Abstract
Purpose
To directly compare the clinical effectiveness of maxillomandibular advancement (MMA) and uvulopalatopharyngoplasty (UPPP)—performed alone and in combination—for the treatment of moderate to severe obstructive sleep apnea (OSA).
Methods
The investigators designed and implemented a retrospective cohort study composed of subjects with moderate to severe OSA (baseline AHI >15). The predictor variable was operative treatment and included: MMA, UPPP, and MMA followed by UPPP (UPPP/MMA). The primary outcome variable was the apnea-hypopnea index (AHI) measured preoperatively and 3–6 months postoperatively. Other variables were grouped into the following categories: demographic, respiratory and sleep parameters. Descriptive and bivariate statistics were computed.
Results
The sample was composed of 106 subjects grouped as follows: MMA (n=37), UPPP (n=34), and UPPP/MMA (n=35) for treatment of OSA. There were no significant differences between the three groups for the study variables at baseline, except for AHI. Surgical treatment resulted in a significant decrease in AHI in each group: MMA (baseline AHI, 56.3 ± 22.6 vs. AHI after MMA, 11.4 ± 9.8; P <0.0001), UPPP/MMA (baseline AHI, 55.7 ± 49.2 vs. AHI after UPPP/MMA, 11.6 ± 10.7; P <0.0001) and UPPP (baseline AHI, 41.8 ± 28.0 vs. AHI after UPPP, 30.1 ± 27.5; P = 0.0057). After adjusting for differences in baseline AHI, the estimated mean change in AHI was significantly larger for MMA compared to UPPP (MMA AHI, −40.5 vs. UPPP AHI, –19.4; P = <0.0001). UPPP/MMA was no more effective than MMA (P = 0.684).
Conclusion
The results of this study suggest that MMA should be the surgical treatment option of choice for most patients with moderate to severe OSA who are unable to adequately adhere to CPAP.
Keywords: Obstructive Sleep Apnea, Maxillomandibular Advancement, Uvulopalatopharyngoplasty
Introduction
Obstructive sleep apnea (OSA) is a common disorder affecting at least 2–4% of the adult population and is associated with hypertension,[1–3] diabetes,[4] cardiovascular disease,[5] a diminished quality of life,[6–8] and an increased rate of motor vehicle accidents;[9] thus making it a significant public health concern.[10] Longitudinal observational cohort studies indicate that severe OSA significantly increases the risk of cardiovascular events,[11] including stroke and death.[12
Continuous positive airway pressure (CPAP) is the accepted first-line therapy for patients with OSA.[13] Significant improvements in objective and subjective sleepiness, quality of life and cognitive function have been demonstrated following the use of CPAP.[13] Furthermore, effective treatment of severe OSA by CPAP reduces the risk of cardiovascular events.[11] The major barrier to achieving clinically effective treatment is non-compliance with CPAP therapy; which leaves the patient untreated and at increased risk for cardiovascular events.
Surgical modifications of the upper airway such as maxillomandibular advancement (MMA) and uvulopalatopharyngoplasty (UPPP) have been used as alternative therapies for patients with OSA who are unable to adhere to CPAP therapy.[14–20] Several observational studies have shown that MMA and UPPP produce an improvement in OSA—although MMA consistently produces a larger decrease in the apnea-hypopnea index (AHI) than UPPP.[15
While some surgeons have used MMA as an isolated primary procedure,[18, 21] others have advocated a phased approach to surgical treatment of OSA.[22] In the phased approach, UPPP and possibly adjunctive procedures, such as genioglossal advancement and/or hyoid repositioning/myotomy [22] are performed as the initial phase of therapy (Phase I). If Phase I surgery does not produce a successful outcome, the patient would then proceed onto Phase II surgery consisting of MMA. It is unknown if phased surgery—comprised of UPPP followed by MMA—provides any additional benefit beyond MMA performed alone as the primary procedure.
To the best of the author’s knowledge, there have been no studies that have directly compared the clinical effectiveness of UPPP and MMA—performed alone and in combination—for the treatment of moderate to severe OSA, as measured by changes in AHI. The authors hypothesize that MMA performed alone is more effective than UPPP alone, and as effective as UPPP followed by MMA, for treatment of moderate to severe OSA. The specific aim of this study was to directly compare the clinical effectiveness of UPPP and MMA—performed alone or in combination—for treatment of moderate to severe OSA.
Methods
Study Design/Sample
The authors designed and implemented a retrospective cohort study composed of patients with moderate to severe OSA (baseline AHI >15) who underwent UPPP and MMA alone or in combination for the treatment of OSA at Vanderbilt University Medical Center (VUMC) between 1997 and 2007; and Henry Ford Hospital (HFH) in Detroit, Michigan, between 1990 and 1996. This research protocol was approved by the Vanderbilt University IRB (Human Research Protection Program) and ethical standards were used to conduct this research.
The following criteria were used for inclusion in the study: 1) Adults 18 years or older, 2) moderate to severe OSA (AHI >15) as determined by an overnight in-laboratory diagnostic polysomnography (PSG), 3) completion of CPAP Titration PSG, 4) inability to tolerate or adhere to CPAP therapy, 5) completion of MMA and/or UPPP for treatment of OSA, and 7) PSG performed 3–6 months following MMA and/or UPPP. Patients who had previous upper airway or craniofacial surgery, not related to the treatment of OSA, were excluded from the study, as well as any individuals who had inadequate existing data at the baseline and postsurgical time intervals. No body mass index (BMI) or anatomic criteria were used to determine inclusion or exclusion of patients from the study.
A focused electronic medical record query identified a cohort of 110 patients with moderate to severe OSA who had undergone MMA and/or UPPP at VUMC. An additional 18 patients were identified that had MMA with or without UPPP at HFH between 1990 and 1996. 82.8% (106/128) of all potential subjects met criteria for inclusion in the study, and the vast majority of patients in the study group were treated at VUMC (85%). There was a nearly equal distribution of the 106 patients into each of the three treatment groups— MMA (n=37), UPPP (n=34), and UPPP followed by MMA (UPPP/MMA) (n=35). All MMA procedures—MMA alone or UPPP/MMA—were consecutively performed by the senior author at either VUMC (n= 56) or HFH (n=16). All patients who underwent UPPP alone had their surgery performed at VUMC— between 1997 and 2007—by multiple surgeons using similar techniques to perform the UPPP. 11.4% (3/35) of patients in the UPPP/MMA group had their UPPP procedure performed at VUMC.
Study Variables
The primary outcome measure used in this study was changes in the AHI after surgical treatment. The predictor variable was operative treatment and included: MMA, UPPP, and MMA followed by UPPP (UPPP/MMA). Secondary outcome measures included changes in: low SaO2%, total sleep time (TST), % sleep efficiency, N1 Sleep %, N2 Sleep %, N3 Sleep % and REM Sleep %. Demographic and other study variables included: age (years), gender, body mass index (BMI; kilograms per meter squared), co-morbid medical conditions, and CPAP Titration AHI.
Surgical Procedures
All MMA procedures were performed using standardized surgical techniques, which included: 1) a total Le Fort I maxillary osteotomy, 2) bilateral sagittal split ramus osteotomies of the mandible, and 3) genioglossal advancement if indicated.[23] The Le Fort 1 total maxillary osteotomy was performed first, using standard techniques[24] with a modified step design.[25, 26] After completion of the osteotomy, the maxilla was down- fractured and mobilized until it could be passively positioned forward into an interim splint, with a planned surgical advancement of approximately 10mm. The maxilla was then fixated with four 2.0 mm L-shaped bone plates, placed at the piriform rim and zygomatico-maxillary buttress regions bilaterally. To minimize over-projection of the naso-maxillary complex, piriform rim re-contouring, anterior nasal spine reduction and a limited septoplasty were completed. Additionally, the soft tissues were managed by placement of an alar base controlling suture and v-y closure of the upper lip. No maxillary bone grafts were used. Bilateral sagittal split ramus osteotomies of the mandible were then completed to advance the mandible a minimum of 10mm, using standard techniques, including the Epker modification[27] of the technique originally developed by Trauner and Obwegeser.[28, 29] The mandibular osteotomies were then fixated in the advanced position by bone plates and mono-cortical screws. A genioglossal advancement was then performed for patients with microgenia, following completion of the maxillary and mandibular osteotomies. The surgical procedure consisted of an osteotomy of the inferior border of the anterior mandible, with the superior border of the osteotomy encompassing the entire attachment of the genioglossus muscle. After completion of the osteotomy the musculoskeletal segment was advanced approximately 10mm. The genioglossal advancement procedure was commonly performed concomitantly with MMA (40.5%; 15/37) or as part of the UPPP/MMA phased protocol (42.9%; 15/35). After the completion of the MMA surgical procedure, standard of care practices were used for perioperative and post-operative patient management. Each patient was maintained in intermaxillary fixation for 3 weeks. The same MMA surgical procedures were used in both the MMA alone and UPPP/MMA treatment groups.
The UPPP procedures were performed by multiple surgeons using the technique, as first described by Fujita.[30] The procedure involves removal of tonsils if present, excision of the uvula/posterior soft palate and redundant mucosa of the anterior and posterior tonsillar pillars, and then closure of the tonsillar pillars. 79.4% (27/34) of patients in the UPPP alone group underwent tonsillectomy, while no patients in the UPPP/MMA reportedly had tonsillectomy performed concomitantly with UPPP. Furthermore, the vast majority of patients in the UPPP alone group underwent concomitant nasal surgery (94.1%; 32/34), while 42.9% (15/35) of patients in the UPPP/MMA group had nasal surgery performed concomitantly with UPPP. Genioglossal advancement was rarely performed (2.9%, 1/34) concomitantly with UPPP.
Polysomnography
All patients in the study underwent a standard multichannel overnight diagnostic PSG at baseline and then completed a follow-up postsurgical PSG about 3–6 months after MMA (mean = 4.0 ± 1.9 months) or UPPP (mean = 4.4 ± 6.1 months). Standard variables were measured for each PSG including: AHI, low SaO2%, TST, % sleep efficiency, N1 Sleep (transition to sleep), N2 Sleep (light sleep), N3 Sleep (deep sleep) , and REM Sleep (dream sleep). An approximate range of normal values for the percentage of total sleep time for each of the sleep stages for adults is as follows: N1—5%, N2—45–55%, N3—13–20% and REM—20%. The CPAP Titration AHI was determined during the CPAP Titration PSG. In no studies were respiratory event related arousals (RERAs) reported. Results of the PSG studies were used to define the presence and severity of OSA at baseline and then to determine the clinical effectiveness of surgical treatment by measuring changes in in the outcome measures after surgery.
Data Collection and Statistical Analyses
As data was collected, it was directly entered into a customized clinical research database (REDCap) that was designed to store all demographic and polysomnographic data collected at the baseline and post-surgical time intervals. Data was then analyzed using the statistical software SAS for Windows (Version 9.1.3, SAS Institute, Cary, NC) and R (www.r-project.org). Descriptive statistical analysis was performed for all demographic and outcome variables at baseline and then again 3–6 months after surgery, and reported as mean ± standard deviation for continuous variables and frequency and percent for categorical variables. Kruskal – Wallis Test and Chi-Square Test were used to test for differences in demographic and outcome measurements between the three surgical groups at baseline. Spearman’s rank correlation coefficients and Mann-Whitney Test were used to assess the association between study variables and baseline AHI. Within each surgical group, the Wilcoxon Signed Rank Test was used to evaluate the unadjusted treatment effect for each of the outcome measures. Kruskal-Wallis statistics were used to test for differences between the three study groups. Changes in AHI were estimated (along with 95% confidence intervals) for each of the surgical treatment groups, using an analysis of covariance (ANCOVA) which adjusted for differences in baseline AHI values. Similar ANCOVA models were used to perform a subgroup analysis to assess the estimated treatment effect of tonsillectomy performed concomitantly with UPPP, and genial advancement performed concomitantly with MMA. For all analyses, P ≤ .05 was considered statistically significant. Additionally, the mean percent reduction in the AHI (baseline AHI minus postoperative AHI divided by baseline AHI), and the proportion of patients reaching specific levels of treatment effectiveness (AHI improved; AHI <20 with 50% reduction in AHI; AHI ≤15; AHI ≤ 5) were determined. Patients were classified for severity of OSA as: normal (AHI ≤ 5), mild (AHI >5–15), moderate (AHI >15–30) or severe (AHI >30).
Results
Baseline Analysis
The study cohort of 106 patients was composed primarily of middle age (mean age, 45.2 ± 10.4 yr.), obese (baseline body mass index, 31.1 ± 5.6 kg/m2) men (78.3%) with severe OSA (baseline AHI, 50.1 ± 33.5) and significant oxyhemoglobin desaturations (baseline low SaO2%, 77.7 ± 13.2). 95% of patients complained of excessive daytime sleepiness prior to surgical treatment. The study cohort was observed to have comorbid medical conditions; most commonly hypertension (32.7%)—with no significant differences in distribution between the three surgical groups (P = 0.3442). Less frequently occurring medical conditions included: depression (16.3%), GERD (16.3%), lung disease (7.7%), hypothyroidism (5.8%), cardiac dysrhythmias (4.8%), diabetes (4.8%), coronary artery disease (2.9%), and a history of myocardial infarction (2.9%) or stroke (2.9%). Sleep architecture for the study cohort was disrupted at baseline. Specific abnormalities in sleep architecture included: decreased TST (baseline, 300.2 ± 119.2 minutes), decreased % Sleep Efficiency (baseline, 82.6 ± 11.2), decreased REM Sleep % (baseline, 11.8 ± 8.1), and increased N1 Sleep % (baseline, 21.5 ± 20.3). CPAP was found to be highly efficacious, as measured by changes in the AHI during the CPAP Titration PSG (CPAP Titration AHI, 4.1 ± 7.7).
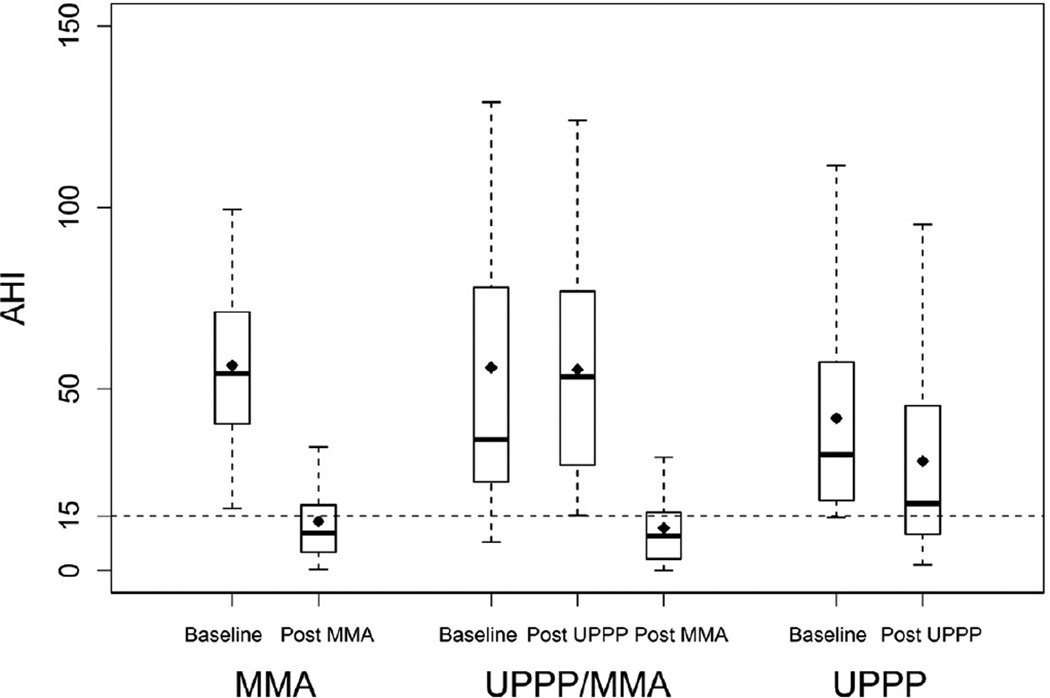
No significant differences at baseline were observed between the three surgical groups for most of the study variables including: age, gender, BMI, low SaO2%, and CPAP Titration AHI (Table 1). However, there were significant differences between the surgical groups for baseline AHI (Table 1). The UPPP group had a significantly lower baseline AHI (P = 0.0063), compared to the MMA and UPPP/MMA groups. The MMA and UPPP/MMA groups were not significantly different for baseline AHI (P = 0.7208), although there was a wider spectrum of baseline disease severity in the UPPP/MMA group (Figure 1). Furthermore, more patients in the MMA group were classified as having severe OSA (89%), compared to either the UPPP/MMA (51%) or UPPP (50%) groups. Additionally, there were no significant associations between baseline AHI and any of the study variables except for gender (P = 0.0025) and low SaO2% (P = 0.0029); where men (baseline AHI, 56.2 ± 36.6) had a higher baseline AHI than women (baseline AHI, 34.4 ± 18.0), and a lower baseline SaO2% was associated with a higher baseline AHI.
Table 1.
BIVARIATE ASSOCIATIONS BETWEEN STUDY VARIABLES AND PREDICTOR VARIABLE AT BASELINE
| Predictor |
||||
|---|---|---|---|---|
| Study Variable | MMA1 (N=37) |
UPPP/MMA1 (N=35) |
UPPP1 (N=35) |
P Value3 |
| Age (yr.) | 44.2 ± 9.0 | 45.3 ± 11.0 | 46.1 ± 12.4 | 0.7407 |
| Men (%)2 | 27 (73.0%) | 31 (88.6%) | 25 (73.5%) | 0.1972 |
| BMI (kg/m2) | 29.8 ± 3.8 | 31.5 ± 5.9 | 33.2 ± 7.7 | 0.1749 |
| AHI | 56.3 ± 22.6 | 55.7 ± 49.2 | 41.8± 28.0 | 0.018 |
| Low SaO2% | 74.2 ± 13.8 | 80.6 ± 9.5 | 76.3 ± 16.6 | 0.109 |
| CPAP Titration AHI | 4.3 ± 5.9 | 2.6 ± 4.4 | 4.0 ± 9.5 | 0.4895 |
Abbreviations: MMA, maxillomandibular advancement; UPPP, uvulopalatopharyngoplasty; BMI, body mass index; AHI, apnea-hypopnea index; SaO2%, oxygen saturation percentage; CPAP, continuous positive airway pressure
Shown are mean ± standard deviation
Number of men (total percentage of men in sample)
P values shown are based on χ2 test and Kruskal-Wallis statistics test for differences between all three study groups
Figure 1.
Intergroup comparisons of changes in AHI following surgical treatment. The bottom and top of the box represent the 25th and 75th percentile, which is bisected by the median value; black diamond represents the mean value; whiskers are used to represent the upper and lower values.
Respiratory Analysis
Surgical treatment resulted in a significant decrease in the AHI for all three groups: MMA (baseline AHI, 56.3 ± 22.6 vs. AHI after MMA, 11.4 ± 9.8; P <0.0001), UPPP/MMA (baseline AHI, 55.7 ± 49.2 vs. AHI after UPPP/MMA, 11.6 ± 10.7; P <0.0001)and UPPP (baseline AHI, 41.8 ± 28.0 vs. AHI after UPPP, 30.1 ± 27.5; P = 0.0057) (Figure 1). However, there was a significant difference in the magnitude of the change in the AHI when comparing MMA to UPPP, but not when comparing MMA to UPPP/MMA (Table 2). Nearly an 80% decrease in AHI was observed for both the MMA (79.8%) and UPPP/MMA (79.2%) groups, while only a 28% decrease was observed following UPPP. After adjusting for baseline differences in AHI, the estimated change in AHI was significantly larger for MMA compared to UPPP; there was no significant difference when comparing MMA and UPPP/MMA (Table 3).
Table 2.
BIVARIATE ASSOCIATIONS BETWEEN CHANGE IN AHI AND PREDICTOR VARIABLE
| Predictor | P Values5 | |||||
|---|---|---|---|---|---|---|
| MMA1 | UPPP/MMA1 | UPPP1 | MMA vs. UPPP2 |
MMA vs. UPPP/MMA3 |
UPPP vs. UPPP/MMA4 |
|
| Mean change in AHI |
−44.9 ± 23.1 | −42.2 ± 48.1 | −11.8 ± 25.8 | <0.0001 | 0.1322 | <0.0001 |
Abbreviations: AHI, apnea-hypopnea index; MMA, maxillomandibular advancement; UPPP, uvulopalatopharyngoplasty
Shown are mean change in AHI [difference between baseline and post-treatment values] ± standard deviation.
Comparison of MMA and UPPP groups.
Comparison of MMA and UPPP/MMA groups.
Comparison of UPPP and UPPP/MMA groups.
P values shown are based on Kruskal-Wallis statistics test for differences between all three treatment groups
Table 3.
BIVARIATE ASSOCIATIONS BETWEEN ADJUSTED CHANGE IN AHI AND PREDICTOR VARIABLE
| Predictor | P Values5 | |||||
|---|---|---|---|---|---|---|
| MMA1 | UPPP/MMA1 | UPPP1 | MMA vs. UPPP2 |
MMA vs. UPPP/MMA3 |
UPPP vs. UPPP/MMA4 |
|
| Estimated Change in AHI |
−40.5 (−46.2,−34.9) | −38.7 (−45.1,−32.4) | −19.4 (−25.3 −13.5) | <0.0001 | 0.684 | <0.0001 |
Abbreviations: AHI, apnea-hypopnea index; MMA, maxillomandibular advancement; UPPP, uvulopalatopharyngoplasty
Shown are estimated change in AHI values and the associated 95% confidence intervals. The estimates were based on an ANCOVA model with baseline AHI values and treatment group indicator as covariates.
Comparison of MMA and UPPP groups.
Comparison of MMA and UPPP/MMA groups.
Comparison of UPPP and UPPP/MMA groups.
P values were derived from an ANCOVA model which adjusted for the differences in AHI values and were not adjusted for multiple comparisons.
95% of patients undergoing MMA or UPPP/MMA showed an improvement in the AHI after surgery, compared to 74% of patients undergoing UPPP. Subjective complaints of excessive daytime sleepiness (EDS) decreased from 95% of patients at baseline to only 6% (3/52) for patients following MMA—performed alone or in combination with UPPP. In contrast, 38% of patients who underwent UPPP continued to report symptoms of EDS following UPPP. Nearly 75% of all patients undergoing MMA (76%) or UPPP/MMA (71%) had an AHI of 15 or less after surgery, while only 41% of UPPP patients had an AHI of 15 or less after surgery. 32% of MMA and UPPP/MMA reached an AHI of ≤5 after surgery, while only 12% of UPPP reached this level of control of OSA. Additionally, 41% (14/34) of the UPPP group had residual severe OSA (AHI >30) following surgery. Four of the UPPP patients with residual severe OSA restarted CPAP therapy after surgery, which left 29% (10/34) of the entire UPPP group with untreated severe residual OSA after surgery.
A significant improvement in low SaO2% occurred in all three groups after surgery. The largest improvement was seen after MMA (baseline, 74.2 ± 13.8 vs. after MMA, 83.6 ± 10.5; P <0.0001), followed by UPPP/MMA (baseline, 80.6 ± 9.5 vs. after UPPP/MMA, 85.7 ± 6.0; P <0.0001) and then UPPP (baseline, 76.3 ± 16.6 vs. after UPPP, 79.0 ± 13.8; P = 0.0057). There was a significant difference in the level of improvement in low SaO2% when comparing MMA to UPPP (p=0.0094), but not when comparing MMA to UPPP/MMA (p=.7193).
Subgroup Analysis
After adjusting for baseline differences in AHI, the estimated change in AHI was considerably larger for those patients who had tonsillectomy performed as a component of UPPP (–19.4; CI: –31.2 to –7.7) , compared to those patients who did not have tonsillectomy (–8.0; CI: – 19.5 to 3.4). This level of difference (11.4; CI –5.12 to 27.9) is clinically significant, but did not meet the level of statistical significance (P = 0.1704). Likewise, after adjusting for baseline differences in low SaO2%, the estimated change in low SaO2% was larger for those patients who did have tonsillectomy (7.5 %; CI: –3.0 to 12), compared to those patients who did not have tonsillectomy (3.5%; CI: –0.5 to 7.6). This level of difference (–4.0; CI: –10.0 to 2.1), did not meet the level of statistical significance (P = 0.1915).
After adjusting for baseline differences in AHI, the estimated change in AHI was not significantly different (P = 0.6533) when comparing those patients who had genioglossal advancement (–28.7; CI: –37.4 to –19.9) performed concomitantly with MMA, to those patients who did not have genioglossal advancement (–31.3; CI: –38.4 to –24.1). Likewise, after adjusting for baseline differences in low SaO2%, there was no statistically significant difference (P = 0.1549) in the estimated change in low SaO2% when comparing those patients who did have genioglossal advancement (2.4: CI: –2.2 to 7.1) to those who did not (6.9; CI: 3.1 to 10.7).
Sleep Analysis
Surgical treatment resulted in an improvement in sleep architecture which occurred to varying degrees amongst the three surgical groups. There was a large increase in TST after MMA (baseline TST, 278.1 ± 129.3 minutes vs. TST after MMA, 350.7 ± 71.3 minutes; P = 0.0007) and UPPP/MMA (baseline TST, 290.7 ± 137 minutes vs. TST after UPPP/MMA, 372.3 ± 54.1; P =0.0532), but no change after UPPP (baseline TST, 300.5 ± 121.6 minutes vs. TST after UPPP, 299.91 ± 127.4; P = 0.2665). There was an increase in REM Sleep % for all three groups, but the changes were statistically significant only for MMA: MMA (baseline REM Sleep %, 10.5 ± 7.9 vs. REM Sleep % after MMA, 16.8 ± 7.2; P = <0.0001), UPPP/MMA (baseline REM Sleep %, 11.7 ± 8.5 vs. REM Sleep % after UPPP/MMA, 15.5 ± 9.5; P =0.8469), and UPPP (baseline REM Sleep %, 12.5 ± 9.1vs. REM Sleep % after UPPP, 15.1 ± 7.4; P = 0.1928). There was a concomitant significant reduction in the N1 Sleep % for both MMA (baseline N1 Sleep %, 21.1 ± 21.1 vs. N1 Sleep % after MMA, 11.0 ± 8.15; P = 0.0067) and UPPP/MMA (baseline N1 Sleep %, 33.1 ± 20.7 vs. N1 Sleep % after UPPP/MMA, 12.8 ± 9.77; P =0.0014), but not for UPPP (baseline N1 Sleep %, 18.7 ± 21.5 vs. N1 Sleep % after UPPP, 13.7 ± 10.0; P = 0.8176). Sleep efficiency improved an insignificant amount in all three surgical groups. There were no significant changes observed in N2 Sleep% and N3 Sleep%.
Discussion
This study was performed to directly compare the clinical effectiveness of MMA and UPPP, performed alone or in combination, for the treatment of moderate to severe OSA in adults, with changes in AHI as the primary outcome measure. The results of this study confirm the author’s hypothesis that MMA performed alone is clinically more effective than UPPP alone, and is equivalent in effectiveness to phased therapy consisting of UPPP followed by MMA, for treatment of moderate to severe OSA. Furthermore, the results of this study suggest that there is no significant clinical benefit to performing genioglossal advancement concomitantly with MMA.
The authors observed analogous findings for the secondary outcome measurements. Overall, improvements in low SaO2%, TST, N1 Sleep % and REM Sleep % were generally observed for each of the three surgical groups, but the magnitude of improvements were consistently larger for MMA compared to UPPP. Furthermore, the performance of UPPP followed by MMA, did not produce a better result than MMA performed alone.
As an adjunct to the American Academy of Sleep Medicine (AASM) Standards of Practice, Caples and coworkers in 2010 reviewed the available literature and performed a meta-analysis of the clinical effectiveness of surgical treatment for OSA in adults, which included MMA and UPPP.[15] The findings of the AASM meta-analysis are comparable to the findings of this study. The meta-analysis showed a mean reduction in AHI of 46.8 (mean baseline AHI, 54.5 to mean AHI after MMA, 7.7) when MMA was performed as the primary surgical procedure; which is similar to the unadjusted mean reduction in AHI of 44.9 (mean baseline AHI, 56.3 to mean AHI after MMA, 11.4) observed for the MMA group in this study. The AASM has published practice parameters for surgery in OSA in adults and recommends that MMA is indicated for surgical treatment of severe OSA in patients who cannot tolerate or who are unwilling to adhere to positive airway pressure therapy, or in whom oral appliances, which are more often appropriate in mild and moderate OSA patients, have been considered and found ineffective or undesirable (Option).[31
The UPPP results of the AASM meta-analysis[15] are also comparable to the results of this study. The AASM reported a mean reduction in AHI of 10.5 (mean baseline AHI 40.3 to mean AHI after UPPP, 29.8), which is equivalent to the unadjusted mean reduction in AHI of 11.7 (mean baseline AHI, 41.8 to mean AHI after UPPP, 30.1) observed for the UPPP alone group in this study. Both the meta-analysis and the findings of this study demonstrated that patients with moderate to severe OSA at baseline will on average have a residual AHI of about 30 following UPPP. Another meta-analysis of UPPP performed by Sher and coworkers[32], indicates that UPPP performed alone in unselected patients is clinically effective in less than 50% of patients with OSA, which is similar to the results of this study. In addition to the relatively modest change in AHI after UPPP, the initial improvements that do occur may diminish over time and in some cases OSA can become worse after UPPP.[33, 34] The potential for worsening of OSA after UPPP was observed in this study where 40% of patients in the UPPP/MMA group had an increase in the AHI after UPPP, while 26% of patients in the UPPP alone group had an increase in AHI after UPPP. The AASM has published practice parameters for UPPP and recommends that UPPP as a sole procedure, with or without tonsillectomy, does not reliably normalize the AHI when treating moderate to severe OSA. [31
Holty and Guilleminault have published a review and meta-analysis for MMA, with the majority of subjects (67%) having undergone UPPP prior to or concurrent with MMA. [17] The pooled MMA results—both MMA alone and UPPP followed by MMA—showed a mean reduction in AHI of 54.4 (mean baseline AHI, 63.9 to mean AHI after MMA, 9.5). These results are similar to the findings of this study, although the mean baseline AHI reported in the meta-analysis was higher, which may explain the larger mean reduction in AHI, as the post-operative AHIs were similar. Both MMA meta-analyses [15, 17] and the results of this study indicate that MMA--performed alone or in combination with UPPP—results in a consistent and significant reduction in AHI. Furthermore, there appears to be no clinically significant difference between MMA performed alone and phased therapy consisting of UPPP followed by MMA.
The phased surgical protocol of UPPP followed by MMA was presumably developed in response to the relatively limited treatment effect of UPPP alone. The reported success rates for phase I surgery have varied between 22.7%[14] and 78%.[35] Overall, success rates for Phase I appear to be similar to those reported for UPPP performed alone. Furthermore, in this study we observed that for those patients who failed UPPP and then proceeded onto MMA; on average UPPP contributed essentially nothing (1% reduction in AHI) to the overall treatment effect of phased therapy. The large reduction in AHI that predictably occurs following MMA will likely overwhelm any treatment effect that UPPP may contribute to the phased approach for management of moderate to severe OSA. Additionally, the results of this study indicate that for those patients undergoing phased treatment, the initial response to UPPP—worsening or improvement in AHI—did not impact or predict the subsequent response to MMA, as there was essentially no difference in the change in AHI resulting from MMA performed alone or following UPPP. In summary, there appears to be no additive benefit to have had UPPP performed prior to MMA, as measured by changes in AHI.
There are other important considerations—beyond the equivalency in clinical effectiveness— that strongly favor using MMA alone over phased therapy as the treatment of choice for moderate to severe OSA. MMA performed as the sole surgical procedure has the potential to provide the most cost effective approach to treatment and will concomitantly expose the patient to less surgical and anesthetic risk, compared to phased therapy. In addition to the problem of limited effectiveness for phase I surgery, it has been reported that only between 26%[36] and 53%[14] of phase I failures proceed onto phase II (MMA). Therefore, adherence to a phased protocol does not appear warranted, especially for those patients with severe apnea, because they will continue to be exposed to increased risk for fatal and non-fatal cardiovascular events[11, 12], if they remain inadequately treated after UPPP.
One of the potential limitations of this investigation is that this is a retrospective observational cohort study design in contrast to the preferred randomized clinical trial—which is a common inherent problem in conducting surgical clinical trials. Although the study population was derived from consecutive convenience sampling, there were an essentially equivalent number of subjects in each treatment group, and the total study population of over 100 patients represents a relatively large group of surgical patients, which enhances the probability that the results of this study are representative of patients undergoing surgical management of OSA. One of the most important limitations to the observational study design is the inability to control confounding variables that may significantly influence the outcome measurements used to assess the effectiveness of treatment. Important known confounding variables associated with treatment effectiveness for OSA include: BMI at the time of treatment, baseline AHI, and comorbid medical conditions (e.g. pulmonary disease). In this study, the potential impact of these confounding variables was relatively well controlled because at baseline there were no significant differences between the three groups for BMI, age, gender and medical conditions. The baseline AHI for the UPPP alone group was significantly lower than the MMA alone and UPPP/MMA groups, so our statistical analysis included adjusting for baseline differences in AHI when we calculated the estimated change in AHI for each of the three surgical groups. Additionally, there was reasonable standardization of the treatment protocols as all MMA procedures were performed by a single surgeon using a standardized surgical protocol and all patients in the UPPP group were treated at a single institution using similar traditional UPPP surgical techniques.
Nasal continuous positive airway pressure (CPAP) is the accepted first-line therapy for patients with OSA, and is presumed to be highly efficacious, virtually eliminating OSA.[13] Therefore, in any clinical care pathway for OSA, all attempts should first be made to enhance the likelihood that the patient can become fully compliant with CPAP therapy—unless there is some compelling reason to perform surgery as the definitive treatment (e.g. a pediatric patient with micrognathia). If the patient cannot adequately adhere to CPAP and remains untreated, surgery should be considered as an important alternative therapy; since a large percentage of patients obtain benefit from surgery, and therefore the patient would have better control of disease than no treatment at all. Even for those patients who are partially compliant with CPAP, MMA may provide better control of disease than partial compliance with CPAP. The authors have previously reported on the effectiveness of treatment apnea-hypopnea index (ET-AHI), that they developed for the purpose of determining a mathematical estimate of the true therapeutic control of OSA of any therapeutic intervention, as measured by changes in the effective AHI in the home setting.[37] The ET-AHI calculations predicted that a CPAP adherence rate of about 85% would be necessary to achieve equivalence with the decrease in AHI resulting from MMA alone, as reported in this study. Therefore, an integrated personalized approach to patient care is needed to insure that each individual with OSA receives clinically effective treatment to minimize adverse cardiovascular and metabolic events, as well as improve their quality of life. The results of this study show that MMA is a clinically effective treatment for patients with moderate to severe OSA who are unable to adhere to CPAP therapy. MMA performed alone is more effective than UPPP alone and as effective as UPPP followed by MMA. Therefore, MMA should be the treatment option of choice for most patients with moderate to severe OSA who are unable to adequately adhere to CPAP.
Acknowledgements
This investigation was supported in part by the Vanderbilt CTSA grant UL1 RR024975 from the National Center for Research Resources, National Institutes of Health and in part by a Research Support Grant Award from Oral and Maxillofacial Surgery Foundation. The authors thank David Adkins for his contributions to data collection and analysis, and Dr. Donna Walls and Ann Marie West for their contributions to data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 3.Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746. [PubMed] [Google Scholar]
- 4.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160:521. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 5.Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- 6.Moore P, Bardwell WA, Ancoli-Israel S, Dimsdale JE. Association between polysomnographic sleep measures and health-related quality of life in obstructive sleep apnea. J Sleep Res. 2001;10:303. doi: 10.1046/j.1365-2869.2001.00264.x. [DOI] [PubMed] [Google Scholar]
- 7.Moyer CA, Sonnad SS, Garetz SL, Helman JI, Chervin RD. Quality of life in obstructive sleep apnea: a systematic review of the literature. Sleep Med. 2001;2:477. doi: 10.1016/s1389-9457(01)00072-7. [DOI] [PubMed] [Google Scholar]
- 8.Reimer MA, Flemons WW. Quality of life in sleep disorders. Sleep Med Rev. 2003;7:335. doi: 10.1053/smrv.2001.0220. [DOI] [PubMed] [Google Scholar]
- 9.George CF, Smiley A. Sleep apnea & automobile crashes. Sleep. 1999;22:790. [PubMed] [Google Scholar]
- 10.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 11.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 12.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 13.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:CD001106. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettega G, Pepin JL, Veale D, Deschaux C, Raphael B, Levy P. Obstructive sleep apnea syndrome. fifty-one consecutive patients treated by maxillofacial surgery. Am J Respir Crit Care Med. 2000;162:641. doi: 10.1164/ajrccm.162.2.9904058. [DOI] [PubMed] [Google Scholar]
- 15.Caples SM, Rowley JA, Prinsell JR, Pallanch JF, Elamin MB, Katz SG, Harwick JD. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conradt R, Hochban W, Brandenburg U, Heitmann J, Peter JH. Long-term follow-up after surgical treatment of obstructive sleep apnoea by maxillomandibular advancement. Eur Respir J. 1997;10:123. doi: 10.1183/09031936.97.10010123. [DOI] [PubMed] [Google Scholar]
- 17.Holty JE, Guilleminault C. Maxillomandibular advancement for the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2010;14:287. doi: 10.1016/j.smrv.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Prinsell JR. Maxillomandibular advancement surgery in a site-specific treatment approach for obstructive sleep apnea in 50 consecutive patients. Chest. 1999;116:1519. doi: 10.1378/chest.116.6.1519. [DOI] [PubMed] [Google Scholar]
- 19.Riley RW, Powell NB, Guilleminault C. Maxillary, mandibular, and hyoid advancement for treatment of obstructive sleep apnea: a review of 40 patients. J Oral Maxillofac Surg. 1990;48:20. doi: 10.1016/0278-2391(90)90174-z. [DOI] [PubMed] [Google Scholar]
- 20.Waite PD, Wooten V, Lachner J, Guyette RF. Maxillomandibular advancement surgery in 23 patients with obstructive sleep apnea syndrome. J Oral Maxillofac Surg. 1989;47:1256. doi: 10.1016/0278-2391(89)90719-2. [DOI] [PubMed] [Google Scholar]
- 21.Hochban W, Conradt R, Brandenburg U, Heitmann J, Peter JH. Surgical maxillofacial treatment of obstructive sleep apnea. Plast Reconstr Surg. 1997;99:619. doi: 10.1097/00006534-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a surgical protocol for dynamic upper airway reconstruction. J Oral Maxillofac Surg. 1993;51:742. doi: 10.1016/s0278-2391(10)80412-4. [DOI] [PubMed] [Google Scholar]
- 23.Boyd SB. Management of obstructive sleep apnea by maxillomandibular advancement. Oral Maxillofac Surg Clin North Am. 2009;21:447. doi: 10.1016/j.coms.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Bell WH. Le Forte I osteotomy for correction of maxillary deformities. Journal of oral surgery. 1975;33:412. [PubMed] [Google Scholar]
- 25.Bennett MA, Wolford L. Maxillary Surgery Technical Considerations. In: Bell WH, editor. Surgical Correction of Dentofacial Deformities: New Concepts. Philadelphia WB: Saunders; 1985. p. 45. [Google Scholar]
- 26.Kaminishi RM, Davis WH, Hochwald DA, Nelson N. Improved maxillary stability with modified Lefort I technique. J Oral Maxillofac Surg. 1983;41:203. doi: 10.1016/0278-2391(83)90084-8. [DOI] [PubMed] [Google Scholar]
- 27.Epker BN. Modifications in the sagittal osteotomy of the mandible. Journal of oral surgery. 1977;35:157. [PubMed] [Google Scholar]
- 28.Obwegeser H. The Indications for Surgical Correction of Mandibular Deformity by the Sagittal Splitting Technique. The British journal of oral surgery. 1964;1:157. doi: 10.1016/s0007-117x(63)80067-0. [DOI] [PubMed] [Google Scholar]
- 29.Trauner R, Obwegeser H. The surgical correction of mandibular prognathism and retrognathia with consideration of genioplasty. I. Surgical procedures to correct mandibular prognathism and reshaping of the chin. Oral surgery, oral medicine, and oral pathology. 1957;10:677. doi: 10.1016/s0030-4220(57)80063-2. [DOI] [PubMed] [Google Scholar]
- 30.Fujita S, Conway W, Zorick F, Roth T. Surgical correction of anatomic azbnormalities in obstructive sleep apnea syndrome: uvulopalatopharyngoplasty. Otolaryngol Head Neck Surg. 1981;89:923. doi: 10.1177/019459988108900609. [DOI] [PubMed] [Google Scholar]
- 31.Aurora RN, Casey KR, Kristo D, Auerbach S, Bista SR, Chowdhuri S, Karippot A, Lamm C, Ramar K, Zak R, Morgenthaler TI. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33:1408. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 33.Hessel NS, Vries N. Increase of the apnoea-hypopnoea index after uvulopalatopharyngoplasty: analysis of failure. Clin Otolaryngol Allied Sci. 2004;29:682. doi: 10.1111/j.1365-2273.2004.00864.x. [DOI] [PubMed] [Google Scholar]
- 34.Sasse SA, Mahutte CK, Dickel M, Berry RB. The characteristics of five patients with obstructive sleep apnea whose apnea-hypopnea index deteriorated after uvulopalatopharyngoplasty. Sleep Breath. 2002;6:77. doi: 10.1007/s11325-002-0077-1. [DOI] [PubMed] [Google Scholar]
- 35.Dattilo DJ, Drooger SA. Outcome assessment of patients undergoing maxillofacial procedures for the treatment of sleep apnea: comparison of subjective and objective results. J Oral Maxillofac Surg. 2004;62:164. doi: 10.1016/j.joms.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Riley RW, Powell NB, Guilleminault C. Obstructive sleep apnea syndrome: a review of 306 consecutively treated surgical patients. Otolaryngol Head Neck Surg. 1993;108:117. doi: 10.1177/019459989310800203. [DOI] [PubMed] [Google Scholar]
- 37.Boyd SB, Walters AS. Effectiveness of Treatment Apnea-Hypopnea Index: A Mathematical Estimate of the True Apnea-Hypopnea Index in the Home Setting. J Oral Maxillofac Surg. 2012 doi: 10.1016/j.joms.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]