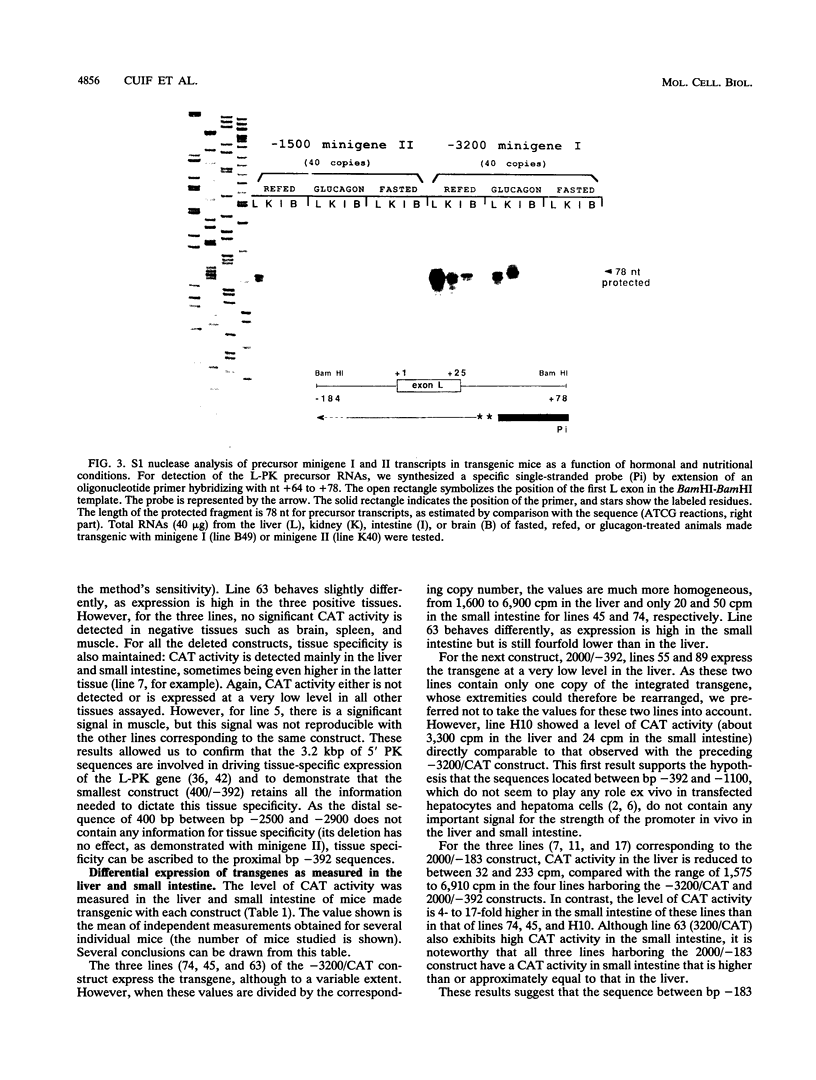
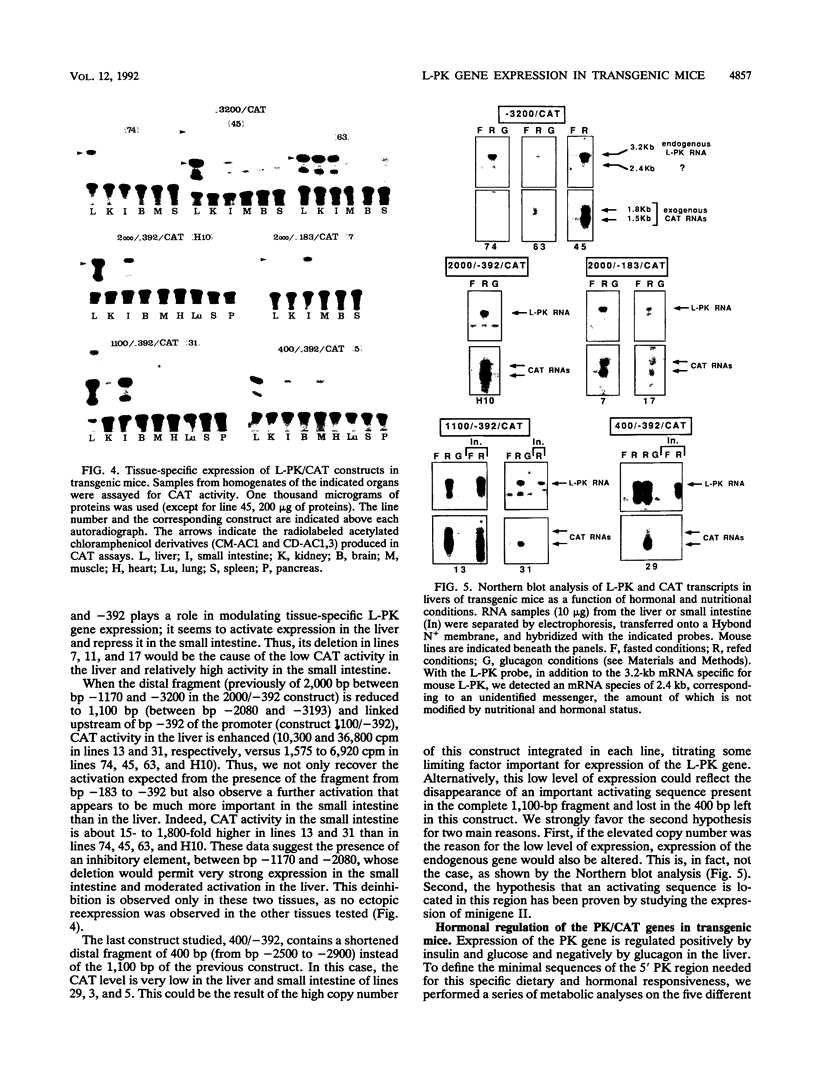
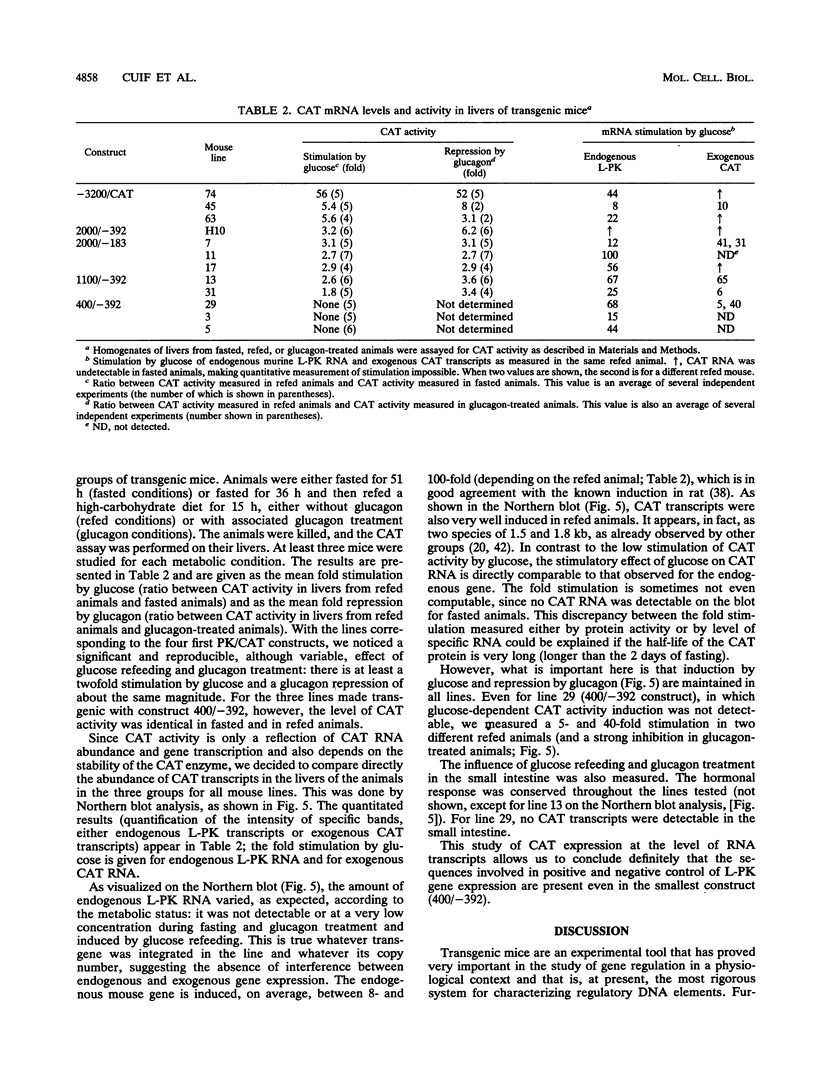
Abstract
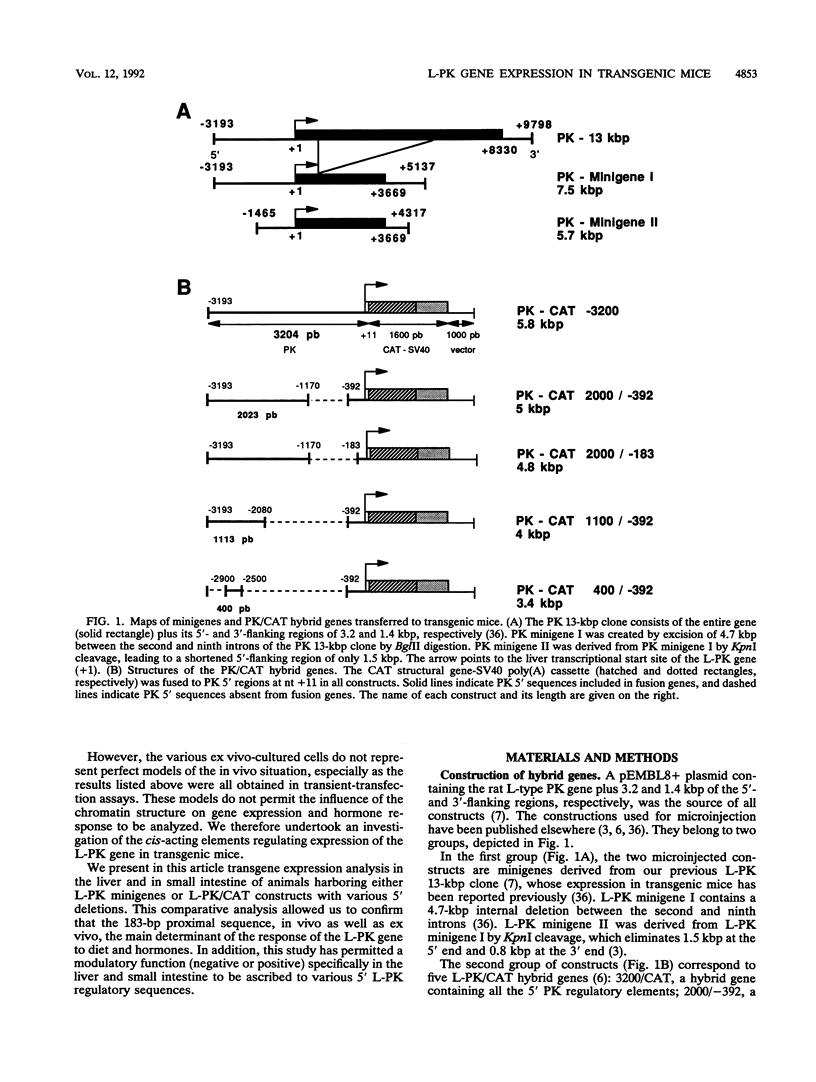
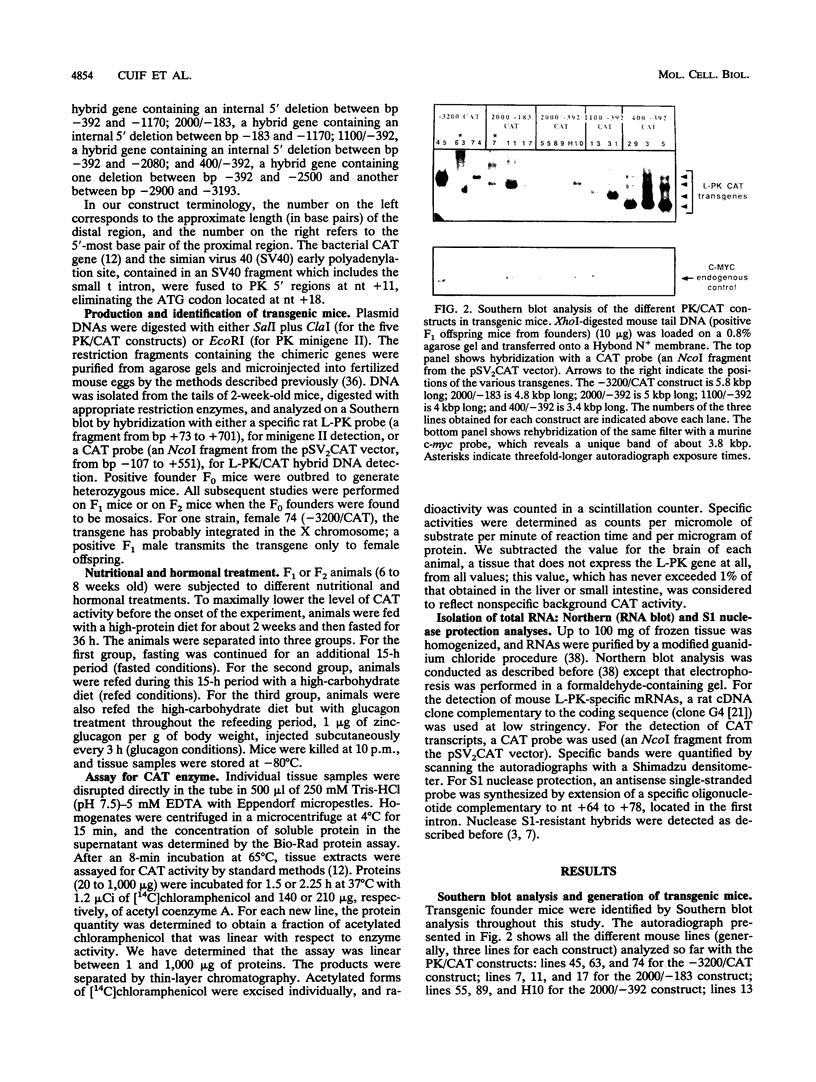
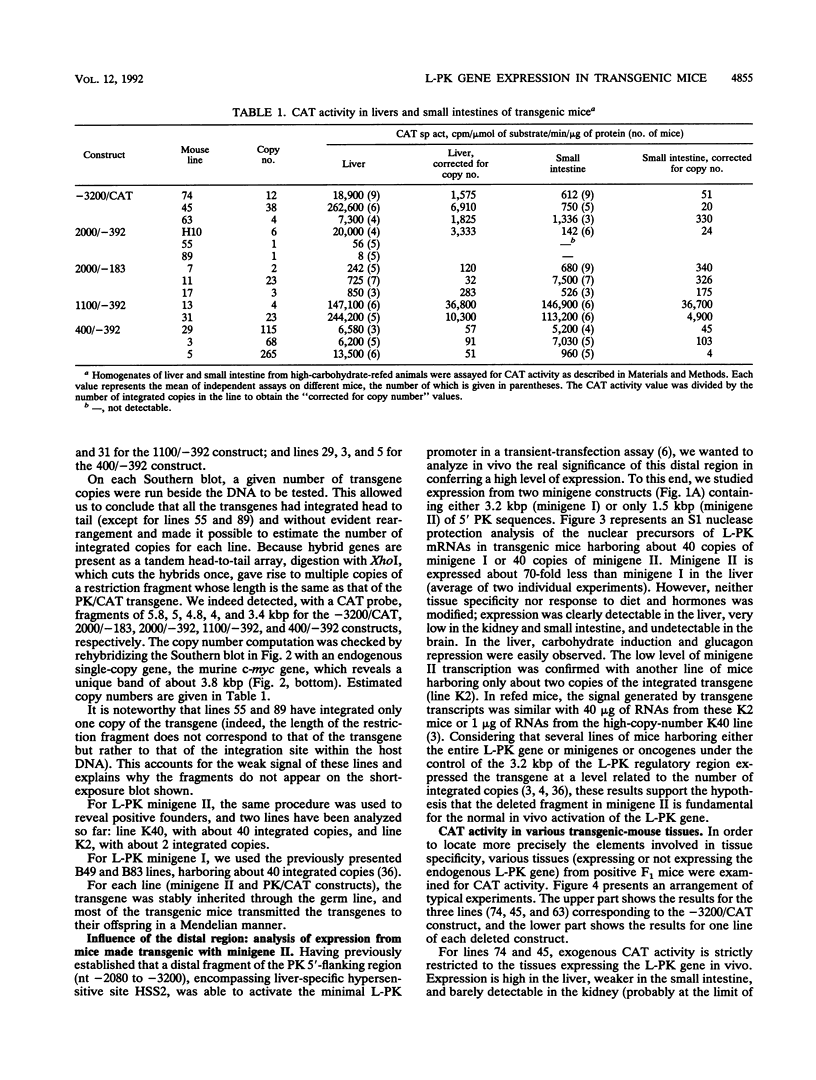
L-type pyruvate kinase (L-PK) is a key enzyme of the glycolytic pathway specifically expressed in the liver and, to a lesser degree, in the small intestine and kidney. One important characteristic of L-PK gene expression is its strong activation by glucose and insulin and its complete inhibition by fasting or glucagon treatment. Having previously established that the entire rat L-PK gene plus 3.2 kbp of 5'-flanking region functions in mice in a tissue-specific and hormonally regulated manner, various deletions of these 3.2 kbp of 5'-flanking regions were tested in transgenic animals to map the cis-acting elements involved in transcriptional gene regulation. Our experiments indicate that the proximal region between -183 and +11 confers tissue specificity and contains all the information necessary for dietary and hormonal control of L-PK gene expression in vivo. We found, however, that the transcriptional activity generated by this proximal promoter fragment can be modulated by distal sequences in a tissue-specific manner. (i) Sequences between bp -183 and -392 seem to play a dual role in the liver and small intestine; they induce L-PK expression in the liver but repress it in the small intestine. (ii) Sequences from bp -392 up to -1170 do not seem to have any additional effect on promoter activity. (iii) Between bp -1170 and -2080, we found a putative extinguisher whose transcriptional inhibitory effect is much more marked in the small intestine than in the liver. (iv) Finally, between bp -2080 and -3200, we identified an activating sequence required for full expression of the gene in the liver.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alemany J., Borras T., de Pablo F. Transcriptional stimulation of the delta 1-crystallin gene by insulin-like growth factor I and insulin requires DNA cis elements in chicken. Proc Natl Acad Sci U S A. 1990 May;87(9):3353–3357. doi: 10.1073/pnas.87.9.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergot M. O., Diaz-Guerra M. J., Puzenat N., Raymondjean M., Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992 Apr 25;20(8):1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet D., Vaulont S., Tremp G., Ripoche M. A., Daegelen D., Jami J., Kahn A., Raymondjean M. DNase-I hypersensitivity analysis of the L-type pyruvate kinase gene in rats and transgenic mice. Eur J Biochem. 1992 Jul 1;207(1):13–21. doi: 10.1111/j.1432-1033.1992.tb17013.x. [DOI] [PubMed] [Google Scholar]
- Cartier N., Miquerol L., Tulliez M., Lepetit N., Levrat F., Grimber G., Briand P., Kahn A. Diet-dependent carcinogenesis of pancreatic islets and liver in transgenic mice expressing oncogenes under the control of the L-type pyruvate kinase gene promoter. Oncogene. 1992 Jul;7(7):1413–1422. [PubMed] [Google Scholar]
- Chambaz J., Cardot P., Pastier D., Zannis V. I., Cladaras C. Promoter elements and factors required for hepatic transcription of the human ApoA-II gene. J Biol Chem. 1991 Jun 25;266(18):11676–11685. [PubMed] [Google Scholar]
- Cognet M., Bergot M. O., Kahn A. cis-acting DNA elements regulating expression of the liver pyruvate kinase gene in hepatocytes and hepatoma cells. Evidence for tissue-specific activators and extinguisher. J Biol Chem. 1991 Apr 25;266(12):7368–7375. [PubMed] [Google Scholar]
- Cognet M., Lone Y. C., Vaulont S., Kahn A., Marie J. Structure of the rat L-type pyruvate kinase gene. J Mol Biol. 1987 Jul 5;196(1):11–25. doi: 10.1016/0022-2836(87)90507-9. [DOI] [PubMed] [Google Scholar]
- Crenshaw E. B., 3rd, Kalla K., Simmons D. M., Swanson L. W., Rosenfeld M. G. Cell-specific expression of the prolactin gene in transgenic mice is controlled by synergistic interactions between promoter and enhancer elements. Genes Dev. 1989 Jul;3(7):959–972. doi: 10.1101/gad.3.7.959. [DOI] [PubMed] [Google Scholar]
- Decaux J. F., Antoine B., Kahn A. Regulation of the expression of the L-type pyruvate kinase gene in adult rat hepatocytes in primary culture. J Biol Chem. 1989 Jul 15;264(20):11584–11590. [PubMed] [Google Scholar]
- Eisenberger C. L., Nechushtan H., Cohen H., Shani M., Reshef L. Differential regulation of the rat phosphoenolpyruvate carboxykinase gene expression in several tissues of transgenic mice. Mol Cell Biol. 1992 Mar;12(3):1396–1403. doi: 10.1128/mcb.12.3.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout R., Tilghman S. M. Configuration of the alpha-fetoprotein regulatory domain during development. Genes Dev. 1988 Aug;2(8):949–956. doi: 10.1101/gad.2.8.949. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Habener J. F. Cyclic AMP response element binding proteins: a cornucopia of transcription factors. Mol Endocrinol. 1990 Aug;4(8):1087–1094. doi: 10.1210/mend-4-8-1087. [DOI] [PubMed] [Google Scholar]
- Hammer R. E., Krumlauf R., Camper S. A., Brinster R. L., Tilghman S. M. Diversity of alpha-fetoprotein gene expression in mice is generated by a combination of separate enhancer elements. Science. 1987 Jan 2;235(4784):53–58. doi: 10.1126/science.2432657. [DOI] [PubMed] [Google Scholar]
- Keller S. A., Rosenberg M. P., Johnson T. M., Howard G., Meisler M. H. Regulation of amylase gene expression in diabetic mice is mediated by a cis-acting upstream element close to the pancreas-specific enhancer. Genes Dev. 1990 Aug;4(8):1316–1321. doi: 10.1101/gad.4.8.1316. [DOI] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen P. R., Harney J. W., Moore D. D. Repression mediates cell-type-specific expression of the rat growth hormone gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8283–8287. doi: 10.1073/pnas.83.21.8283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Liou G. I., Geng L., al-Ubaidi M. R., Matragoon S., Hanten G., Baehr W., Overbeek P. A. Tissue-specific expression in transgenic mice directed by the 5'-flanking sequences of the human gene encoding interphotoreceptor retinoid-binding protein. J Biol Chem. 1990 May 25;265(15):8373–8376. [PubMed] [Google Scholar]
- Lone Y. C., Simon M. P., Kahn A., Marie J. Complete nucleotide and deduced amino acid sequences of rat L-type pyruvate kinase. FEBS Lett. 1986 Jan 20;195(1-2):97–100. doi: 10.1016/0014-5793(86)80138-7. [DOI] [PubMed] [Google Scholar]
- Mendel D. B., Hansen L. P., Graves M. K., Conley P. B., Crabtree G. R. HNF-1 alpha and HNF-1 beta (vHNF-1) share dimerization and homeo domains, but not activation domains, and form heterodimers in vitro. Genes Dev. 1991 Jun;5(6):1042–1056. doi: 10.1101/gad.5.6.1042. [DOI] [PubMed] [Google Scholar]
- Nasrin N., Ercolani L., Denaro M., Kong X. F., Kang I., Alexander M. An insulin response element in the glyceraldehyde-3-phosphate dehydrogenase gene binds a nuclear protein induced by insulin in cultured cells and by nutritional manipulations in vivo. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5273–5277. doi: 10.1073/pnas.87.14.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Granner D. K. Regulation of gene expression by insulin. Biochem J. 1991 Sep 15;278(Pt 3):609–619. doi: 10.1042/bj2780609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Ogier H., Munnich A., Lyonnet S., Vaulont S., Reach G., Kahn A. Dietary and hormonal regulation of L-type pyruvate kinase gene expression in rat small intestine. Eur J Biochem. 1987 Jul 15;166(2):365–370. doi: 10.1111/j.1432-1033.1987.tb13524.x. [DOI] [PubMed] [Google Scholar]
- Petropoulos C. J., Rosenberg M. P., Jenkins N. A., Copeland N. G., Hughes S. H. The chicken skeletal muscle alpha-actin promoter is tissue specific in transgenic mice. Mol Cell Biol. 1989 Sep;9(9):3785–3792. doi: 10.1128/mcb.9.9.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J. Insulin regulation of the glucagon gene is mediated by an insulin-responsive DNA element. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7224–7227. doi: 10.1073/pnas.88.16.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Robinson M. O., McCarrey J. R., Simon M. I. Transcriptional regulatory regions of testis-specific PGK2 defined in transgenic mice. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8437–8441. doi: 10.1073/pnas.86.21.8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Son H. J., Shahan K., Rodriguez M., Derman E., Costantini F. Identification of an enhancer required for the expression of a mouse major urinary protein gene in the submaxillary gland. Mol Cell Biol. 1991 Aug;11(8):4244–4252. doi: 10.1128/mcb.11.8.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K. S., Towle H. C. Localization of the carbohydrate response element of the rat L-type pyruvate kinase gene. J Biol Chem. 1991 May 15;266(14):8679–8682. [PubMed] [Google Scholar]
- Tremp G. L., Boquet D., Ripoche M. A., Cognet M., Lone Y. C., Jami J., Kahn A., Daegelen D. Expression of the rat L-type pyruvate kinase gene from its dual erythroid- and liver-specific promoter in transgenic mice. J Biol Chem. 1989 Nov 25;264(33):19904–19910. [PubMed] [Google Scholar]
- Tripodi M., Abbott C., Vivian N., Cortese R., Lovell-Badge R. Disruption of the LF-A1 and LF-B1 binding sites in the human alpha-1-antitrypsin gene has a differential effect during development in transgenic mice. EMBO J. 1991 Nov;10(11):3177–3182. doi: 10.1002/j.1460-2075.1991.tb04879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulont S., Munnich A., Decaux J. F., Kahn A. Transcriptional and post-transcriptional regulation of L-type pyruvate kinase gene expression in rat liver. J Biol Chem. 1986 Jun 15;261(17):7621–7625. [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Kahn A., Raymondjean M. Analysis by cell-free transcription of the liver-specific pyruvate kinase gene promoter. Mol Cell Biol. 1989 Oct;9(10):4409–4415. doi: 10.1128/mcb.9.10.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaulont S., Puzenat N., Levrat F., Cognet M., Kahn A., Raymondjean M. Proteins binding to the liver-specific pyruvate kinase gene promoter. A unique combination of known factors. J Mol Biol. 1989 Sep 20;209(2):205–219. doi: 10.1016/0022-2836(89)90273-8. [DOI] [PubMed] [Google Scholar]
- Yamada K., Noguchi T., Matsuda T., Takenaka M., Monaci P., Nicosia A., Tanaka T. Identification and characterization of hepatocyte-specific regulatory regions of the rat pyruvate kinase L gene. The synergistic effect of multiple elements. J Biol Chem. 1990 Nov 15;265(32):19885–19891. [PubMed] [Google Scholar]
- Yamada K., Noguchi T., Miyazaki J., Matsuda T., Takenaka M., Yamamura K., Tanaka T. Tissue-specific expression of rat pyruvate kinase L/chloramphenicol acetyltransferase fusion gene in transgenic mice and its regulation by diet and insulin. Biochem Biophys Res Commun. 1990 Aug 31;171(1):243–249. doi: 10.1016/0006-291x(90)91383-4. [DOI] [PubMed] [Google Scholar]