC, USA).
To test for possible effects of gender and anxiety diagnosis, two post-hoc regression analyses were performed for all significant effects. The first regression analysis included gender, CTQ scores, and their interaction as predictor variables and percent signal change from the significant clusters as the outcome variable. The second regression analysis was similar but included anxiety diagnosis, CTQ scores, and the anxiety X CTQ score interaction as predictors.
3. Results
In our sample of adults with an inhibited temperament, 56% (n = 10) of participants reported significant maltreatment exposure on at least one subscale; the mean CTQ total score was 13.56 (SD = 14.32). Within the maltreatment subscales, the most often reported form of childhood maltreatment was physical abuse, with 33% of participants reporting physical abuse. Seventeen percent of participants reported significant emotional neglect, 11% reported emotional abuse, 22% reported physical neglect, and 11% reported sexual abuse. Only two participants reported no maltreatment of any kind. The remaining six participants reported subthreshold levels of maltreatment (see Table 1).
Table 1. Childhood Maltreatment Frequency.
| Maltreatment Frequency | |||
|---|---|---|---|
|
| |||
| None | Minimal | Present | |
| Childhood Maltreatment | n (%) | n (%) | n (%) |
| Emotional Abuse | 4 (22%) | 12 (67%) | 2 (11%) |
| Emotional Neglect | 5 (28%) | 10 (56%) | 3 (17%) |
| Physical Abuse | 7 (39%) | 5 (28%) | 6 (33%) |
| Physical Neglect | 11 (61%) | 3 (17%) | 4 (22%) |
| Sexual Abuse | 16 (89%) | 0 (0%) | 2 (11%) |
| Maltreatment Total | 2 (11%) | 6 (33%) | 10 (56%) |
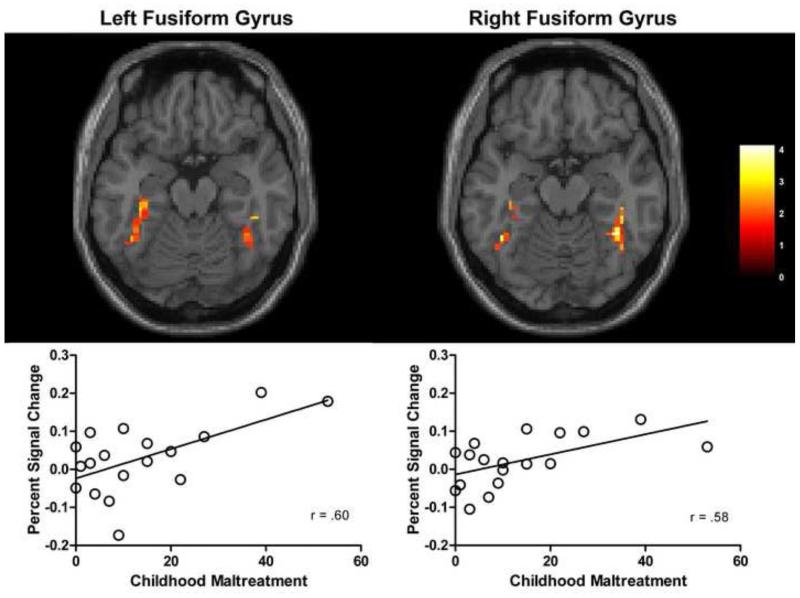
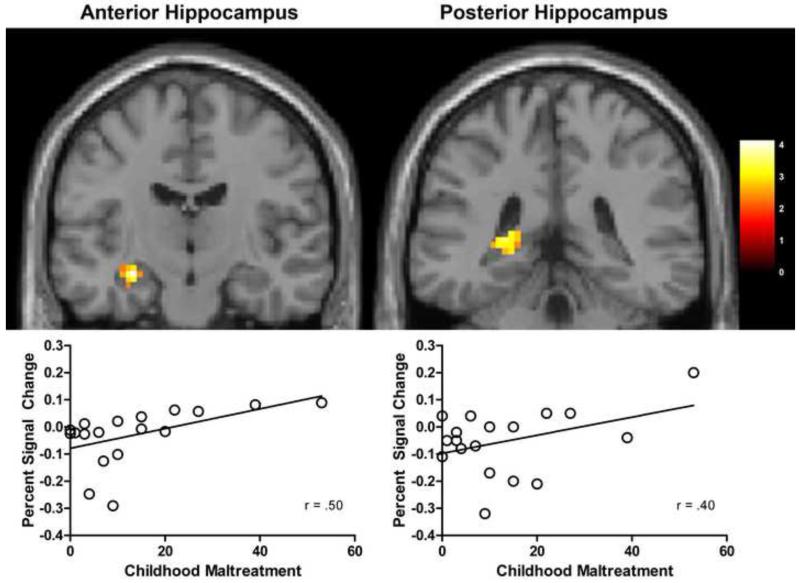
To investigate the role of childhood maltreatment in novel face processing in inhibited individuals, we examined the correlation between maltreatment frequency and BOLD signal in novel relative to familiar faces. Childhood maltreatment frequency was positively correlated with BOLD signal to novel faces in both the fusiform gyrus and the hippocampus. In the fusiform gyrus, CTQ total scores were positively correlated with BOLD signal in clusters of both the left and right gyri (Figure 1). In the hippocampus, CTQ total scores were correlated with BOLD signal in the left hippocampus, with significant clusters in both the anterior hippocampus and posterior hippocampus (Figure 2). CTQ scores were not correlated with activation in either the right hippocampus or the bilateral amygdala (correlations for the anatomical amygdala ROI both r = .10). CTQ scores were not correlated with the familiar > novel contrast in the regions of interest and the exploratory whole brain analyses revealed no significant positive or negative correlations in other brain regions.
Figure 1. Fusiform face area (FFA) BOLD signal is correlated with maltreatment exposure.
The figure shows regions in the bilateral fusiform gyri where BOLD signal is positively correlated with CTQ total scores, p <0.05. Left cluster size = 49 voxels, peak voxel at −42, −51, −15. Right cluster size = 35 voxels, peak voxel at 39, −48, −15. Images are displayed on a standard single subject MNI brain. The color bar shows t-values.
Figure 2. Hippocampal BOLD signal to novel faces is correlated with maltreatment exposure.
The figure shows regions in the a) left anterior hippocampus and b) left posterior hippocampus where BOLD signal is positively correlated with CTQ total scores, p <0.05. Anterior hippocampus: cluster size = 39 voxels, peak voxel at −27, −15, −15. Posterior hippocampus: cluster size = 22 voxels, peak voxel at −24, −39, 6. . Images are displayed on a standard single subject MNI brain. The color bar shows t-values.
To provide greater clarity regarding the study findings we performed several post-hoc analyses. First, to determine the relative contributions of response to novel faces and familiar faces, we performed post-hoc correlations between CTQ scores, BOLD signal to novel faces (vs. fixation) and BOLD signal to familiar faces (vs. fixation). For all of the significant clusters, the significant relationship between CTQ scores and BOLD signal was based on the difference between response to novel and familiar faces, such that CTQ scores were not significantly correlated with either response to novel faces alone or familiar faces alone (Supplemental Figure 1).
Next, we performed post-hoc analyses to examine potential effects of gender. CTQ scores were similar between males and females (p = .72). For the post-hoc regression analyses of gender effects, CTQ score was the only significant predictor for the bilateral fusiform gyri and the anterior hippocampus. However, for the posterior hippocampus, none of the predictors were significant, suggesting that gender, CTQ and the gender X CTQ interaction shared substantial variance.
Post-hoc analyses were also performed to examine potential effects of anxiety diagnosis. Participants with an anxiety diagnosis had higher CTQ scores than participants without a diagnosis (p = .06, trend). For the post-hoc regression analyses of anxiety effects, CTQ score was the only significant predictor for the bilateral fusiform gyri and the anterior hippocampus. However, for the posterior hippocampus, anxiety group, CTQ score, and the anxiety X CTQ score interaction were all significant predictors. The interaction was accounted for by a stronger correlation between CTQ score and posterior hippocampus activation in the anxiety group (r = .84) than in the non-anxiety group (r = .23).
To examine the potential effects of outliers, we reanalyzed the data after removing the subject with the highest CTQ score. All significant and null findings remained unchanged except for the right fusiform gyrus. In the right fusiform gyrus, the previously significant cluster (35 voxels) was reduced to 20 voxels and no longer met the cluster-based threshold for a corrected p < .05.
Finally, to determine whether the observed effects of maltreatment were specific to individuals with an inhibited temperament, we performed correlations between CTQ score and percent signal change in the left hippocampus, left FFA, and right FFA in the uninhibited group. CTQ scores were not significantly correlated with percent signal change (all ps > .25).
4. Discussion
In adults with an inhibited temperament, childhood maltreatment exposure was significantly correlated with greater activation to novel relative to familiar faces in the fusiform gyrus and hippocampus. Both the fusiform gyrus and hippocampus are associated with novelty detection and face processing, thus correlations between childhood maltreatment severity and BOLD signal in these areas may indicate a heightened sensitivity to novelty in inhibited individuals with reported childhood maltreatment. Our findings suggest that even modest exposure to childhood maltreatment in adults with inhibited temperament is associated with differences in a sensory feed-forward mechanism, suggesting a possible gene x environment interaction on a neural network involved in social-emotional stimulus processing. These neural alterations may manifest behaviorally as hypervigilance to novelty or potential threat that is associated with childhood maltreatment in inhibited adults.
Heightened response to novel faces in the fusiform gyrus, which includes the fusiform face area (FFA), was correlated with reported childhood maltreatment exposure. While behavioral studies have shown alterations in emotional face perception in those with maltreatment history (Pollak et al., 2000; Masten et al., 2008; Gibb, Schofield, and Coles, 2009; Leist and Dadds, 2009), there has been little investigation of alterations in FFA function in populations with childhood maltreatment history. Smaller FFA gray matter volume has been reported in adolescents (Edmiston et al., 2011) and young adults (Tomoda et al., 2009) with reported childhood maltreatment, suggesting that the fusiform face area may also be altered structurally in childhood maltreatment. The findings of increased fusiform gyrus activation to novel faces in this study may reflect increased detection sensitivity or hypervigilance to novel faces in inhibited individuals with a history of childhood maltreatment. This hypothesis is consistent with behavioral findings reporting that maltreated children detect fearful faces more quickly than non-maltreated controls (Masten et al., 2008). Hypervigilance to potential threat in individuals exposed to maltreatment, coupled with an inherited temperamental predisposition to avoid novelty and to interpret novelty as potentially threatening, may explain the increased signal change finding in the novel-familiar face contrast observed in this study.
Positive correlations between CTQ scores and response to novel relative to familiar faces in the hippocampus were also prominent findings in this study. Alterations in hippocampal structure have long been associated with exposure to stress, trauma, and childhood maltreatment (Bremner et al., 1997; Woon and Hedges, 2008). Increasingly, fMRI studies have suggested increased hippocampal activation in individuals with post-traumatic stress disorder (PTSD)—both during recall (Piefke et al., 2007; Hayes et al., 2011; St Jacques et al., 2011) and exposure to negatively valenced stimuli (Thomaes et al., 2009; Felmingham et al., 2010). A core feature of PTSD is attentional bias to threatening or potentially threatening stimuli, and inhibited temperament has been associated with both increased risk for the development of PTSD as well as PTSD symptom severity (Myers et al., 2012a; Myers et al., 2012b). Previous findings suggest that adults with maltreatment exposure show increased attentional bias—another marker of heightened sensitivity—to mildly threatening stimuli such as angry faces (Bradley et al., 1999; Mogg and Bradley, 2002; Pollak and Sinha, 2002). Taken together, these findings suggest that hippocampal alterations may be associated with maltreatment or traumatic event exposure, particularly in individuals with heritable risk factors, such as inhibited temperament.
Although several prior studies have shown differences in amygdala activation during emotional face processing following childhood maltreatment (Maheu et al., 2010; Grant et al., 2011; Tottenham et al., 2011; Dannlowski et al., 2012; van Harmelen et al., 2012), we were unable to detect significant effects in this study. One possible explanation is that we used neutral faces in our study and the previously reported amygdala effects may be limited to emotional faces. Studies of amygdala volume in maltreated children also report mixed findings, with both reported increases (Tottenham et al., 2010) and decreases (Driessen et al., 2000; Edmiston et al., 2011) in amygdala volume. The heterogeneity in functional and structural findings may be due to differences in timing and duration of exposure to the stressor, both factors that may alter the developmental trajectory of the amygdala (Tottenham and Sheridan, 2009) as well as the age of assessment. Future studies should assess the onset and duration of maltreatment exposure in addition to maltreatment type and severity to determine the effect of these timing factors on amygdala function.
While the study findings indicate a positive relationship between childhood maltreatment and brain activation to novel, relative to familiar faces, it is important to note that there was substantial variance in brain activation. In the regions studied, activation ranged from negative (familiar > novel) to flat to positive. Thus, the key finding is that childhood maltreatment predicted individual differences in brain response to novel faces. Because this is an initial report of this effect, it is important to conduct further studies to clarify the nature of the effect and provide additional clues about underlying mechanisms.
Reporting of childhood maltreatment in this sample was relatively mild and within normative ranges. Therefore the study findings suggest that even mild childhood maltreatment may contribute to changes in perception of social cues via alterations in a sensory-limbic network in individuals with an inhibited temperament. However, given the rates of anxiety disorders in our sample and the higher reports of childhood maltreatment in those with an anxiety disorder, it is possible that the increased response to novel faces is caused by the anxiety disorder. Response to novel faces in the posterior hippocampus was influenced by both anxiety and childhood maltreatment; however, the other significant clusters were uniquely predicted by childhood maltreatment even after controlling for anxiety.
There were several study limitations. Trauma incidence and severity were based on retrospective self-report, which may be biased; however, a recent study has shown that biases are minimal with retrospective self-report of childhood maltreatment (Fergusson et al., 2011). Also since the CTQ only assesses the presence of past childhood maltreatment, an assessment of duration or timing of maltreatment experiences was not possible. Another limitation is that we did not explicitly measure cognitive ability in this study; however, there were no obvious cognitive deficits in any participants based on performance during clinical interview. Finally, a non-inhibited control group was not explicitly included in this study, although our post-hoc analyses suggest that the observed effects were specific to the inhibited group. Future studies with larger sample sizes should directly test for gene X environment effects.
In summary, we propose that early exposure to maltreatment may prime sensory-limbic regions to be highly attuned to novelty especially in individuals with inherited risk, such as those with an inhibited temperament. The observed neural differences are consistent with behavioral findings in face processing and increased vigilance for threat, suggesting that heightened activation in the fusiform gyrus and hippocampus may underlie these behavioral differences. Furthermore, these neural differences may represent one mechanism by which incidence of anxiety disorders is increased in an already at risk population (Schwartz et al., 1999; Hirshfeld-Becker et al., 2007; Simon et al., 2009; Essex et al., 2010). Future work is needed to clarify the biological mechanisms of risk and resilience in both inhibited individuals and those exposed to childhood maltreatment to better provide prevention, assessment, and treatment of this vulnerable population.
Supplementary Material
Acknowledgements
We thank the research participants. Portions of this work were presented at the Workshop on Prosocial Behavior, Emory University, Atlanta, October 2011. This work was supported in part by funding from the National Institutes of Health (K01-MH083052 to JUB; Vanderbilt CTSA grant UL1 RR024975-01 from NCRR/NIH); and the Vanderbilt University Institute of Imaging Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Bengner T, Malina T. Remembering versus knowing during face recognition in unilateral temporal lobe epilepsy patients with or without hippocampal sclerosis. Brain and Cognition. 2008;68(2):148–156. doi: 10.1016/j.bandc.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nsr078. doi:10.109 (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience. 2009;10(10):145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Social Cognitive and Affective Neuroscience. 2011;6(5):621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. Neuroimage. 2010;50(3):1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology. 1999;38(Pt 3):267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological Psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage. 2002;16:s497. [Google Scholar]
- Brunet PM, Heisz JJ, Mondloch CJ, Shore DI, Schmidt LA. Shyness and face scanning in children. Journal of Anxiety Disorders. 2009;23(1):909–914. doi: 10.1016/j.janxdis.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Cohen P, Brook J. Family factors related to the persistence of psychopathology in childhood and adolescence. Psychiatry. 1987;50(4):332–345. doi: 10.1080/00332747.1987.11024365. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Aroit V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Petersen D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57(12):1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatric and Adolescent Medicine. 2011;165(12):1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. American Journal of Psychiatry. 2010;167(1):40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer E, Peduto A, Bryant R. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. Journal of Abnormal Psychology. 2010;119(1):241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Boden JM. Structural equation modeling of repeated retrospective reports of childhood maltreatment. International Journal of Methods in Psychiatric Research. 2011;20(2):93–104. doi: 10.1002/mpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F, Perez J, McGuire P, Politi P. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009;34(6):418–432. [PMC free article] [PubMed] [Google Scholar]
- Gauthier I, Nelson CA. The development of face expertise. Current Opinion in Neurobiology. 2001;11(2):219–224. doi: 10.1016/s0959-4388(00)00200-2. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Unraveling mechanisms for expert object recognition: bridging brain activity and behavior. Journal of Experimental Psychology: Human Perception and Performance. 2002;28(2):431–446. doi: 10.1037//0096-1523.28.2.431. [DOI] [PubMed] [Google Scholar]
- Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Childhood Maltreatment. 2009;14(2):148–156. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45(1):32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: a behavior-genetic perspective. Biological Psychiatry. 2000;48(12):1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Grant MM, Cannistraci C, Hollon SD, Gore J, Shelton R. Childhood trauma history differentiates amygdala response to sad faces within MDD. Journal of Psychiatric Research. 2011;45(7):886–895. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nature Neuroscience. 2004;7(5):555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE. Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology. 2001;25:766–776. doi: 10.1016/S0893-133X(01)00278-0. [DOI] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmäki V, Hari R. Cognitive response profile of the human fusiform face area as determined by MEG. Cerebral Cortex. 2000;10(1):69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Hayes JP, LaBar KS, McCarthy G, Selgrade E, Nasser J, Dolcos F, VISN 6 Mid-Atlantic MIRECC workgroup. Morey RA. Reduced hippocampal and amygdala activity predicts memory distortions for trauma reminders in combat-related PTSD. Journal of Psychiatric Research. 2011;45(5):660–669. doi: 10.1016/j.jpsychires.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, Rosenbaum JF. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. Journal of Developmental and Behavioral Pediatrics. 2007;28(3):225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- Jetha MK, Zheng X, Schmidt LA, Segalowitz SJ. Shyness and the first 100 ms of emotional face processing. Social Neuroscience. 2012;7(1):74–89. doi: 10.1080/17470919.2011.581539. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Blanding NQ, Norrholm SD, Duncan E, Bradley B, Ressler KJ. Childhood abuse is associated with increased startle reactivity in adulthood. Depression and Anxiety. 2009;26(11):1018–1026. doi: 10.1002/da.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Development. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Lanius RA, Bluhm R, Lanius U, Pain C. A review of neuroimaging studies in PTSD: heterogeneity of response to symptom provocation. Journal of Psychiatric Research. 2006;40(8):709–729. doi: 10.1016/j.jpsychires.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Leist T, Dadds MR. Adolescents’ ability to read different emotional faces relates to their history of maltreatment and type of psychopathology. Clinical Child Psychology and Psychiatry. 2009;14(2):237–250. doi: 10.1177/1359104508100887. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A,, Öhman A, Department of clinical neuroscience. Psychology section. Karolinska Institute The Karolinska Directed Emotional Faces. 1998 [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JYF, Ackerman JP, Pine DS, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cognitive Affective and Behavioral Neuroscience. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Masten CL, Guyer AE, Hodgdon HB, McClure EB, Charney DS, Ernst M, Kaufman J, Pine DS, Monk CS. Recognition of facial emotions among maltreated children with high rates of post-traumatic stress disorder. Child Abuse and Neglect. 2008;32(1):139–153. doi: 10.1016/j.chiabu.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research and Therapy. 2002;40(12):1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Myers CE, Van Meenen KM, Servatius RJ. Behavioral inhibition and PTSD symptoms in veterans. Psychiatry Research. 2012a;196(2-3):271–276. doi: 10.1016/j.psychres.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers CE, Van Meenen KM, McAuley JD, Beck KD, Pang KCH, Servarius RJ. Behaviorally inhibited temperament is associated with severity of post-traumatic stress disorder symptoms and faster eyeblink conditioning in veterans. Stress. 2012b;15(1):31–44. doi: 10.3109/10253890.2011.578184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paivio SC, Cramer KM. Factor structure and reliability of the Childhood Trauma Questionnaire in a Canadian undergraduate student sample. Child Abuse and Neglect. 2004;28(8):889–904. doi: 10.1016/j.chiabu.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Pedreira C, Mormann F, Kraskov A, Cerf M, Fried I, Koch C, Quiroga RQ. Responses of human medial temporal lobe neurons are modulated by stimulus repetition. Journal of Neurophysiology. 2010;103(1):97–107. doi: 10.1152/jn.91323.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piefke M, Pestinger M, Arin T, Kohl B, Kastrau F, Schnitker R, Vohn R, Weber J, Ohnhaus M, Erli HJ, Perlitz V, Paar O, Petzold ER, Flatten G. The neurofunctional mechanisms of traumatic and non-traumatic memory in patients with acute PTSD following accident trauma. Neurocase. 2007;13(5):342–357. doi: 10.1080/13554790701851494. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Developmental Psychology. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences USA. 2002;99(13):9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children’s recognition of facial displays of emotion. Developmental Psychology. 2002;38(5):784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Development and Psychopathology. 1992;4(2):301–321. [Google Scholar]
- Robinson JL, Reznick JS, Kagan J, Corley R. The heritability of inhibited and uninhibited behavior - a twin study. Developmental Psychology. 1992;28:1030–1037. [Google Scholar]
- Rossion B, Schlitz C, Crommelinck M. The functionally defined right occipital and fusiform “face areas” discriminate between novel from visually familiar faces. Neuroimage. 2003;19(3):8777–883. doi: 10.1016/s1053-8119(03)00105-8. [DOI] [PubMed] [Google Scholar]
- Rutishauser U, Mamelak AN, Schuman EM. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron. 2006;49(6):805–813. doi: 10.1016/j.neuron.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. Journal of Traumatic Stress. 2001;14(4):843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biological Psychiatry. 2003;53(10):854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Simon NM, Herlands NN, Marks EH, Mancini C, Letamendi A, Li Z, Pollack MH, Van Ameringen M, Stein MB. Childhood maltreatment linked to greater symptom severity and poorer quality of life and function in social anxiety disorder. Depression and Anxiety. 2009;26(11):1027–1032. doi: 10.1002/da.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. Journal of Psychiatric Research. 2011;45(5):630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomaes K, Dorrepaal E, Draijer NP, de Ruiter MB, Elzinga BM, van Balkom AJ, Smoor PL, Smit J, Veltman DJ. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Research. 2009;171(1):44–53. doi: 10.1016/j.pscychresns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Tomoda A, Navalta CP, Polcari A, Sadato N, Teicher MH. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biological Psychiatry. 2009;66(7):642–648. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Developmental Psychology. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Millner A, Galvan A, Davidson MC, Elgsti IM, Thomas KM, Freed PJ, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Psychology. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, Demenescu LR, van der Wee NJ, Veltman DJ, Aleman A, van Buchem MA, Spinhoven P, Penninx BW, Elzinga BM. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Social Cognitive and Affective Neuroscience. 2012 doi: 10.1093/scan/nss007. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Walker EA, Gelfand A, Katon WJ, Koss MP, Von Korff M, Bernstein D, Russo J. Adult health status of women with histories of childhood abuse and neglect. American Journal of Medicine. 1999;107(4):332–339. doi: 10.1016/s0002-9343(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Wright CI, Martis B, Schwartz CE, Shin LM, Fischer H,H, McMullin K, Rauch SL. Novelty responses and differential effects of order in the amygdala, substantia innominata, and inferior temporal cortex. Neuroimage. 2003;18(3):660–669. doi: 10.1016/s1053-8119(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Hale LA, D’Esposito M, Knight RT. Rapid prefrontal-hippocampal habituation to novel events. Journal of Neuroscience. 2004;24(23):5356–5363. doi: 10.1523/JNEUROSCI.4587-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.