Abstract
The visuomotor functions of the superior colliculus depend not only on direct inputs from the retina, but also on inputs from neocortex. As mammals vary in the areal organization of neocortex, and in the organization of the number of visual and visuomotor areas, patterns of corticotectal projections vary. Primates in particular have a large number of visual areas projecting to the superior colliculus. As tree shrews are close relatives of primates, and they are also highly visual, we studied the distribution of cortical neurons projecting to the superior colliculus by injecting anatomical tracers into the colliculus. Since projections from visuotopically organized visual areas are expected to match the visuotopy of the superior colliculus, injections at different retinotopic locations in the superior colliculus provide information about the locations and organization of topographic areas in extrastriate cortex. Small injections in the superior colliculus labeled neurons in locations within areas 17 (V1) and 18 (V2) that are consistent with the known topography of these areas and the superior colliculus. In addition, the separate locations of clusters of labeled cells in temporal visual cortex provide evidence for five or more topographically organized areas. Injections that included deeper layers of the superior colliculus also labeled neurons in medial frontal cortex, likely in premotor cortex. Only occasional labeled neurons were observed in somatosensory or auditory cortex. Regardless of tracer injection location, we found that unlike primates, a substantial projection to the superior colliculus from posterior parietal cortex is not a characteristic of tree shrews.
Keywords: superior colliculus, tectum, cortex, evolution
INTRODUCTION
The superior colliculus is a key structure involved in integrating visual, auditory, and somatosensory information for orienting movements (Schiller et al., 1971, Casagrande et al., 1972; Harting et al., 1973; Stein et al., 1976; Werner et al., 1997; McPeek and Keller, 2004) that are important for navigating environments, avoiding predators, and foraging for food. Differences in how a particular species responds to sensory stimuli to navigate their environment will likely be reflected in the organization of inputs to the superior colliculus. Cortical projections to the superior colliculus have been studied in a wide range of species within the Euarchotoglire clade, which includes primates, lagomorphs, tree shrews and rodents.
In primates, such as New World (Cusick, 1988; Collins et al., 2005) and Old World monkeys (Fries, 1984; Lock et al., 2003), and prosimian galagos (Baldwin and Kaas, 2012), mostly visual and visuomotor areas project to the superior colliculus with visual areas projecting primarily to the superficial layers, and visuomotor areas projecting to deeper layers of the superior colliculus. Few, if any, projections arise from somatosensory areas outside of the region of S2/PV, nor do projections arise from primary motor cortex (Collins et al., 2005; Fries 1984; Baldwin et al., 2012). In contrast, in rodents such as rats and mice, the superior colliculus receives projections from primary somatosensory and motor areas of cortex, as well as from visual areas (Wise and Jones, 1977; Olavarria and Van Sluyters, 1982; Cadusseau and Roger, 1985; Welker et al., 1988; Harvey and Worthington, 1990; Hofsteter and Ehret, 1992; Inoue et al., 1992; Miyashita et al., 1994; Hoffer et al., 2005; Triplett et al., 2009; Aronoff et al., 2010). These nocturnal rodents rely heavily on their whiskers in order to navigate their immediate environments, while tree shrews, much like primates, navigate their environment visually. Here we consider the cortical projection pattern to the superior colliculus in tree shrews, which are highly visual mammals and are closely related to both primates and rodents as members of the Euarchontoglire clade (Murphy et al., 2001; Meredith et al., 2011). It is likely that the organization of cortical inputs to the superior colliculus of tree shrews reflects not only features found in other members of the Euarchotoglire clade, but also specializations reflecting their diurnal highly visual niche. Tree shrews have a cone-dominated retina, a large superior colliculus, and a sizeable region of visual cortex that includes large primary and secondary areas, as well as an expanded temporal visual cortex (Kaas, 2002; Wong and Kaas, 2009).
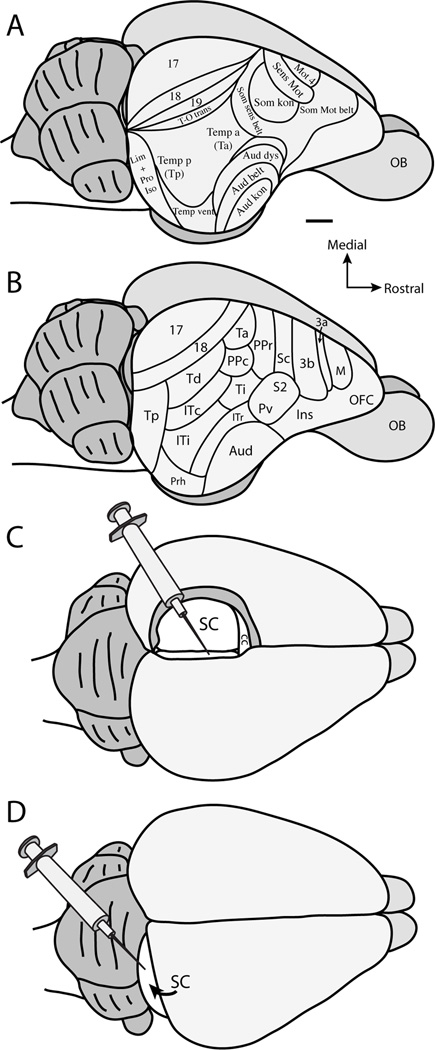
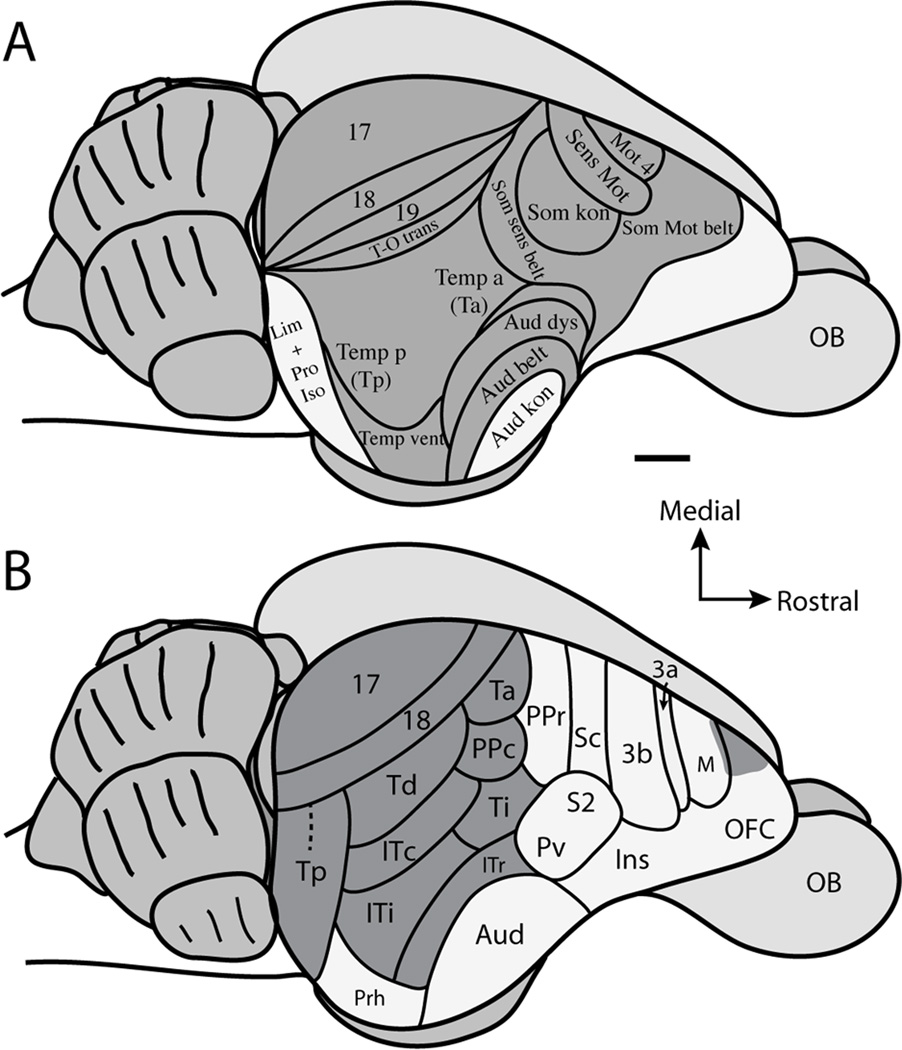
The current understanding of cortical projection patterns to the superior colliculus in tree shrews is largely based on the study of Casseday et al. (1979). These investigators divided the cortex of tree shrews into areas based on cytoarchitecture (Fig. 1A), as well as descriptions of cortical organization in tree shrews derived from patterns of cortical connections (Diamond et al., 1970; Harting et al., 1973; Casseday et al., 1976; Oliver and Hall 1978). However, our understanding of the cortical organization of tree shrews has changed substantially since the report of Casseday et al., (1979) (Fig. 1B). For instance, cortical areas in frontal cortex, including motor and prefrontal cortex, have been further defined using single unit electrode mapping, architecture, and anatomical experiments (Remple et al., 2006, 2007), and our understandings of the location and organization of areas of somatosensory cortex have also been refined and characterized (Sur et al., 1980; 1981; Remple et al., 2006, 2007). Concepts of cortical organization within the visual portions of temporal cortex have been further defined in studies of connections and cortical architecture (Sesma et al., 1984; Lyon et al., 1998; Chomsung et al., 2010), while the core auditory region of temporal cortex has been defined by microelectrode mapping (Kaas, 2011). Finally, Wong and Kaas (2009) have architectonically analyzed the areal organization of tree shrew cortex using a multitude of histological techniques. The results of all of these studies have produced a substantially different map of the cortical organization (Fig. 1B) than the map described by Casseday et al., (1979) (Fig. 1A). Therefore, the functional implications of the corticotectal projection patterns in tree shrews need further consideration and reinterpretation.
Figure 1.
Organization scheme of tree shrew cortex based on A. Casseday et al., 1979, and B. adapted from Wong and Kaas (2009). C. Illustration of anatomical tracer placement after aspiration of the contralateral hemisphere and retraction of the ipsilateral hemisphere to the injected superior colliculus. D. Illustration of anatomical tracer placement after retraction of the occipital lobe to visualize the caudal aspect of the superior colliculus. See Table 2 for abbreviations. Scale bar is 2mm.
In the present study, cortical projections to the superior colliculus in tree shrews were studied using anatomical retrograde tracer injections into the superior colliculus. We were able to create injection sites that were small and were located at different topographical locations, as well as at different depths, within the superior colliculus. We analyzed cortical projections to the superior colliculus in flattened preparations of the cortical sheet in order to gain an areal view of the full distribution of cortical projections across all cortical areas. The main goal of this study was to assess the full distribution of cortical projections to the superior colliculus in tree shrews and relate the pattern of projections to known anatomical cortical borders. Additionally, we expected to provide information about the visuotopic organization of temporal and inferotemporal cortical areas by correlating the locations of labeled cells to the topographical locations of injection sites within the superior colliculus as described by Lane et al. (1971). Our results revealed that the primary projections to the superior colliculus in tree shrews arise from visual and visuomotor cortical areas, with few projections from auditory and somatosensory areas. The projections from visual areas 17 and 18 were within topographic locations that largely matched the topographic placement of the injections within the superior colliculus. Additionally, single injections into the superior colliculus labeled multiple patches of labeled neurons in temporal and inferotemporal cortex that were in register with areal divisions of tree shrew cortex suggested by Wong and Kaas’s (2009) architectonic study. Finally, projections from frontal cortex may reflect motor regions that are associated with head or forepaw movements, but are likely outside of primary motor cortex (Remple et al., 2006).
METHODS
Injections of anatomical tracers were placed in the superior colliculus of five tree shrews to reveal the distribution of corticotectal projections. All surgical procedures were approved by the Vanderbilt University Animal Care and Use Committee or the Institutional Animal Care and Use Committee of the University of Louisville and were in accordance with the NIH Guide for the care and use of laboratory animals.
Surgical procedures and injections
Surgical procedures have been described elsewhere (Baldwin et al., 2011; Baldwin and Kaas, 2012; Wei et al., 2011). Briefly, tree shrews were initially anesthetized with an intramuscular injection of ketamine (100 mg/kg) and xylazine (6.7 mg/kg), and were maintained at anesthetic levels during surgical procedures either with isoflurane (0.5–2%) or additional supplements of ketamine and xylazine every 45 minutes. All procedures were performed under aseptic conditions. Once anesthetized, the tree shrews were positioned in a stereotaxic frame. An incision was made along the midline of the skull, and a small craniotomy was made over the occipital lobe and the dura was reflected. After this, one of the following procedures was used to place injections. In the first procedure, the medial wall of the right superior colliculus was visualized after aspiration of the left occipital pole, and retraction of the medial wall of the right hemisphere (Fig. 1C; cases 09–62, 10–18, and 10–20). In the second, the occipital lobe was retracted in order to visualize the caudal aspect of the superior colliculus (Fig. 1D, Case 03–34). Once the superior colliculus was visible, injections of 0.2–0.8µl of cholera toxin subunit B (CTB: Molecular Probes Invitrogen, Carlsbad, CA; 10% in distilled water) or fluoro-ruby (FR; Molecular Probes Invitrogen; 10% in distilled water) were made using a Hamilton syringe fitted to a glass pipette beveled to a fine tip. As a third procedure, (Case 11–35), a glass pipette containing a BDA and CTB mixture (5% BDA and 1% desalted CTB in 0.1M phosphate buffer: tip diameter 2.5 µm) was lowered vertically through cortex, and the tracer was injected iontophoretically (2 µA positive current for 20 minutes) into both the left and right superior colliculi at varying locations. After tracer injections were complete, gelfoam was placed in the region of aspirated cortex, the cortex was covered with gelfilm, and the opening of the skull was sealed with an artificial bone flap made of dental cement. The incision site was closed and the tree shrews were carefully monitored during recovery from anesthesia, given Buprenex (0.03 mg/kg IM) as an analgesic, and were returned to their home cage with food and water.
Tissue processing and data analysis
After a 5 to 7 day survival period, the tree shrews were given a lethal injection of sodium pentobarbital (250 mg/kg) and, when areflexic, were perfused with phosphate buffered saline (PBS; pH 7.4) followed by 2% paraformaldehyde in PBS and 2% paraformaldehyde in PBS with 10% sucrose. The brain was removed and the cortex flattened after being separated from underlying brain structures and placed in 4% paraformaldehyde for 1 hour. The brainstem and thalamus were placed in 4% paraformaldehyde for 1 to 2 hours. After postfixation, brain tissue was placed in PBS with 30% sucrose for 12–24 hours at 4 °C for cryoprotection. The cortex was cut parallel to the pia surface, and the brainstem and thalamus were cut coronally at a thickness of 40 µm on a freezing microtome. Cortical sections were divided into three or four series of every third or fourth section. One series was mounted directly onto glass slides without further processing for the visualization of neurons labeled with the FR tracer. Another series was processed for CTB using an immunohistochemical protocol (Baldwin et al., 2011: See Table 1 for antibody characterization). The third and fourth series were processed for cytochrome oxidase (Wong-Riley, 1979) or myelin (Gallyas, 1979). The brainstem and thalamus sections were saved in series of five with one series mounted directly onto glass slides for FR injection site analysis; one series processed to reveal CTB injection sites; and a third processed for CO; while the fourth and fifth series were processed for acetylcholinesterase (AChE: Geneser-Jensen and Blackstad, 1971), Nissl, or saved for another study.
Table 1.
Antibody Characterization
| Antigen | Immunogen | Manufacturer | Dilution Factor |
|---|---|---|---|
| Cholera toxin subunit B | Purified CTB isolated from Vibrio Cholerae | List Biological Laboratories Inc. (Campbell, CA), goat polyclonal #703 | 1:5000 |
Locations of retrogradely labeled cell bodies were plotted using a Neurolucida system (MicroBrightField, Williston, VT). Cortical tissue overlying the superior colliculus injection sites was analyzed for possible tracer contamination within our processed tissue and during dissection. Photomicrographs of tissue sections were taken using a DMX1200F digital camera mounted to a Nikon microscope (Nikon Inc., Melville, NY) or were taken with Qimaging EXi Aqua digital camera (Surrey, BC, Canada) mounted to a Leica microscope. Photographs were adjusted for brightness and contrast using Adobe Photoshop but were otherwise unaltered. The locations of injection sites and retrogradely labeled cells in sections processed for CTB or FR were aligned with sections processed for architectonic features using common blood vessels. Injection site locations relative to superior colliculus layers were determined by alignment with sections processed for CO, Nissl, or AChE, while borders of cortical areas were determined using CO- or myelin-stained sections.
Injection site identification
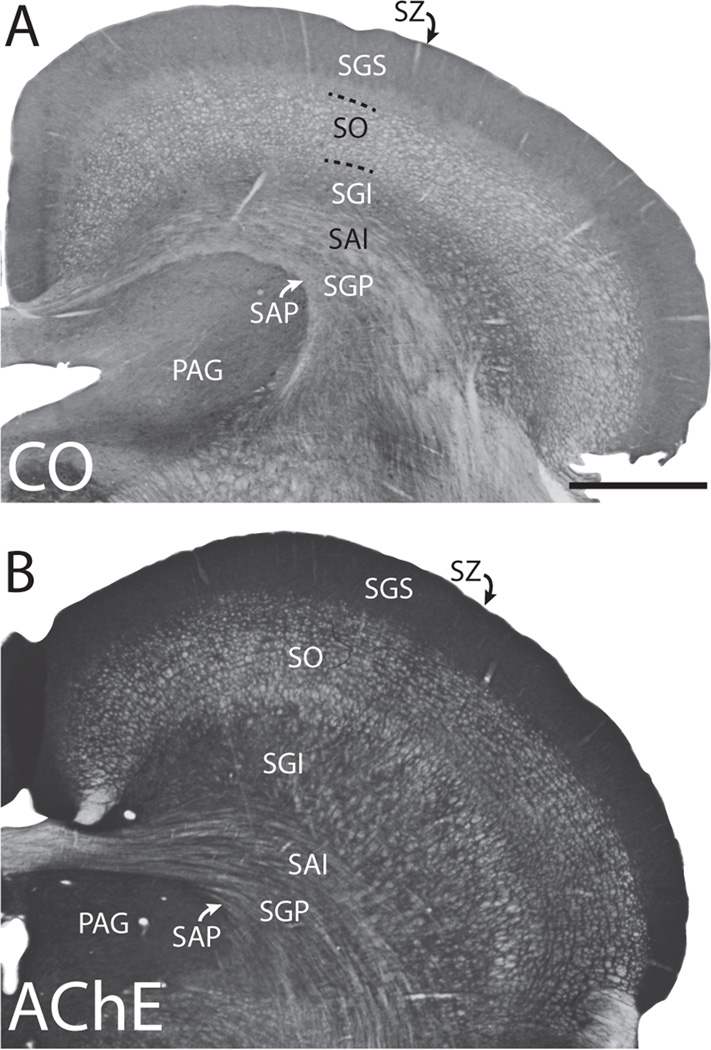
The seven main layers of the superior colliculus were identified using CO and AChE staining (Fig. 2) and were similar to laminar descriptions by Lee and Hall (1995). The superficial layers, including the stratum zonale (SZ), stratum giseum superficiale (SGS), and stratum opticum (SO) are primarily associated with visual sensory functions; while the deeper layers are associated with higher order visual functions, the integration of sensorimotor inputs, as well as various motor functions (Casagrande et al., 1972; Harting et al., 1973; Casagrande and Diamond, 1975; Raczkowski et al., 1976; Albano et al., 1978). Often our injections involved multiple layers; however, we were still able to compare cases with mainly superficial injections that included the upper half of the SGI (cases 09–62, 03–34, 11-35RH), and cases with injection sites that included deeper, or all, layers of the superior colliculus (cases 11-35LH, 10–18, 10–20).
Figure 2.
Laminar organization of the superior colliculus as revealed through cytochrome oxidase (CO) staining (top section) and acetylcholinesterase (AChE) staining (bottom section). The seven main layers of the superior colliculus are the stratum zonale (SZ), the stratum grisium superficiale (SGS), the stratum opticum (SO), the stratum griseum intermediate (SGI), the stratum album intermedium (SGI), the stratum griseum profundum (SGP), and the stratum album profundum (SAP). Also shown is the periaqueductal grey (PAG). Medial is left, and dorsal is up. Scale bar is 1mm.
The visuotopic organization of the superior colliculus in tree shrews has been determined in microelectrode recording experiments (Lane et al., 1971). The medial superior colliculus contains cells responsive to stimuli within the upper visual field, and the lateral superior colliculus contains cells responsive to stimuli within the lower visual field. The caudal aspect of the superior colliculus represents the peripheral visual field, with central vision represented more rostrally. We did not individually determine the visuotopic location of the injection sites but instead based these locations on the maps of Lane et al. (1971) after reconstructing the injection site locations within a dorsal view of the superior colliculus.
For all but two injections, we found no evidence of cortical contamination. However, cases 11-35L and 11-35R did show slight contamination within area 17. Previous reports on cortical connections in tree shrews suggest that only areas 18, Td, and Tp share connections with area 17 (Sesma et al., 1984; Lyon et al., 1998) and therefore we still present cases 11-35R and 11-35L. For case 11-35R, the contamination of area 17 was minor; however, 11-35L may have significant contamination of area 17, as suggested by two distinct foci of label within areas 18, Tp, and Td. This case still provides useful information on the organization of temporal visual areas that do not receive projections from area 17 (Sesma et al., 1984; Lyon et al., 1998).
Determining the locations of labeled cells
Most cortical cells projecting to the superior colliculus in tree shrews arise from layer 5 (Casseday et al., 1979). Though it is difficult to locate the laminar position of labeled cells when cortex is cut parallel to the pia surface, no labeled cells were present within our most superficial sections of cortex, and, instead, were present predominantly within the bottom half of our samples, likely below layer 4.
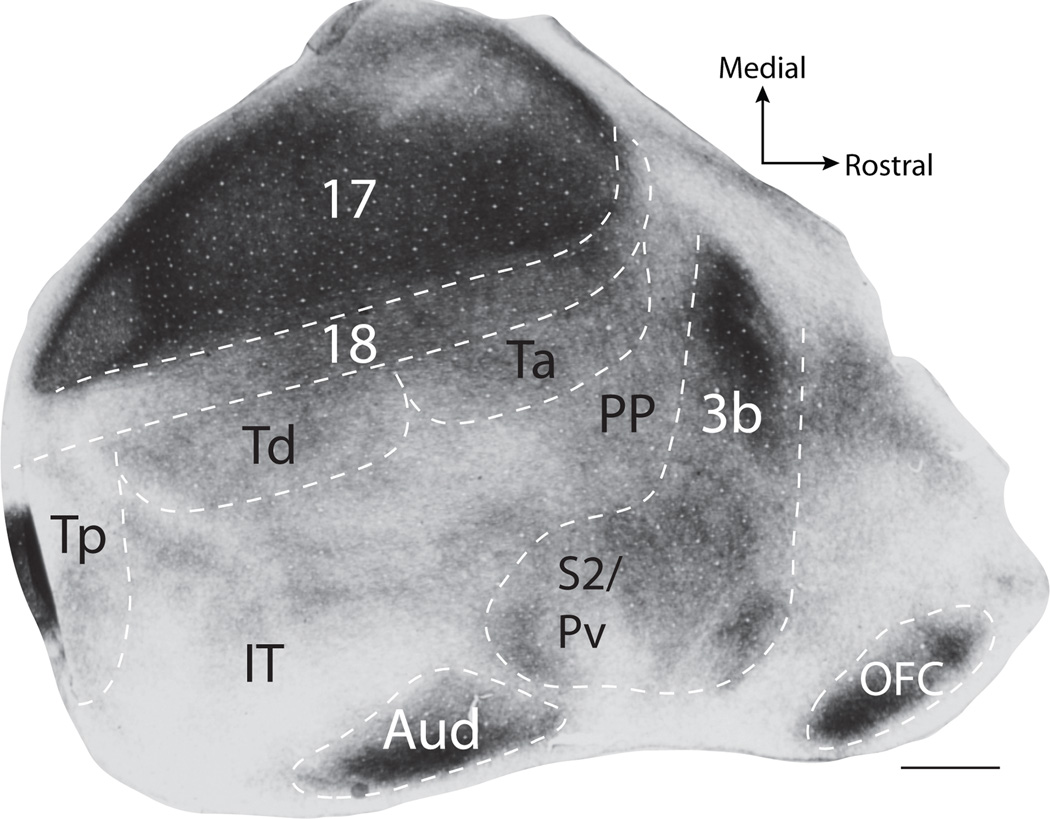
We identified cortical areas in the flattened cortex of tree shrews using tissue sections processed for CO or myelin (Fig. 3), or by relating the position of labeled cells to cortical maps described by Wong and Kaas (2009) (Fig. 1B). Areas 17, 3b, S2/PV, auditory cortex (Aud), as well as the orbitofrontal cortex (OFC) were identified by their characteristic dark CO and myelin staining patterns (Wong and Kaas, 2009) (Fig. 3). Area 18 stains less darkly for myelin than area 17, but more darkly than rostral and lateral cortical areas. Determining the boundaries for subdivisions of temporal cortex (Tp, Td, Ta, Ti, and IT) was more difficult using CO and myelin. Thus, we estimated the locations of these borders after those of Wong and Kaas (2009), who used additional histological staining techniques to determine border locations. To be conservative, we avoided placing most of these borders in our illustrations and only indicated the expected locations of areas, but we included schematics of the borders by Wong and Kaas (2009) in the figures for reference.
Figure 3.
Cortical architecture revealed by myelin staining. Area 17, auditory cortex (Aud), area 3b, and the orbital frontal cortex (OFC) stain darkly for myelin, while PV/S2 and area 18 stain slightly less darkly but more so than surrounding cortical tissue. The dark myelination caudal to Tp is likely a result of uneven folding within this region of cortex. Scale bar is 2mm.
RESULTS
Patterns of corticotectal projections in tree shrews were revealed by placing injections of tracers in the superior colliculus. For this study, ten anatomical tracer injections were placed into six superior colliculi of five tree shrews. Of these cases, five injections involved superficial and intermediate layers of the superior colliculus, while five injections involved superficial, intermediate, and deep layers of the superior colliculus. The depths of injections were determined by aligning coronal sections processed for tracers with adjacent anatomical sections processed for CO, or AChE. Results from the superficial injection cases are presented first. We expected visual areas to project to superficial layers of the superior colliculus and frontal visuomotor areas to project to deeper layers.
Corticotectal projections
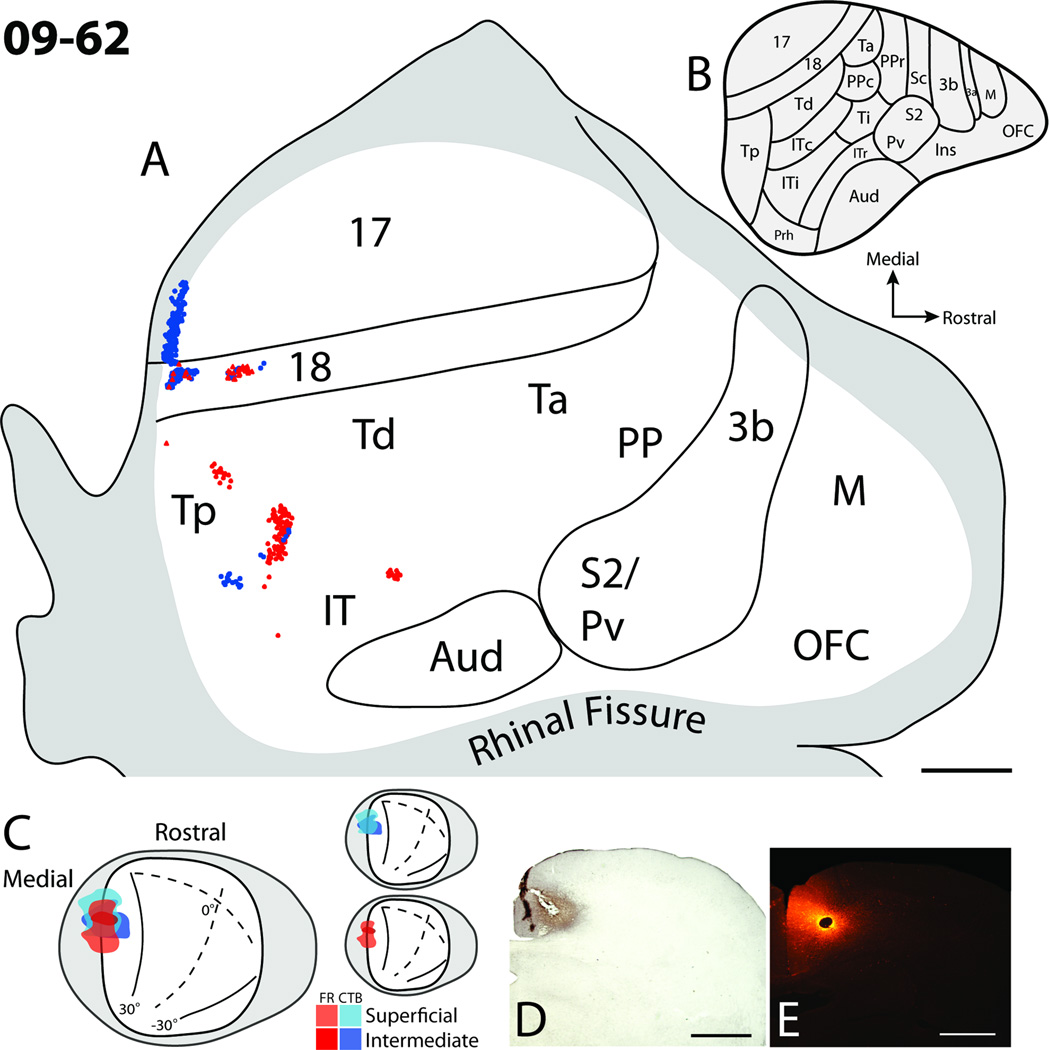
Case 09–62 (Fig. 4), contained the most superficial injections. The injection sites were located within the lower SGS, SO, and dorsal most aspect of the SGI. Both CTB and FR injections overlapped one another along the medial wall of the superior colliculus (Fig. 4), a location that represents paracentral vision of the upper visual field (Lane et al., 1971). As the injection cores were small in this case, limited numbers of cells were labeled in cortex. Labeled cells in cortex were present within areas 17, 18, Tp, as well as a few patches of cells located within the IT region. Cells within area 17 and 18 were in locations of upper visual field representations close to the border between 17 and 18 representing the vertical meridian (Kaas et al., 1972), consistent with the retinotopic locations of the injection sites. Area 18 contained two patches of labeled cells, which may reflect the presence of modular subdivisions within area 18 (Sesma et al., 1984; Lyon et al., 1998). Few labeled FR cells were found in area 17, and this is likely because the injection site was centered within the SO of the superior colliculus and did not include much of the upper SGS, which is known to receive striate projections (Harting and Noback, 1971; Casseday et al., 1979; Huerta et al., 1985). No labeled cells were present within motor, somatosensory, or auditory cortical areas. Thus, injections including the SGS and SO labeled cells within early visual areas such as 17 and 18, as well as some temporal visual areas.
Figure 4.
Cortical projections to the superficial and intermediate layers of the superior colliculus in case 09–62. A. The distribution of retrogradely labeled cells within the flattened cortex after fluoro-ruby (FR) and cholera toxin subunit B (CTB) injections into the superior colliculus. Solid lines represent borders determined using myelin stained sections, and the grey shaded region represents cortex that was either along the medial or ventral surfaces of the brain. Red dots represent the locations of retrogradely labeled FR cell bodies, while blue dots represent the locations of retrogradely labeled CTB cell bodies. B. Proposed cortical areas for tree shrews (Wong and Kaas, 2009). C. Dorsal view of the superior colliculus showing the locations of injection sites. Red hues represent the location of the FR injection site, while blue hues represent the location of the CTB injection site. Darker hues indicate the location of injection sites deeper within the superior colliculus. C. Photomicrographs of the CTB (D) and FR (E) injection sites in coronal sections through the superior colliculus. Scale bar for A is 2mm, and D and E is 1mm.
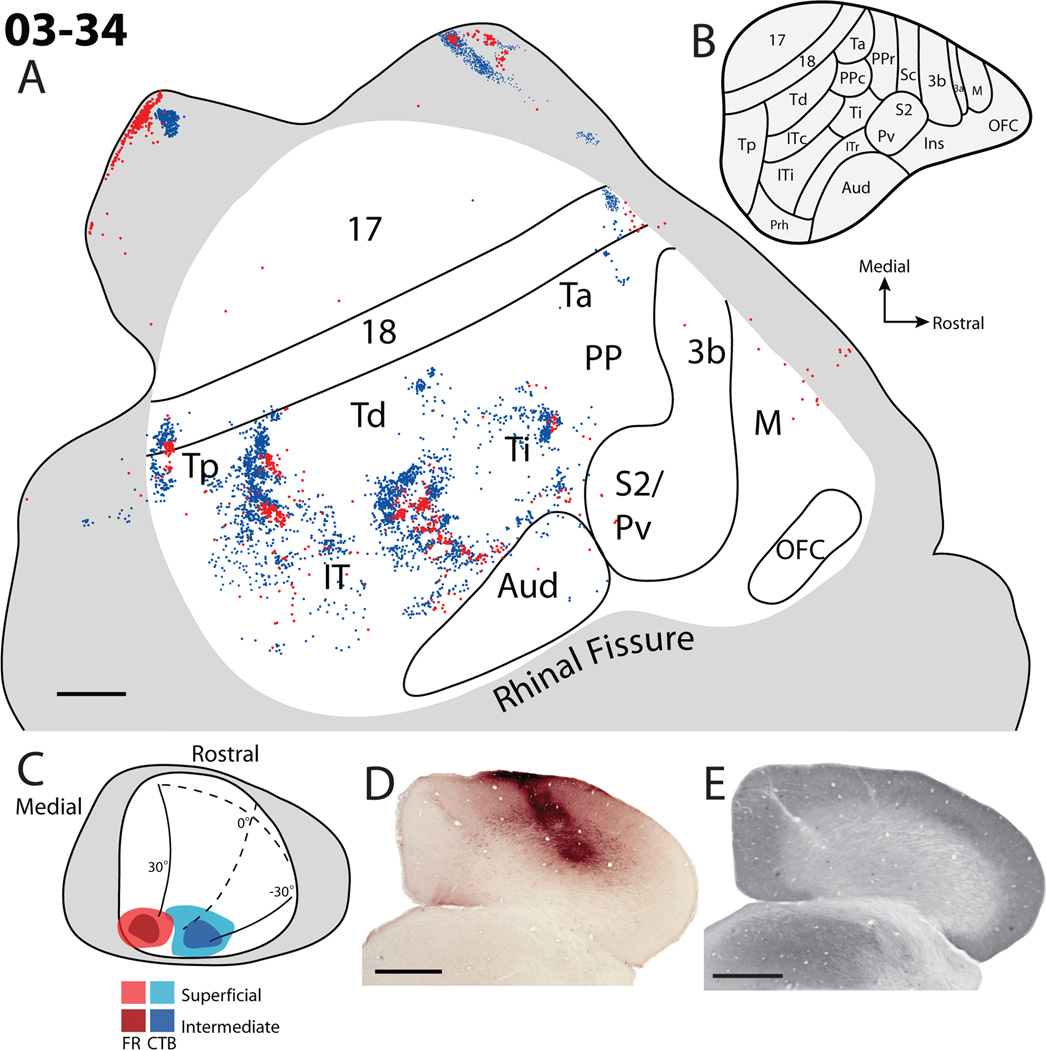
The second case, 03–34 (Fig. 5), contained injection sites that were slightly deeper within the superior colliculus. Both CTB and FR injections were within the most caudal aspect of the superior colliculus representing peripheral vision. The injection sites included superficial layers of the superior colliculus as well as the SGI. The location of the FR injection site was within the upper visual field within the representation of peripheral vision, while the CTB injection site was near the representation of the horizontal meridian including both upper and lower visual fields. The two injection sites did not overlap. Labeled cells were located within area 17, 18, Tp, Td, Ta, Ti and possibly the posterior parietal cortex; multiple patches were also located within IT and the outer most edge of auditory cortex. No cells were located within area 3b, S2/PV, or OFC. Additionally, a few scattered FR cells were located along the rostral medial wall of frontal cortex, possibly including premotor cortex. Within cortical areas, patches of labeled cells for each of the two injections were displaced from one another and rarely overlapped, suggesting the locations of borders and a retinotopic organization pattern throughout temporal and occipital cortex. Most importantly, a band of cortex between Td and auditory cortex has repeating patches of neurons labeled by the two injections, suggesting that peripheral vision is represented back to back near the IT/Ti border regions (compare label in Fig. 5A with map in 5B). The appearance of multiple patches of alternating repeating patterns of reversed label within this region suggests a modular organization within these areas, and a complex visuotopic organization. A similar array of alternating patches of label for the two injections extended mediolaterally along the presumptive border of Td and Tp. Patches of labeled neurons representing peripheral vision were also located along the caudal aspect of Tp and rostrally between Ti and PP. Only one patch of labeled cells for each tracer was apparent within Tp. A band of cells was located ventral to Td, possibly within ITc (compare label in Fig. 5A with map in 5B). Finally, patches of labeled neurons representing peripheral vision were located in the ventral part of area 17 that was unfolded in the flattened cortex. This part of area 17 is known to represent peripheral vision (Kaas et al., 1972). Other labeled neurons were located in the rostral and caudal aspects of area 18: locations that represent peripheral vision. The scattering of labeled cells in lateral IT adjacent to auditory cortex suggests a lack of visuotopy in this region.
Figure 5.
Cortical projections to the superficial and intermediate layers of the superior colliculus in case 03–34. A. Reconstruction of the labeled cells within flattened cortex. Blue dots represent the location of retrogradely labeled CTB cell bodies, while red dots represent the location of retrogradely labeled fluoro-ruby cell bodies. B. The architectonically defined areas within the tree shrew cortex as determined by Wong and Kaas, 2009. C. Dorsal view of the injection site locations within the superior colliculus. D. The CTB injection site within a coronal section through the caudal aspect of the superior colliculus. E. A brain section adjacent to D that has been processed for cytochrome oxidase (CO). Scale bar A is 2mm, D and E is 1mm.
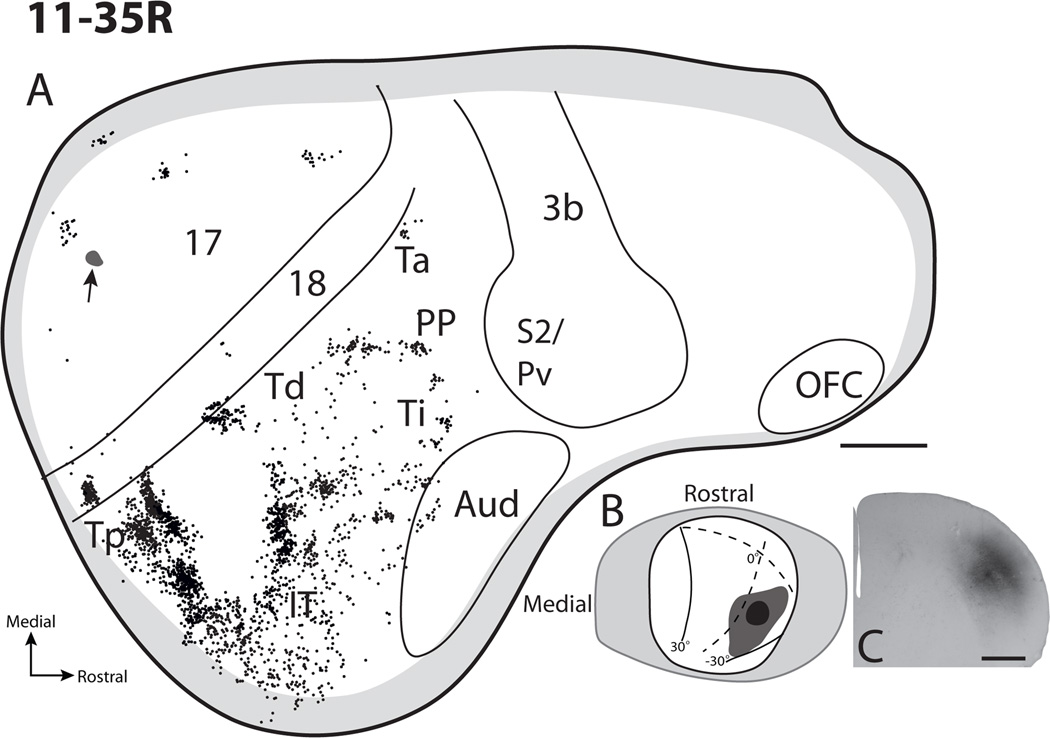
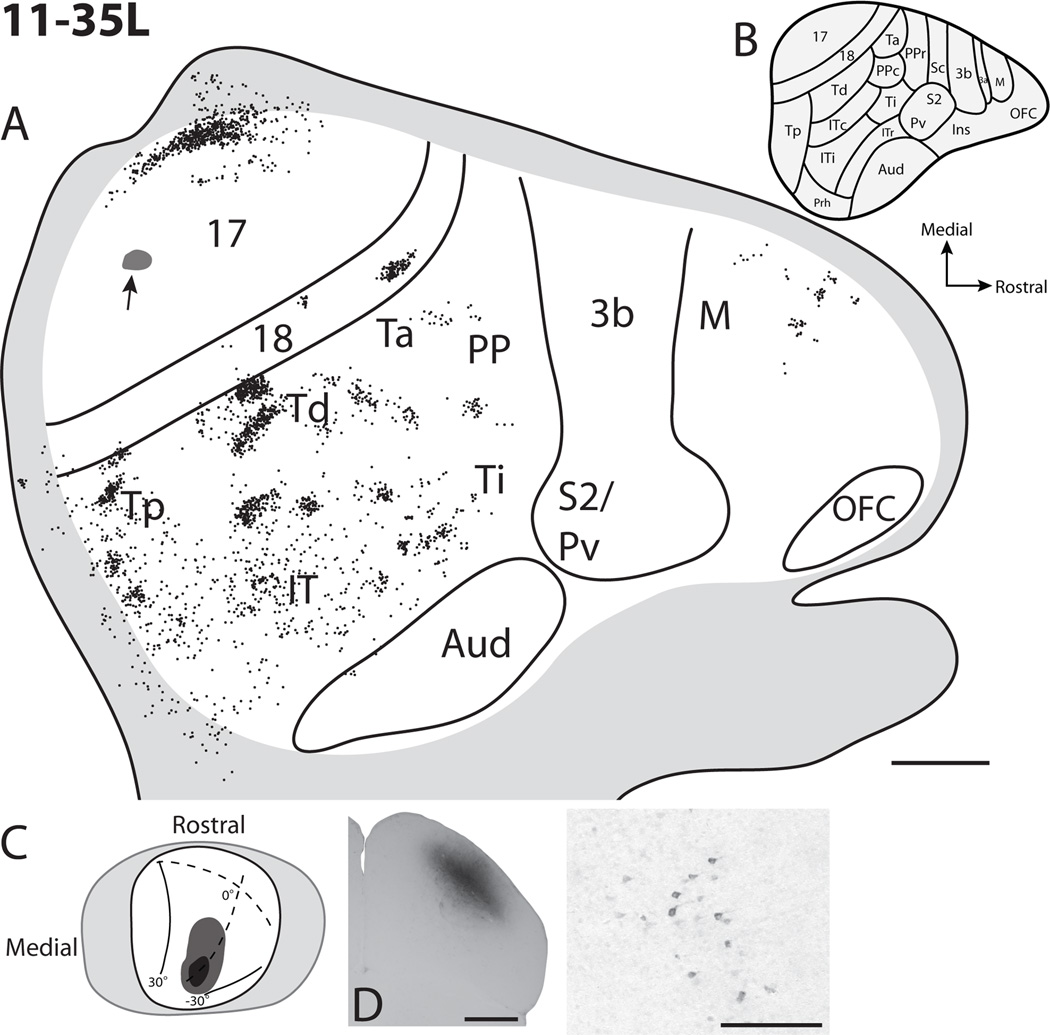
The next set of superior colliculus injections (11-35R and 11-35L, Figs. 6 and 7) were iontophoretically placed in the superior colliculi of the left and right hemispheres of the same tree shrew. These two injections involved the lateral half of the superior colliculus within the intermediate and deeper tectal layers. Since it was not possible to visualize the lateral half of the superior colliculus by ablating part of the opposite cerebral hemisphere, injections into the lateral superior colliculus were placed by penetrating the overlying visual cortex with a glass pipette. While injections were successfully placed in the superior colliculus of each midbrain using this procedure, a slight contamination of parts of striate cortex along the course of the pipette penetration occurred in each attempt. Results are included here because the amount of labeled transport to neurons elsewhere in cortex appeared to be quite small, and because the labeled neurons outside of area 17 would only be in locations known to project to area 17 (areas 18, Tp, and Td: Sesma et al., 1984; Lyon et al., 1998) and not other areas projecting to the superior colliculus. Thus, the injections provide useful additional information.
Figure 6.
Cortical projections of the superficial and intermediate layers of the superior colliculus in case 11-35R. A. The distribution of retrogradely labeled CTB cells throughout cortex after an injection into the central lateral superior colliculus. The small black shaded region in area 17 with an arrow shows the location of the penetration track used for tracer placement. B. Dorsal view reconstruction of the injection site in the superior colliculus with a visuotopic map as described by Lane et al. (1971) superimposed. C. A coronal section through the superior colliculus that has ben processed for CTB to indicate the depth of the CTB injection site. Scale bar for A is 2mm, C is 1mm.
Figure 7.
Cortical projections to the superficial, intermediate, and deep layers of the superior colliculus in case 11-35L. A. Reconstruction of the distribution of labeled cells within flattened cortex of the left hemisphere. The cortex has been flipped to appear as the right hemisphere ease comparisons of cortical label with other cases. The small black area with an arrow in area 17 represents the location of the penetration track used for tracer placement. B. Cortical organization map adapted from Wong and Kaas 2009. C. Dorsal view of the superior colliculus with the visuotopic map of Lane et al. (1971) superimposed. In this case the darker grey represents the core of the injection site, while the light grey represents the tracer spread. D. Photomicrograph of a portion of the CTB injection site in a coronal view of the superior colliculus. E. An example of CTB labeled neurons in cortex. Scale bar for A is 2mm, D is 1mm, and E is 0.5mm.
Of the two injections in the superior colliculus in case 11–35, the more lateral and superficial injection was in the right superior colliculus, 11-35R (Fig 6). The injection core was focused within the SO and SGI, while avoiding the SGS. As a result, very little label was in area 17 and 18, which project to the SGS; and the labeled neurons in these areas may reflect some involvement of the SGS, or in part, the slight contamination of area 17. However, the dense patches of labeled neurons in cortex lateral to area 18 completely, or nearly completely, reflect the injection in a lateral part of the superior colliculus that represents paracentral vision of the lower visual quadrant. Three patches of label were observed caudally within temporal cortex. Two patches of labeled cells were observed within area Tp with one patch seemingly denser than the more caudal patch of cells. An additional patch of labeled cells was present lateroventral to these two patches, and was possibly also within Tp. Similar to previous cases a patch of labeled cells was observed within Td, as well as the Ti-Ta region. The scattering of labeled neurons in IT cortex provides further evidence that this cortex is likely visual but without much visuotopic organization. While a few labeled neurons were in auditory cortex, none were in somatosensory cortex or posterior parietal cortex. Additionally, as in previous cases with injections that did not penetrate beyond the SGI, no labeled cells were observed in frontal cortex.
The injection in the left superior colliculus of case 11–35, 11-35L (Fig. 7), involved the middle of the superior colliculus representing paracentral to peripheral vision close to the horizontal meridian. The injection core included the lower SGS, SO, and much of the SGI. Labeled neurons were observed in the expected topographic location of area 17; while labeled cells within the rostral aspect of area 18 correspond to paracentral vision of the lower visual quadrant near the horizontal meridian, roughly matching the injection site in the superior colliculus. While some of the patches of labeled neurons in Tp and Td could reflect the slight contamination of area 17, the bulk of the label in temporal, parietal, and frontal cortex likely reflect projections to the superior colliculus. Patches of label in this case provide further evidence for retinotopic areas in the upper region of temporal cortex, a lack of retinotopy in ventral IT, and a projection from dorsomedial frontal cortex to the deeper layers of the superior colliculus.
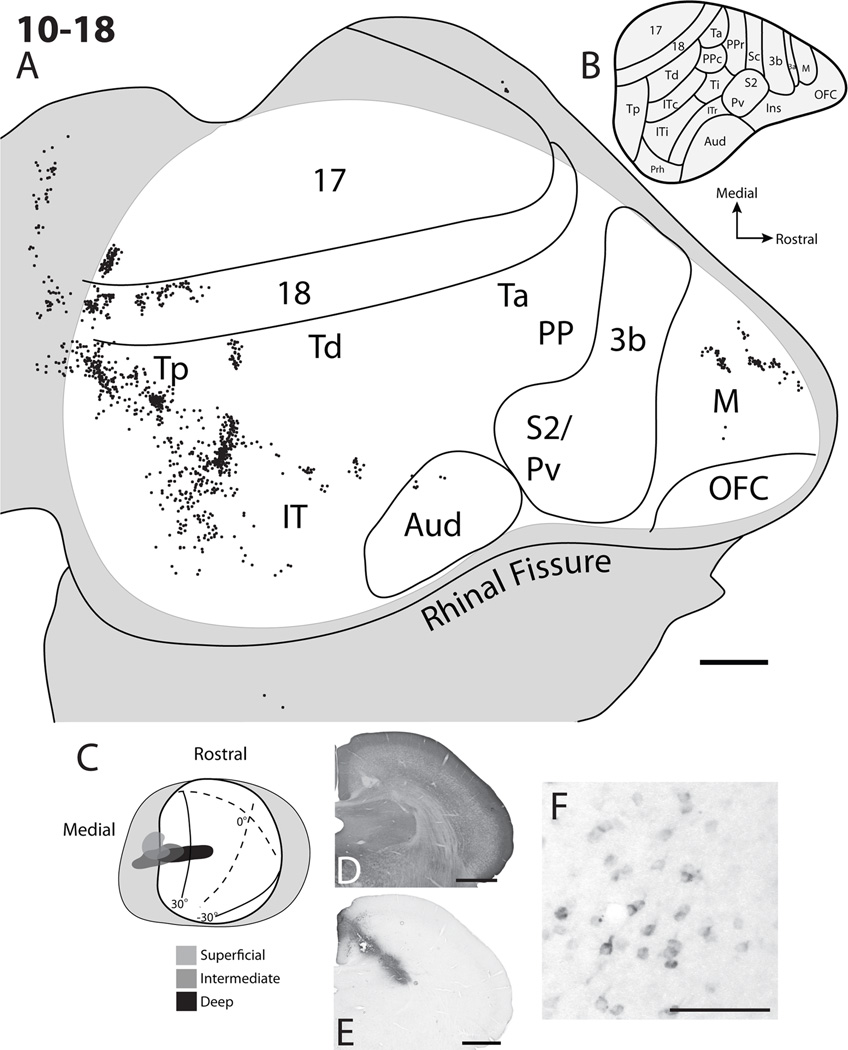
The CTB injection core for case 10–18 (Fig. 8) extended into the deepest layers of the superior colliculus. Two injections at different depths within the superior colliculus were made along a diagonal trajectory arising from the medial wall and were positioned within the upper SGI as well as the deep layers of the superior colliculus. There was a slight gap in the spread of the injection core within the lower SGS (Fig. 8E). Additionally, some tracer spread into the dorsal-most aspect of the central grey (Fig. 8E). Labeled cells were found in area 17, 18, Tp, Td, IT, and the frontal cortex. The foci of labeled cells were in the upper visual field representations within areas 17 and 18 and were close to the border between areas 17 and 18 representing the vertical meridian, consistent with the injection site location near the medial margin in the superficial layers of the superior colliculus. Similar to cases 09–62 (Fig. 4) and 10–20 (Fig. 9), multiple patches of labeled cells were present within area 18, suggesting a modular organization. Also, as in case 10–20, two patches of labeled cells were present within the region of Tp, with one more focused patch located rostromedial to a more diffuse patch. The rostromedial patch is further away from the rostral border of area 18 than the more diffuse patch as in case 10–20 (Fig. 9). Cells labeled within the Td region were located close to the rostral border of area 18. Again, a band of cells was located ventral to the Td region, but rostral to the Tp region within IT, much as in case 10–20 (Fig. 9). However, the gap between the cell clusters in this region was much larger for case 10–18. Labeled cells within the ventral IT region were scattered, suggesting a lack of a retinotopic organization. Foci of retrogradely labeled cells within frontal cortex were in locations similar to those observed in case 10–20 (Fig. 9), with one patch located caudolaterally to the more rostromedial patch. These cells were likely within a premotor area rostromedial to primary motor cortex (Remple et al., 2006, 2007), but some of the more caudally labeled cells could be within the most rostral aspect of primary motor cortex. Surprisingly, no cells were located within the Ta/Ti/PP region. Again, no cells were present within somatosensory areas 3b, S2/PV, orbital frontal cortex, and only a few cells were within the architectonically defined auditory cortex. Overall, the results observed in case 10–18 were similar to case 10–20 with multiple patches of labeled cells within the region of Tp and area 18; labeled cells within IT and frontal cortex; and a lack of labeled cells within somatosensory, primary motor, and auditory cortex.
Figure 8.
Cortical projections to the superficial, intermediate, and deep layers of the superior colliculus in case 10–18. A. The distribution of retrogradely labeled cells within the flattened cortex after a cholera toxin subunit B (CTB) injection into the superior colliculus. Black dots represent retrogradely labeled cells, solid lines are borders determined based on myelin stained sections and grey shaded areas represent unfolded cortex along the medial wall and ventral surfaces of the brain. B. Cortical organization map adapted from Wong and Kaas (2009). C. Dorsal view of the superior colliculus on the visuotopic map of Lane et al. (1971) superimposed. Grey area represents the medial and lateral aspects of the superior colliculus unfolded. Darker shades of grey within the superior colliculus represent intermediate and deep layers of the superior colliculus included in the CTB injection site. D. A coronal brain section stained for CO. E An adjacent CTB stained section through the injection site within the superior colliculus. F. An example of CTB labeled neurons in cortex. Scale bar for A is 2mm, D and E is 1mm, and F is 0.25mm.
Figure 9.
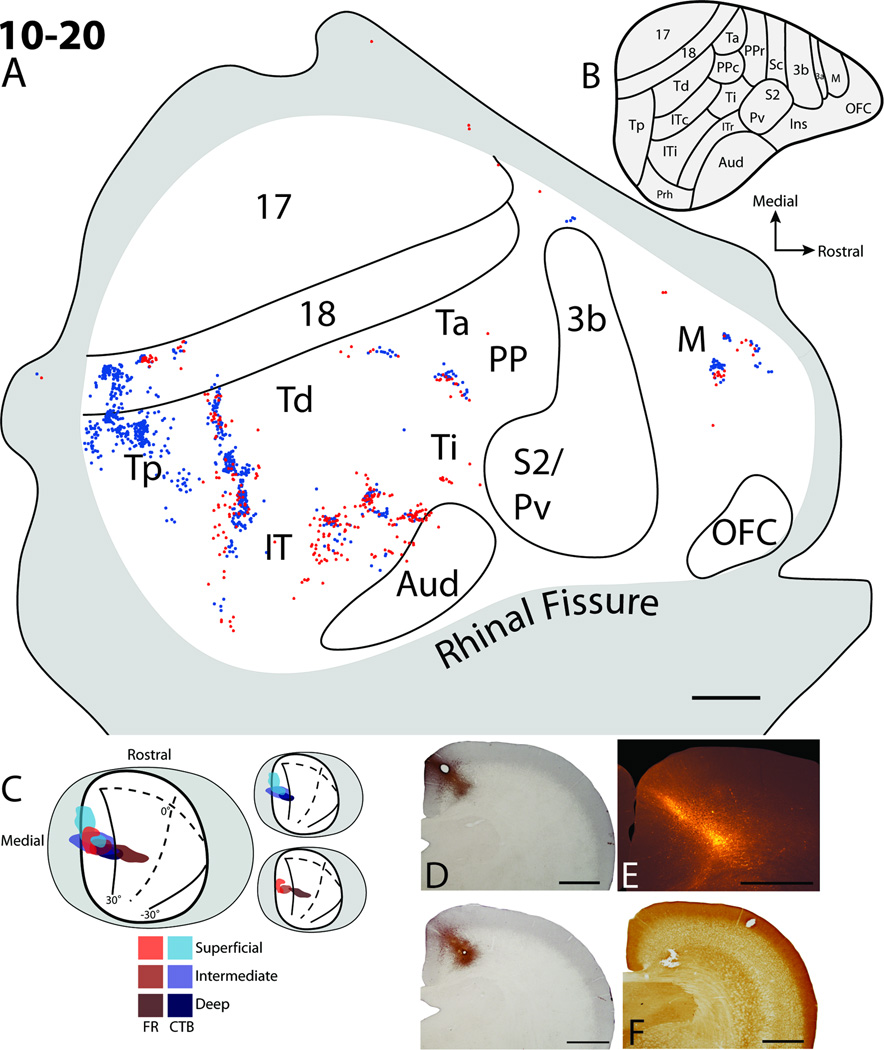
Cortical projections to the superficial, intermediate, and deep layers of the superior colliculus in case 10–20. A. The distributions of retrogradely labeled cells within the flattened cortex after fluoro-ruby (FR) and cholera toxin subunit B (CTB) injections into the superior colliculus. Solid lines represent borders of areas determined using myelin stained sections. Red dots represent the locations of retrogradely labeled FR cells. Blue dots represent the locations of retrogradely labeled CTB cells. The grey shaded area represents cortex that was either along the medial or ventral surfaces of the brain. B. Cortical areas from Wong and Kaas (2009). C. Dorsal view of the superior colliculus with the visuotopic map of Lane et al. (1971) superimposed. Grey area represents the medial and lateral aspects of the superior colliculus folded out. The red hue areas represent the FR injection site while the blue hue areas represent the location of the CTB injection site. Darker hues indicate the location of the injection sites at deeper levels within the SC. D. The CTB injection site in coronal sections through the superior colliculus. E. The FR injection site in a coronal section through the superior colliculus. F. A coronal section adjacent to E stained for cytochrome oxidase. The hole in the tissue shows the second CTB injection focus within the SGI of the superior colliculus. Scale bar for A is 2mm, D, E, and F is 1mm.
The injection cores for our final case (10–20 Fig. 9) extended into the deepest layers of the superior colliculus (Fig 9 D–F) and were in similar topographic locations as the injection sites for cases 09–62 (Fig. 4) and 10–18 (Fig. 8). The full extent of the CTB injection core covered all layers of the superior colliculus. This extent was achieved by placing tracer injections at two different depths along the injection tract. One focus was within the superficial layers of the superior colliculus (Fig. 9D), while the other was within the SGI (Fig. 9F). The two foci for the FR injection were within the lower SO/upper SGI, and the deep layers of the superior colliculus (Fig. 9E). Both CTB and FR injections were positioned diagonally into the superior colliculus from the medial wall, thus the injection included the medial superficial layers and progressed more laterally into the deeper layers of the superior colliculus. The FR injection site did not include much of the SGS. The lack of CTB cells within striate cortex was surprising given that one of the foci of the injection was within the lower SGS (Fig. 9D). Possibly the relevant part of area 17 representing the paracentral upper visual field was lost during flattening and processing. Unlike the previous case (10–18, Fig. 8), there does not appear to be a gap in the spread of the CTB injection site across the layers of the superior colliculus, which could be why there were labeled cells within the PPc and Ta region for this case (Fig. 9) and not for case 10–18 (Fig. 8). Other, focused patches of labeled cells, representing the parafoveal upper visual field, were present within areas 18, TP, Td; regions within medial IT and within the Ta/PPc region; while a few labeled cells were scattered within the lateral IT region. The more focused patches of label suggest that 18, Tp, Td, and PPc have topographic representations, while the scattering of labeled cells suggests that lateral IT does not contain a topographic representation. As in the cases 09–62 (Fig. 4), and 10–18 (Fig. 8), multiple patches of label were observed along the length of area 18, suggesting a possible modular characteristic of this area in trees shrews (Sesma et al., 1984). Within the Tp region, multiple clusters of cells were apparent with one dense patch located rostromedially, and a second more diffuse patch located caudolaterally, similar to cases 11-35R (Fig 6) and 10–18 (Fig. 8). The two closely spaced patches of labeled cells in Tp were relatively far away from the line of cells observed in Td. The line of labeled cells within Td extended ventrally into IT, with a slight gap between cells in Td and those within the IT region. The more ventral patch of cells could be all within ITc as described by Wong and Kaas (2009), within ITi, or another area within IT (compare label in Fig. 9A with map in Fig. 9B). Because of the close clustering of CTB labeled cells with FR labeled cells relative to the close positions of the injection sites, it is likely that this IT region has at least a crude topographic organization pattern. Two patches of labeled cells were also located more rostrally within the IT/Ti region, with an additional more rostral patch of labeled cells located close to the border of auditory cortex. These patches could represent cells within areas Ti, ITi, and ITr (compare Fig. 9A with map in Fig. 9B). Two patches of label were present within the frontal cortex (Fig. 9), similar to case 10–18 (Fig. 8). A dense patch of labeled cells was located rostrolaterally, and a more diffuse patch of labeled cells was located more medially. These patches were likely within premotor or prefrontal cortical areas, and not within primary motor cortex (Remple et al., 2006, 2007) because of their distance of over 2mm rostral to the area 3b border. No labeled cells were present in orbital frontal cortex, somatosensory, primary motor, and only a few labeled cells were scattered along the border of auditory cortex similar to all other cases. Overall, this case provides information on projection patterns of labeled cells to all layers of the superior colliculus. Major differences between this case and the first case (09–62), with similarly placed injection sites, are that labeled cells were observed within frontal cortex, and multiple patches of labeled cells were within area TP. Additionally, the distribution of labeled cells within temporal and parietal areas was more dense than that observed for 09–62.
In summary, injections into the superior colliculus revealed corticotectal projecting cells within occipital and temporal visual areas, with multiple focused patches of cells suggesting retinotopic organization patterns within them. However, the consistent pattern of scattered cells throughout much of ventral IT suggests that this region is not retinotopicaly organized. Additionally, injection sites that involved deeper layers of the superior colliculus resulted in labeled cells within frontal cortex, but these labeled cells were likely outside of primary motor cortex (Remple et al., 2006; 2007). Labeled cells were only occasionally observed within auditory cortex and the parietal region of cortex including posterior parietal cortex.
DISCUSSION
In the present study, we examined the areal distribution of cortical areas projecting to the superior colliculus in tree shrews and compared the locations of labeled tectal projecting cells with our current understanding of the organization of cortical areas in tree shrew neocortex (Wong and Kaas, 2009). Our injections were placed at different depths of the superior colliculus to reveal differences between projection patterns to the superficial layers of the superior colliculus and those to deeper layers. Additionally, we placed injection sites at various retinotopic locations within the superior colliculus, so that patterns of labeled cells could suggest topographic subdivisions of temporal visual cortex, as well as to test if there are differences in cortical projections to different quadrants of the superior colliculus. The results indicate that the majority of cortical cells projecting to the superior colliculus in tree shrews arise from visual or visuomotor cortex. After injections into the superficial layers of the superior colliculus, labeled cells were found in occipital and temporal visual areas, while deeper injections labeled neurons in frontal cortex, likely in premotor cortex. Our results also provide some insight into the topographic layout of occipital and temporal cortical areas that seems to correlate well with the architectonic subdivisions of cortex described by Wong and Kaas (2009). Finally, very few neurons were observed in auditory cortex and only a few cells were observed within somatosensory cortex in only one case (Fig. 5). No cells were observed in orbital frontal, or primary motor cortex.
Some of our results are similar to those reported in a previous study of cortical projections to the superior colliculus (Casseday et al., 1979). Casseday et al., (1979) reported differences in extrastriate cortical inputs to rostral and caudal aspects of the intermediate layers of the superior colliculus. However, their report suggested that cells within extrastriate visual cortex were labeled only after injections were placed within the rostral and not the caudal portion of the superior colliculus. In contrast, our injections within the caudal superior colliculus, consistently labeled neurons in extrastriate visual areas (Figs. 4–9). Additionally, Casseday et al. (1979) described projections as from a single area, area 19, along the lateral border of V2 (Figs. 1A, 10A), while multiple patches of labeled neurons along the outer border of V2 for single injections in the present cases is more consistent with the presence of multiple visual areas along the lateral border of V2 as proposed in more recent reports (Sesma et al., 1984; Lyon et al., 1998, Wong and Kaas, 2009). Finally, unlike Casseday et al (1979), we found few, if any, cells projecting to the superior colliculus from somatosensory cortex or the primary motor cortex. This difference in conclusions does not seem to reflect major differences in illustrated results, but rather current interpretations of the locations of somatosensory and motor cortical areas. Thus, a better understanding of the functional organization of neocortex in tree shrews has allowed a more accurate view of the projections patterns to the superior colliculus. This information is useful in that it can provide insights on the possible functional characteristics of the superior colliculus in tree shrews, and indicate differences in collicular organization among members of the Euarchotoglire clade. Additionally, our present results further define the organization of cortical areas in tree shrews by providing support for previously proposed areas and suggesting additional divisions.
Figure 10.
Summary and comparison of the locations of cortical areas projecting to the superior colliculus (dark grey shaded regions) based on the study by Casseday et al., 1979 (A), and the summary of results from the current study (B). In Casseday et al., 1979 projections were reported from somatosensory and motor regions, while in the current study only projections from prefrontal cortex, rostral to motor cortex, were observed, with very few to no projections from primary motor or somatosensory cortex. See Figure 1 for abbreviations. Scale bar is 2mm.
Occipital cortical areas
Injections of superficial and intermediate layers of the superior colliculus mainly labeled cells in visual areas within the occipital and temporal cortex. Neurons in area 17 were only labeled when the SGS was included within the injection site (Figs. 4, 5, 7, and 8) and few labeled cells were observed in area 17 when it was not included (Figs. 6, and 9). This is consistent with the results of previous experiments indicating that area 17 projects to the SGS as well as the dorsal-most aspect of the SO in tree shrews (Harting and Noback, 1971; Casseday et al., 1979; Huerta et al., 1985). The locations of labeled cells were in the retinotopic map of area 17 (Kaas et al., 1972) reflected the locations of the injection sites in the retinotopic map of the superior colliculus (Lane et al., 1971). The locations of the labeled neurons in area 18, or V2, also reflected the locations of the injection sites in the superior colliculus in a retinotopically congruent pattern. Thus, superior colliculus injections close to the representation of the vertical meridian within the upper visual field resulted in retrogradely labeled cells located close to the 17/18 border (Figs. 4 and 8). Superior colliculus injections located in the peripheral visual field representation resulted in labeled cells located in the most caudal aspect of area 17, as well as along the most rostral border of 18 (Figs. 5 and 7), locations displaced from the 17/18 border. Also, injections within the upper visual field representations of the superior colliculus resulted in labeled cells within the caudolateral half of area 18, while injections within the lower visual field representation resulted in labeled cells within the most rostromedial half of area 18, or V2 (Kaas et al., 1972). As in tree shrews, projections from occipital areas 17 and 18 have been widely reported for other mammals including rodents (Sefton et al., 1981; Olavarria and Van Sluyters, 1982; Harvey and Worthington, 1990; Rhodes et al., 1991), cats (Updyke, 1977), and various primates (Fries, 1984; Lock et al., 2003; Collins et al., 2005; Baldwin and Kaas, 2012).
Our results did not provide evidence for an area 19 along the rostrolateral border of area 18. Such an area was described by Casseday et al. (1979), but has not been included in more recent reports on tree shrew cortical organization (Sesma et al., 1984; Lyon et al., 1998, Wong and Kaas, 2009). Architectonic evidence for an area 19 implies the existence of a single, third visual area, V3, along the outer border of area 18. Compelling evidence for such an area V3 exists for primates (See Lyon and Kaas, 2002 for review) and cats (Hubel and Wiesel, 1965; Donaldson and Whitteridge, 1977). However, in tree shrews, several areas appear to be located along the outer border of area 18 (Sesma et al., 1984; Lyon et al., 1998, Wong and Kaas, 2009). Therefore, it is likely that an area V3 evolved independently in cats and primates.
Temporal cortical areas
Along the rostral border of area 18, dorsal temporal cortex has been divided into the temporal posterior (Tp), temporal dorsal (Td), and temporal anterior (Ta) areas (Sesma et al., 1984; Lyon et al., 1998; Wong and Kaas, 2009) (Fig. 1B). All injections into the superior colliculus in the present study labeled cells within Tp and area Td, with the exception of case 09–62 (Fig. 4). Labeled cells within Ta after superior colliculus injections were more variable, though labeled cells were observed after injections involving intermediate layers of the superior colliculus (Figs. 5, 6, 7, 9), except for case 10–18 (Fig. 8) which may have had a gap of tracer spread within the lower SGS and SAI. Previously, Casseday et al. (1979) concluded that projections to the superior colliculus from extrastriate visual cortex terminate in intermediate and deep layers below the SGS, and that such projections terminated mainly within the rostral aspect of the superior colliculus. In the present study, neurons were labeled in dorsal temporal cortex when injections involved the intermediate layers of the superior colliculus below the SGS. However, our results differ from those of Casseday et al. (1979) in that labeled cells were present in dorsal temporal areas even for our most caudal injections into the superior colliculus, indicating that temporal visual areas project to both rostral and caudal locations within the superior colliculus.
The distribution of labeled neurons in temporal regions Tp, Td, and IT are in cortical locations previously shown to receive inputs from the dorsal and central nuclei of the pulvinar in tree shrews (Lyon et al., 2003; Chomsung et al., 2010). These same nuclei receive projections from the superior colliculus (Luppino et al., 1986; Chomsung et al., 2008). Thus, the superior colliculus provides inputs via the pulvinar to cortical areas that project back to the superior colliculus. We describe the pattern of labeled cells for subdivisions of the temporal cortex below.
We found that Ta had few projections to the superior colliculus relative to the projections from Tp and Td, and that these projections likely terminate within the intermediate layers of the superior colliculus. Because of the weak projections to the superior colliculus, Ta likely only has weak influences on the superior colliculus functions relative to areas Tp and Td. Area Ta also has only weak connections with area 17, but stronger connections with area 18 (Sesma et al., 1984; Lyon et al., 1998), as well as connections with forelimb regions of primary motor cortex (Remple et al., 2007). Thus, Ta has been considered a visuomotor area and could be related to the posterior parietal motor areas described in primates (Stepniewska et al., 2005; Gharbawie et al., 2011).
We found projections from Td to the superior colliculus in all cases except case 09–62 (Fig. 4). The location of labeled neurons in Td after variably located injections in the superior colliculus provide evidence that Td represents much of the contralateral visual hemifield and is retinotopically organized because labeled cells were focused in patches; and when injections were made into separate locations in the superior colliculus, separate, non-overlapping patches of cells were located within Td (Fig. 5). There is previous evidence that the upper visual field is represented caudolaterally and the lower visual field is represented rostromedially in Td and that the visuotopic organization of Td relative to Tp is in a serial repeating pattern, meaning that there is no reversal in the visuotopy across the Tp/Td border (Sesma et al., 1984; Chomsung et al., 2010). Our present results are consistent with this hypothesis, as the distance between the most mediodorsal patch of cells within Tp was consistently displaced by 2mm from the patch of labeled cells within Td (Figs. 5–9). Because Td is located along the border of the middle aspect of area 18, has strong connections with area 17, and stains moderately for myelin, Td may be a homolog of the middle temporal area in primates (Sesma et al., 1984; Lyon et al., 1998). However, MT in primates is displaced rostrally from the area 18 border and Td is not. This and other differences challenge the conclusion that Td is homologous to MT in primates (Kaas and Preuss, 1993). Td connections with the superior colliculus do not provide evidence for or against Td being a homologue of MT because connections between MT and the superior colliculus in primates is variable across primate species, with strong connections observed in New and Old World monkeys (Graham et al., 1979; Fries, 1984; Lock et al., 2003; Collins et al., 2005), but weak or no connections between MT and the superior colliculus observed in galagos (Baldwin and Kaas, 2012).
Tp is located along the most caudolateral aspect of area 18. Multiple patches of label were observed within the region of Tp after single tracer injections into the superior colliculus suggesting that Tp is comprised of more than one retinotopically organized area or is modularly organized. Thus two clearly distinct patches of labeled cells were present within the Tp region after single CTB injections in cases 11-35R (Fig. 6) and 10–18 (Fig 8). For cases 03–34 (Fig. 5) and 11-35L (Fig. 7) where single patches were present along an axis parallel to the V2 border, the injection sites were very caudal within the superior colliculus. In contrast, cases with more rostral superior colliculus injections resulted in patches of labeled cells that were more spread apart within Tp (Figs. 6, 8, and 9) suggesting that there could be a reversal between the two domains within Tp at the representation of peripheral vision. Therefore, it is likely that Tp does consist of two topographically organized areas. Tp has connections with areas 17, and 18, with the connections between area 18 and Tp being denser (Sesma et al. 1984; Lyon et al., 1998). Sesma et al. (1984) also suggested from cortical connection patterns that Tp is composed of two visual areas, but architectonic evidence for two separate areas has not been presented (Sesma et al., 1984; Lyon et al., 1998; Wong and Kaas, 2009). In cases 11-35L (Fig. 7) and 11-35R (Fig. 6), where injections were placed iontophoretically in more lateral locations of the superior colliculus, labeled cells were present ventral to the patches of labeled cells in dorsal Tp close to the rostral border of area 18 after injections in medial locations in the superior colliculus. Label in this location may be a result of cortical contamination of area 17 during tracer placement into the superior colliculus, or could be a result of injections into more lateral aspects of the superior colliculus. Label in ventral Tp was not apparent in case 03–34 (Fig. 5) with a lateral peripheral injection.
The visuotopic organization of Tp and Td do not appear to be mirror images that reverse at the Tp/Td border, as patches of label were consistently separated from one another by 2 to 3mm regardless of the injection site location within the superior colliculus. A lack of a reversal between Td and Ta also seems likely.
Inferior temporal cortical areas
In early architectonic studies of tree shrews, the inferior temporal cortex was not subdivided (Zilles et al., 1978). More recently, IT cortex has been subdivided into three areas by Remple et al. (2007) based on differences in connections with cortical motor areas and by architectonic characteristics. Wong and Kaas (2009) defined four architectonic subdivisions of the inferior temporal cortex: inferior temporal cortex (IT), caudal IT (ITc), inferior IT (ITi), and rostral IT (ITr), along with another cortical area, the temporal inferior area (Ti). Injections that involved intermediate layers of the superior colliculus labeled cells within all of these areas, similar to results of Casseday et al (1979). Multiple isolated patches of labeled cells were present within different locations after single injections in the superior colliculus (Figs. 5, 6, 7, and 9). Injections in more peripheral locations produced label that was spatially close together within the center of IT (Fig. 5), while more rostral injections produced label at more distantly displaced locations (Figs. 6, 8, and 9).
The connection patterns suggest that more peripheral vision is represented along the ITi and Ti border with more central vision represented away from the border (See Fig. 5 and 9). We consistently observed a strong band of labeled cells just ventral to cells within Td, which was likely within ITc as described by Wong and Kaas (2009). In most cases, this patch of label was separated from cells within Td (Figs. 6, 7, 8 and 9), but in other cases these patches were close to each other (Fig. 5). Finally, labeled cells within ventral IT were diffusely scattered for all cases, suggesting that this area does not have a visuotopic organization pattern. Overall, the results are consistent with architectonic and cortical projection patterns that IT cortex has 4 or 5 subdivisions.
Posterior Parietal Cortex
Projections from the parietal cortical region to the superior colliculus were identified in three cases (Figs. 5, 6, and 7). Yet, there were few labeled neurons relative to temporal and even frontal cortical areas. Similar findings were also observed by Casseday et al. (1979). In primates, dense projections from the posterior parietal cortex were observed in galagos (Baldwin and Kaas, 2012), New World monkeys (Collins et al., 2005) and Old World monkeys (Fries, 1984, Lock et al., 2003). This difference may reflect the proportionately larger posterior parietal region of primates, as well as a greater involvement in visuomotor functions (Kaas et al., 2011). In both primates and tree shrews, posterior parietal cortex receives inputs from visual cortex and projects to frontal motor cortex (Remple et al., 2007).
Somatosensory Cortex
We found only a few sparse cells, all in one case (Fig. 5), projecting to the superior colliculus within somatosensory areas. Although Casseday et al. (1979) concluded that neurons in somatosensory cortex project to the superior colliculus, interpretations of the organization of cortex in tree shrews have since changed (compare Fig. 1A to 1B). In Casseday et al. (1979), labeled cells were observed within the S2/PV region of today’s schemes of cortical areas in tree shrews (Wong and Kaas, 2009) after injections within intermediate layers of the superior colliculus, and labeled cells were found within the region of S1, 3a, and motor areas after deeper injections within the superior colliculus. The injections into the superior colliculus that resulted in the most labeled cells within somatosensory and motor cortex of Casseday et al. (1979) were made into more lateral locations within the superior colliculus than in our cases, that involved deeper layers of the superior colliculus (Fig. 10). However, labeled cells within somatosensory and motor cortex were also observed with more medial injections (Casseday et al., 1979). One possible reason for these differences between the results of Casseday et al. (1979) and the current study could be in the way injections were made. Casseday et al. (1979) placed tracers into the superior colliculus by going horizontally through the cerebellum, and brainstem including the inferior colliculus, while our tracers were placed vertically into the superior colliculus after cortical aspiration, retraction, or through other visual cortical areas. It could be that the label observed in Casseday et al. (1979) is a result of contamination of other brainstem structures with connections to somatosensory and motor areas. However, further studies involving injections into somatosensory cortex, or into deep layers of the lateral superior colliculus need to be conducted in order to determine if there are or are not connections between the somatosensory cortex and the superior colliculus in tree shrews.
Our results also differ from reports in rodents where projections from whisker fields in primary and secondary somatosensory are prominent (Wise and Jones, 1977; Rhoades et al., 1981). However, the present results in tree shrews are similar to results from primates (Fries, 1975; Collins et al., 2005; Baldwin et al., 2012), where few cortical inputs originate from primary somatosensory areas. Nevertheless the S2/Pv region has projections to the deep layers of the superior colliculus in galagos and monkeys (Collins et al., 2005; Wu et al., 2005; Baldwin et al., 2012).
Auditory Cortex
Auditory cortex of tree shrews includes a core and belt regions, both staining heavily for myelin (Oliver and Hall, 1975; Casseday et al, 1976; Oliver and Hall, 1978; Wong and Kaas, 2009). In the present report we combined both belt and core areas into a single auditory region (Aud). Few cells were found in auditory cortex after either superficial or deep injections, with the majority of such cells along the caudal borders of Aud. Casseday et al. (1976; 1979) reported projections from the auditory core to the inferior colliculus, but no projections to the superior colliculus. However, projections from the auditory belt and auditory dysgranular regions to intermediate and deep layers of the superior colliculus were described. The illustrated results suggest that most of these auditory projections of Casseday et al. (1979) are from the ITr of Wong and Kaas (2009) and thus are consistent with our present results. In primates, few projections to the superior colliculus arise from the auditory core, belt, or parabelt regions (Fries, 1984; Lock et al., 2003; Collins et al., 2005; Baldwin et al., 2012). Yet there are projections to the superior colliculus from a region between visual and auditory cortex known as the temporal parietal area (Tpt), which is thought to be involved in auditory processing and had connections with posterior parietal and frontal areas (Preuss and Goldman-Rakic, 1991; Stepniewska et al., 2009).
Motor and Frontal cortex
We found labeled cells within frontal cortex after injections that involved deep layers of the superior colliculus. Primary motor cortex in tree shrews has been divided into M1 and M2 regions (Remple et al., 2006, 2007; Wong and Kaas 2009) based on differences in connections, stimulation thresholds, and architecture. Here we combined M1 and M2 into a single motor area, M after injections that involved the deep layers of the superior colliculus. Two patches were observed, with one patch more rostromedial than the other (Figs. 5–7). Though the most caudal patch of cells in frontal cortex (Figs. 8 and 9) could be within the motor cortex, it is likely the patches of labeled cells lie within premotor and prefrontal cortex. The most rostral border of M2 is 2mm or less away from the rostral border of area 3b (Remple et al., 2006; 2007) and labeled cells in this study were located more than 1.5mm away from the rostral 3b border. Casseday et al. (1979) found that deep superior colliculus injections labeled cells in similar locations. It could be that the more rostral patch of labeled cells in tree shrews is within an eye or gaze movement region, as such gaze fields are present within this region of rodents (Hall and Lindholm, 1974; Donoghue and Wise, 1982; Neafsey et al., 1986; Rapisarda, 1990; Stuesse and Newman, 1990; Tsumori, 2001). However, such a field was not observed in the microstimulation experiments of Remple et al. (2006). Thus, the presence of a frontal eye field, as in primates, remains uncertain in tree shrews. In rodents, direct projections from motor cortex to the superior colliculus are present (Miyashita et al., 1994). However, few, if any corticotectal projections arise from primary motor areas in primates (Collins et al., 2005; Fries et al., 1984; Baldwin et al., 2012). While there are strong tectal projections from the frontal eye fields in New and Old World monkeys (Collins et al., 2005; Fries, 1984), prosimian primates have weaker frontal eye field projections with stronger projections arising from other prefrontal areas (Baldwin and Kaas, 2012).
Conclusions
The superior colliculus, or optic tectum, is found in all vertebrates; however, the specific function of this structure may vary depending on differences in afferent and efferent connections. The neocortex can influence the functional properties of the superior colliculus through projections to the superior colliculus, which then in turn projects to motor centers and the visual thalamus. Because of their phylogenetic position, it is important to compare features of brain organization in tree shrews with those in primates and rodents. Our present results provide evidence that, in tree shrews, cortical visual and visuomotor inputs influence the superior colliculus more so than cortical inputs from the somatosensory, auditory, or motor cortices. This finding is more congruent with observations in primates than most rodents. In primates, the inputs to the superior colliculus are more visual in nature and do not contain many inputs from somatosensory, auditory, or motor areas (Fries, 1984; Lock et al., 2003; Collins et al., 2005; Baldwin and Kaas, 2012). In contrast, projections to the superior colliculus from somatosensory cortex, primarily from the barrel field, are observed in rodents (Wise and Jones, 1977; Rhoades, 1981; Olavarria and Van Sluyters, 1982; Cadusseau and Roger, 1985; Welker et al., 1988; Harvey and Worthington, 1990; Hoffer et al., 2005; Aronoff et al., 2010; etc.). However, such connections may differ in the highly visual rodents such as squirrels.
Table 2.
List of abbreviations
| 17 | Area 17 |
| 18 | Area 18 |
| 19 | Area 19 |
| 3b | Primary somatosensory area |
| AChE | Acetylcolinesterase |
| Aud | Auditory cortex |
| Aud Kon | Auditory konio cortex |
| Aud Belt | Auditory belt |
| Aud Dys | Auditory dysgranular cortex |
| CO | Cytochrome oxidase |
| IT | Inferior temporal cortex |
| Lim | Limbic cortex |
| Mot 4 | area 4 of motor cortex |
| M | motor cortex |
| OFC | orbital frontal cortex |
| Pro Iso | proisocortex |
| PV | parietal ventral area |
| S2 | secondary somatosensory area |
| Sc | caudal somatosensory area |
| SC | superior colliculus |
| Sens mot | sensory-motor cortex |
| Sens kon | somatic koniocortex |
| Som sens belt | somatic sensory belt |
ACKNOWLEDGEMENTS
We thank Laura Trice and Arkadiusz Slusarczyk for help with histological procedures, and Mary Feurtado, Nicole Young, and Na Zhou for surgical assistance.
This research was supported by grants from the National Eye Institute, RO1 EY-02686 (J.H.K), RO1 EY-016155 (M.E.B and H.M.P.), and CORE grants P30 EY-008126.
Footnotes
Conflict of Interest Statement
All authors report that there are no conflicts of interest.
Role of Authors
MKLB, HW, JLR, and HMP helped in the designing of the experiment, collecting and analyzing the data, and writing the manuscript. MEB and JHK were involved in designing the experiment, analyzing the data, and writing the manuscript.
REFERENCES
- Abplanalp P. Some subcortical connections of the visual system in tree shrews and squirrels. Brain Behav and Evol. 1970;3:155–168. doi: 10.1159/000125468. [DOI] [PubMed] [Google Scholar]
- Abplanalp P. The neuroanatomical organization of the visual system in tree shrew. Folia Primatol. 1971;16:1–34. doi: 10.1159/000155389. [DOI] [PubMed] [Google Scholar]
- Albano JE, Humphrey AL, Norton TT. Laminar organization of receptive-field properties in tree shrew superior colliculus. J Neurophysiol. 1978;41:1140–1164. doi: 10.1152/jn.1978.41.5.1140. [DOI] [PubMed] [Google Scholar]
- Albano JE, Norton TT, Hall WC. Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Res. 1979;173:1–11. doi: 10.1016/0006-8993(79)91090-4. [DOI] [PubMed] [Google Scholar]
- Aronoff R, Matyas F, Celine M, Carine C, Schneider B, Peterson CCH. Long-range connectivity of mouse primary somatosensory barrel cortex. Eur J Neurosci. 2010;31:2221–2233. doi: 10.1111/j.1460-9568.2010.07264.x. [DOI] [PubMed] [Google Scholar]
- Balaram P, Hackett TA, Kaas JH. VGLUT2 mRNA and protein expression in the visual thalamus and midbrain of prosimian galagos (Otolemur garnetti) Eye and Brain. 2011;3:81–98. doi: 10.2147/EB.S16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Wong P, Reed JL, Kaas JH. Superior colliculus connections with visual thalamus in gray squirrels (Sciurus carolinensis): Evidence for four subdivisions within the pulvinar complex. J Compe Neurol. 519:1071–1094. doi: 10.1002/cne.22552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin MKL, Kaas JH. Cortical projections to the superior colliculus in prosimian galagos (Otolemur garnetti) J Compe Neurol. 2012 doi: 10.1002/cne.23025. EPub Dec 15, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford ME, Hall WC. Collateral projections of predorsal bundle cells of the superior colliculus in rat. J Comp Neurol. 1989;283:86–106. doi: 10.1002/cne.902830108. [DOI] [PubMed] [Google Scholar]
- Cadusseau J, Roger M. Afferent projections to the superior colliculus in the rat, with special attention to the deep layers. J Himforsch. 1985;26:667–681. [PubMed] [Google Scholar]
- Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF. Superior colliculus of the tree shrew: A structural and functional subdivision into superficial and deep layers. Science. 1972;177:444–447. doi: 10.1126/science.177.4047.444. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Diamond IT. Ablation study of the superior colliculus in the tree shrew (Tupaia glis) J Comp Neurol. 1975;156:207–238. doi: 10.1002/cne.901560206. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Diamond IT, Harting JK. Auditory pathways to the cortex in Tupaia glis. J Comp Neurol. 1976;166:303–340. doi: 10.1002/cne.901660304. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Jones DR, Diamond IT. Projections from cortex to tectum in the tree shrew Tupaia glis. J Comp Neurol. 1979;185:253–291. doi: 10.1002/cne.901850204. [DOI] [PubMed] [Google Scholar]
- Collins CE, Lyon DC, Kaas JH. Distribution across cortical areas of neurons projecting to the superior colliculus in New World monkeys. Anat Rec Part A. 2005;285A:619–627. doi: 10.1002/ar.a.20207. [DOI] [PubMed] [Google Scholar]
- Chomsung RD, Petry HM, Bickford ME. Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. J Comp Neurol. 2008;510:24–46. doi: 10.1002/cne.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsung RD, Wei H, Day-Brown JD, Petry HM, Bickford ME. Synaptic organization of connections between the temporal cortex and pulvinar nucleus of the tree shrew. Cereb Cortex. 2010;20:997–1011. doi: 10.1093/cercor/bhp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick CG. Anatomical organization of the superior colliculus in monkeys: cortical pathways for visual and visuomotor functions. Prog Brain Res. 1988;75:1–15. doi: 10.1016/s0079-6123(08)60461-6. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Snyder M, Killackey H, Jane J, Hall WC. Thalamocortical projections in the tree shrew (Tupaia glis) J Comp Neurol. 1970;139:273–306. doi: 10.1002/cne.901390303. [DOI] [PubMed] [Google Scholar]
- Donaldson IML, Witteridge D. The nature of the boundary between cortical visual areas II and III in the cat. Proc Roy Soc B. 1977;199:445–462. doi: 10.1098/rspb.1977.0153. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Wise SP. The motor cortex of the rat: cytoarchitecture and microstimulation mapping. J Comp Neurol. 1982;212:76–88. doi: 10.1002/cne.902120106. [DOI] [PubMed] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Gallyas F. Inputs from motor and premotor cortex to the superior colliculus of the macaque monkey. Behav Brain Res. 1979;18:95–105. doi: 10.1016/0166-4328(85)90066-x. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetylcholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Gharbawie OA, Stepneiwska I, Qi H, Kaas JH. Multiple parietal-frontal pathways mediate grasping in macaque monkeys. J Neurosci. 2011;31:11660–11677. doi: 10.1523/JNEUROSCI.1777-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J, Lin CS, Kaas JH. Subcortical projections of six visual cortical areas in the owl monkey, Aotus trivirgatus. J Comp Neurol. 1979;187:557–580. doi: 10.1002/cne.901870307. [DOI] [PubMed] [Google Scholar]
- Hall WC, Lindholm EP. Organization of motor and somatosensory neocortex in the albino rat. Brain Res. 1974;66:23–38. [Google Scholar]
- Harting JK, Noback CR. Subcortical projections from the visual cortex in the tree shrew (Tupaia glis) Brain Res. 1971;25:21–33. doi: 10.1016/0006-8993(71)90564-6. [DOI] [PubMed] [Google Scholar]
- Harting JK, Hall WC, Diamond IT, Martin GF. Anterograde degeneration study of the superior colliculus in Tupaia glis: Evidence for a subdivision between superficial and deep layers. J Comp Neurol. 1973;148:361–386. doi: 10.1002/cne.901480305. [DOI] [PubMed] [Google Scholar]
- Harting JK, Hall WC, Diamond IT, Martin GF. Anterograde degeneration study of cortical projections of the lateral geniculate and pulvinar nuclei in the tree shrew (Tupaia glis) J Comp Neurol. 1973;150:393–440. doi: 10.1002/cne.901500403. [DOI] [PubMed] [Google Scholar]
- Harvey AR, Worthington DR. The projections from different visual cortical areas to the rat superior colliculus. J Comp Neurol. 1990;298:281–292. doi: 10.1002/cne.902980303. [DOI] [PubMed] [Google Scholar]
- Hoffer ZS, Arantes H, Roth R, Alloway KD. Functional circuits mediating sensorimotor integration: quantitative comparisons of projections from rodent barrel cortex to M1 cortex, neostriatum, superior colliculus, and pons. J Comp Neurol. 2005;488:82–100. doi: 10.1002/cne.20579. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J Neurophysiol. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Weber JT, Rothstein LR, Harting JK. Subcortical connections of area 17 in the tree shrew: an autoradiographic analysis. Brain Res. 1985;340:163–170. doi: 10.1016/0006-8993(85)90788-7. [DOI] [PubMed] [Google Scholar]
- Inoue K, Terashima T, Inoue Y. Postnatal development of corticotectal projection from the visual cortex of the mouse. Okajimas Folia Anat Jpm. 1992;68:319–331. doi: 10.2535/ofaj1936.68.6_319. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Convergence in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav and Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- Kaas JH. The evolution of the auditory cortex: The core areas. In: Winer JA, Schreiner CE, editors. Auditory Cortex. Spring Science-Business; 2011. [Google Scholar]
- Kaas JH, Hall WC, Killackey H, Diamond IT. Visual cortex of the tree shrew (Tupaia glis): architectonic subdivisions and representations of the visual field. Brain Res. 1972;42:491–496. doi: 10.1016/0006-8993(72)90548-3. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Preuss TM. Mammal Phylogeny; Placentals Eds Szalay et al. New York: Springer-Verlag; 1993. Archontan affinities as reflected in the visual system. [Google Scholar]
- Kaas JH, Gharbawie OA, Stepniewska I. The organization and evolution of dorsal stream multisensory motor pathways in primates. Frontiers in Neurosci. 2011 Jun 13; doi: 10.3389/fnana.2011.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RH, Allman JM, Kaas JH. Representation of the visual field in the superior colliculus of the grey squirrel (sciurus carolinensis) and the tree shrew (tupaia glis) Brain Res. 1971;26:277–292. [PubMed] [Google Scholar]
- Lee P, Hall WC. Interlaminar connections of the superior colliculus in tree shrew. II: Projections from the superficial gray to the optic layer. Vis Neurosci. 1995;12:573–588. doi: 10.1017/s0952523800008464. [DOI] [PubMed] [Google Scholar]
- Lock TM, Baizer JS, Bender DB. Distribution of corticotectal cells in macaque. Exp Brain Res. 2003;151:455–470. doi: 10.1007/s00221-003-1500-y. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH. Cortical connections of striate and extrastriate visual areas in tree shrews. J Comp Neurol. 1998;401:109–128. doi: 10.1002/(sici)1096-9861(19981109)401:1<109::aid-cne7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Connectional evidence for dorsal and ventral V3, and other extrastriate areas in prosimian primate, galago garnetti. Brain Behav and Evol. 2002a;59:114–129. doi: 10.1159/000064159. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Kaas JH. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J Comp Neurol. 2002b;449:281–297. doi: 10.1002/cne.10297. [DOI] [PubMed] [Google Scholar]
- Lyon DC, Jain N, Kaas JH. The visual pulvinar in tree shrews I. Multiple subdivisions revealed through acetylcholinesterase and Cat-301 chemoarchitecture. J Comp Neurol. 2003;467:593–606. doi: 10.1002/cne.10939. [DOI] [PubMed] [Google Scholar]
- May PJ, Porter JD. The laminar distribution of macaque tectobulbar and tectospinal neurons. Vis Neurosci. 1992;8:257–276. doi: 10.1017/s0952523800002911. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Keller EL. Deficits in saccade target selection after inactivation of superior colliculus. Nat Neurosci. 2004 Jul;7:757–763. doi: 10.1038/nn1269. [DOI] [PubMed] [Google Scholar]
- Meredith RW, Janecka JE, Gatesy J, Ryder OA, Fisher CA. Impacts of cretaceous terrestrial revolution in KPg extinction on mammal diversification. Science. 2011;334:521–524. doi: 10.1126/science.1211028. [DOI] [PubMed] [Google Scholar]
- Miyashita E, Keller A, Asanuma H. Input-output organization of the rat vibrissal motor cortex. Exp Brain Res. 1994;99:223–232. doi: 10.1007/BF00239589. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Eizirik E, O’Brien JO, Standhope MJ, DeJong WW, Springer MS. Resolution of the early placental mammal radiation using Baysian phylogenetics. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- Neafsey EJ, Bold EL, Haas G, Hurley-Glus KM, Quirk G, Silvert CF, Terreberry RR. The organization of the rat motor cortex: a microstimulation mapping study. Brain Res. 1986;396:77–96. doi: 10.1016/s0006-8993(86)80191-3. [DOI] [PubMed] [Google Scholar]
- Olavarria J, Van Sluyters RC. The projection from striate and extrastriate cortical areas to the superior colliculus in the rat. Brain Res. 1982;242:322–336. doi: 10.1016/0006-8993(82)90318-3. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Hall WC. Subdivisions of the medial geniculate body in tree shrews (Tupaia glis) Brain Res. 1975;86:217–227. doi: 10.1016/0006-8993(75)90698-8. [DOI] [PubMed] [Google Scholar]
- Oliver DL, Hall WC. The medial geniculate body of three shrew, Tupaia glis. II. Connections with the neocortex. J Comp Neurol. 1978;182:459–494. doi: 10.1002/cne.901820306. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS. Ipsilateral cortical connections of granular frontal cortex in the strepsirhine primate galago, with comparative comments on anthropoid primates. J Comp Neurol. 1991;310:507–549. doi: 10.1002/cne.903100404. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Casagrande VA, Diamond IT. Visual neglect in the tree shrew after interruption of descending projections of the deep superior colliculus. Expt Neurol. 1976;50:14–29. doi: 10.1016/0014-4886(76)90231-4. [DOI] [PubMed] [Google Scholar]
- Rapisarda C, Palmeri A, Aicardi G, Sapienza S. Multiple representations of the body and input-output relationships in the agranular and granular cortex of the guinea pig. Somatosens Mot Res. 1990:289–314. doi: 10.3109/08990229009144710. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Lyon DC, Stepniewska I, Kaas JH. Organization of frontoparietal cortex in the tree shrew (Tupaia belangeri). I. Architecture, microelectrode maps, and corticospinal connections. J Comp Neurol. 2006;497:133–154. doi: 10.1002/cne.20975. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Lyon DC, Stepniewska I, Kaas JH. The organization of the frontal parietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Rhoades R. Cortical and spinal somatosensory input to the superior colliculus in the golden hamster: An anatomical and electrophysiological study. J Comp Neurol. 1981;195:415–432. doi: 10.1002/cne.901950304. [DOI] [PubMed] [Google Scholar]
- Samorajski T, Ordy JM, Keefe JR. Structural organization of the retina in the tree shrew (Tupaia glis) J Cell Biol. 1966;28:489–504. doi: 10.1083/jcb.28.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller PH, Sandell JH, Maunsell JH. The effect of frontal eye field and superior colliculus lesions on saccadic latencies in the rhesus monkey. J Neurophysiol. 1987;57:1033–1049. doi: 10.1152/jn.1987.57.4.1033. [DOI] [PubMed] [Google Scholar]
- Stein BE, Goldberg SJ, Clamann HP. The control of eye movements by the superior colliculus in the alert cat. Brain Res. 1976;118:469–474. doi: 10.1016/0006-8993(76)90314-0. [DOI] [PubMed] [Google Scholar]
- Sefton AJ, Mackay-Sim A, Baur LA, Cottee LJ. Cortical projections to visual centers in the rat: an HRP study. Brain Res. 1981;215:1–13. doi: 10.1016/0006-8993(81)90487-x. [DOI] [PubMed] [Google Scholar]
- Sesma MA, Casagrande VA, Kaas JH. Cortical connections of area 17 in tree shrews. J Comp Neurol. 1984;230:337–351. doi: 10.1002/cne.902300303. [DOI] [PubMed] [Google Scholar]
- Snyder M, Diamond IT. The organization and function of the visual cortex in tree shrew. Brain Behav and Evol. 1968;1:244–288. [Google Scholar]
- Stepneiwska I, Fang PC, Kaas JH. Microstimulation reveals sub regions for different complex movements in posterior parietal cortex of prosimian galagos. PNAS. 2005;102:4878–4883. doi: 10.1073/pnas.0501048102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska I, Cerkevich CM, Fang PC, Kaas JH. Organization of the posterior parietal cortex in galagos: II Ipsilateral cortical connections of physiologically identified zones within anterior sensorimotor region. J Comp Neurol. 2009;517:783–807. doi: 10.1002/cne.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuesse SL, Newman DB. Projections from the medial agranular cortex to brain stem visuomotor centers in rats. Exp Brain Res. 1990;80:532–544. doi: 10.1007/BF00227994. [DOI] [PubMed] [Google Scholar]
- Sur M, Weller RE, Kaas JH. Representation of the body surface in somatosensory area I of tree shrews, Tupaia glis. J Compe Neurol. 1980;194:71–95. doi: 10.1002/cne.901940105. [DOI] [PubMed] [Google Scholar]
- Sur M, Weller RE, Kaas JH. The organization of somatosensory area II in tree shrews. J Comp Neurol. 1981;201:121–133. doi: 10.1002/cne.902010109. [DOI] [PubMed] [Google Scholar]
- Triplett JW, Owens MT, Yamada J, Lemke G, Cang J, Stryker M, Feldheim DA. Retinal input instructs alignment of visual topographic maps. Cell. 2009;139:175–185. doi: 10.1016/j.cell.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumori T, Yokota S, Ono K, Yasui Y. Organization of projections from the medial agranular cortex to the superior colliculus in the rat: a study using anterograde and retrograde tracing methods. Brain Res. 2001;903:168–176. doi: 10.1016/s0006-8993(01)02437-4. [DOI] [PubMed] [Google Scholar]
- Updyke BV. Topographic organization of the projections from areas 17, 18, and 19 onto the thalamus, pretectum, and superior colliculus in the cat. J Comp Neurol. 1977;173:185–204. doi: 10.1002/cne.901730106. [DOI] [PubMed] [Google Scholar]
- Wei H, Masterson SP, Petry HM, Bickford ME. Diffuse and specific tectopulvinar terminals in the tree shrew: synapses, synapsins, and synaptic potentials. PLoS One. 2011 doi: 10.1371/journal.pone.0023781. Epub Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker E, Hoogland PV, Van der Loos H. Organization of feedback and feedforward projections of the barrel cortex: a PHA-L study in the mouse. Exp Brain Res. 1988;73:411–435. doi: 10.1007/BF00248234. [DOI] [PubMed] [Google Scholar]
- Werner W, Dannenberg Sabine, Offmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115:191–205. doi: 10.1007/pl00005690. [DOI] [PubMed] [Google Scholar]
- Wiener SI. Laminar distribution and patchiness of cytochrome oxidase in mouse superior colliculus. J Comp Neurol. 1986;244:137–148. doi: 10.1002/cne.902440202. [DOI] [PubMed] [Google Scholar]
- Wise SP, Jones EG. Somatotopic and columnar organization in the corticotectal projection of the rat sensory cortex. Brain res. 1977;133:223–235. doi: 10.1016/0006-8993(77)90760-0. [DOI] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the tree shrew Tupaia belangeri. Anat Rec. 2009;292:994–1027. doi: 10.1002/ar.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–29. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wu CW, Bichot N, Kaas JH. Somatosensory areas S2 and PV project to the superior colliculus in prosimian primate, Galago garnetti. Somat Mot Res. 2005;22:221–231. doi: 10.1080/08990220500262661. [DOI] [PubMed] [Google Scholar]
- Zilles K. A quantitative approach to cytoarchitectonics. I. The areal pattern of the cortex of Tupaia belengeri. Anat Embryol (Berl) 1978;153:195–212. doi: 10.1007/BF00343374. [DOI] [PubMed] [Google Scholar]