Abstract
We report the first documented case of IgG4-related inflammatory pseudotumours (IPTs) along the bilateral oculomotor nerves. A man in his 60s complained of decreased vision. He exhibited bilateral optic nerve atrophy without any extraocular movement deficits. MRI revealed enhanced masses that reached from the bilateral cavernous sinus to within the bilateral orbits. The tumours extended along the lines of the bilateral oculomotor nerves. The patient's serum level of IgG4 was high, 147 mg/dl. A biopsy specimen showed inflammatory cell-rich lesions against a collagenous stroma. Immunostaining revealed infiltration of CD138-positive plasma cells, which were mainly IgG and IgG4 positive. The IgG4/IgG ratio was greater than 0.4. These factors led us to a diagnosis of IgG4-related IPTs. Oral administration of prednisolone (30 mg/day) was started 3 months after the operation and continued for 6 months with gradual tapering. The tumour was significantly reduced by prednisolone.
Background
The diagnosis of inflammatory pseudotumours (IPTs) in the lungs was first described in the 1980s. IPTs are a rare form of tumour characterised by non-neoplastic proliferation of inflammatory cells against a collagenous stroma. IPTs are mostly found in the lungs or the upper respiratory tract, but intracranial occurrence of IPTs is fewer than 70 reported cases.1
More recently, immunoglobulin G4 (IgG4)-related disease has been characterised clinically. IgG4-related disease is marked by elevated serum IgG4 levels and significant infiltration of IgG4-positive plasma cells, along with sclerosis of the lesions. This phenomenon was first described in 2001 as autoimmune pancreatitis.2 In recent years, IgG4-related disease has been found to be associated with IPTs in the pancreas, lung, liver, breast and rarely in intracranial regions. Reports of intracranial IgG4-related IPTs are extremely rare, except in cases of hypophysitis. To our knowledge, this is the first documented case of IgG4-related IPTs along the bilateral oculomotor nerves.
Case presentation
A 63-year-old man was referred to us by an ophthalmologist in August 2011. He was evaluated for decreased vision in both of his eyes and optic nerve atrophy with intracranial mass along the oculomotor nerves. He had complained of decreased vision during the previous 3 years. His visual acuity was 0.8 in 2008, and had decreased to 0.3 by August 2011. The patient was in good health for the most part. His medical history only included chronic sinusitis. A physical examination was unremarkable. Eye examination revealed sluggish light reflex in both the eyes, full visual fields and full extraocular movements. There were no other neurological defects.
Investigations
Laboratory tests were within normal limits except for serum IgG4, 147 mg/dl. Levels of IgG, IgA and IgM were all within normal limits at 1003, 302 and 75 mg/dl, respectively. Hormone levels of FT3, FT4 and TSH were all within normal limits at 3.4 pg/ml, 1.4 ng/dl and 0.74 μIU/ml, respectively.
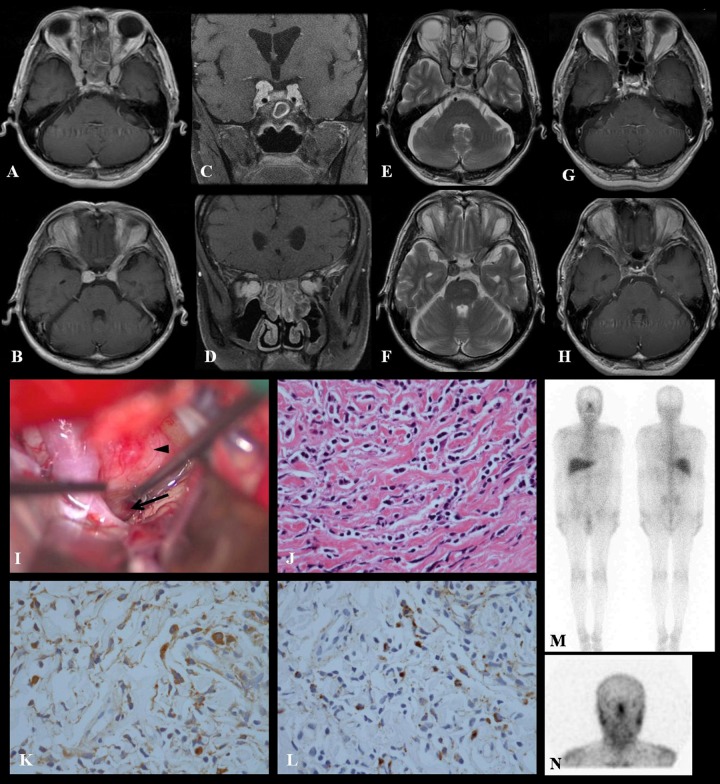
MRI revealed homogeneously enhanced masses that reached from the bilateral cavernous sinus to within the bilateral orbits. T2 intensity of the tumour was low. The tumour seemed to extend along the lines of the bilateral oculomotor nerves. Both oculomotor lesions seemed to be apart from each other in coronal MRI imaging. Both optic nerves were adjacent to the tumour in each intraorbital muscle cone, but neither optic nerve was compressed in subdural space. We also observed an enhanced thick dura mater along with enhanced mass margin that we characterised as hypertrophic pachymeningitis. MRI also revealed marked sinusitis, but there seemed to be no continuity between sinusitis and pachymeningitis. Head CT showed an enhanced mass without any calcification, an enlarged superior orbital fissure and slightly compressed optic canal. His decreased vision seemed to be due to compression of the optic nerves in intraorbital muscle cones or optic canals. Whole-body CT examination with contrast revealed no significant signs of neoplastic lesions. Gallium scintigram showed an accumulation in the head lesion and hilar area (figure 1).
Figure 1.
Preoperative axial and coronal T1-weighted MR images with Gd contrast showed homogeneously enhanced masses that extended from the bilateral cavernous sinus to within the bilateral orbits (A–D). Preoperative axial T2-weighted images (E and F). Post-steroid treatment imaging studies of axial T1-weighted MR images showing marked reduction of the Gd-enhanced tumour (G and H). Photograph of operative views by the right pterional approach (I). The arrowhead indicates the location of the tumour, which continues to the oculomotor nerve (black arrow). Histological studies stained with H&E, high-power views showed a mixture of lymphocytes and plasma cells without cytological atypia, original magnification ×400 (J). Immunohistochemical staining, demonstrating IgG- and IgG4-positive plasma cells. IgG+ plasma cells are shown in high-power view (×400) (K). IgG4+ plasma cells are also shown in high-power view (×400) (L). Greater than 40% of IgG+ plasma cells are IgG4+, and there are >10 IgG4+ plasma cells visible in high-power view. Gallium scintigram showing an accumulation in the head lesion and hilar area (M and N).
Differential diagnosis
Our differential diagnosis based on history and examinations included schwannoma, IgG4-related IPTs, neurolymphomatosis, metastasis, granulomatous disease and Erdheim-Chester disease. We performed a surgical biopsy of the tumour for diagnosis. The tumour was fairly firm, non-aspiratable, milky-white in colour and clearly continuing to the oculomotor nerve. The optic nerve was not compressed by the tumour in the subdural space. Thickened dura mater was observed near the lesion. A biopsy specimen was taken from the right superior-lateral side of the tumour. The specimen was small but sufficient for diagnosis. A frozen section showed fibrotic tissue with infiltration of lymphocytes and plasma cells without cellular atypia. Paraffin-embedded sectioning also showed inflammatory cell-rich lesions against a collagenous stroma. The inflammatory cells in the sample included a mixture of lymphocytes and plasma cells without cytological atypia. Immunostaining showed marked infiltration of CD138-positive plasma cells, which were mainly IgG and IgG4 positive. The IgG4/IgG ratio was greater than 0.4, and more than 10 IgG4-positive cells were visible under high magnification. Neither S-100 protein-positive nor ALK-1 protein-positive cells were observed. Pathological diagnosis was IgG4-related inflammatory pseudotumour.
Treatment
The patient complained of supraduction deficit and ptosis of the right eye after the biopsy. Visual acuity of both eyes remained unchanged. Oral administration of prednisolone (30 mg/day) was started 3 months after the operation. Prednisolone was postponed for 3 months because of the patient's wishes. The patient was treated with prednisolone 30 mg/day for 3 weeks, and then gradually tapered to 10 mg/day by 6 months after the surgery.
Outcome and follow-up
His supraduction deficit and ptosis of the right eye gradually improved in 9 months follow-up. His visual acuity had not worsened and the tumour was significantly reduced at 9 months after biopsy. Serum IgG4 also decreased to 83 mg/dl.
Discussion
The new concept of IgG4-related systemic disease was proposed in Japan.2 It seems to be more frequent in Asian countries. Reports of patients with IgG4-related pachymeningitis have been restricted to Asia. However, the aetiology and mechanism of elevated serum IgG4- and IgG4-positive plasma cells infiltration in IgG4-related IPTs is still unclear. IgG4 is not suspected to cause IgG4-related disease, but rather it is probably elevated because of some other underlying mechanism.3 There had been no well-established diagnostic criteria for systemic IgG4-related disease and IgG4-related IPTs. IgG4-related disease study groups of the Ministry of Health, Labour and Welfare in Japan proposed the following diagnostic criteria: (1) serum IgG4 concentration >135 mg/dl and (2) >40% of IgG-positive plasma cells being IgG4-positive and >10 IgG4-positive cells simultaneously imaged on a high-powered field biopsy sample.4 Furthermore, the diagnosis of IgG4-related disease should only be reached after other differential diagnoses have been excluded. Our patient's serum IgG4 and ratio of IgG4/IgG were 147 mg/dl, and >50%, respectively. We found no evidence for the other diseases listed on the differential diagnosis. Therefore, our case was consistent with definite IgG4-related disease established by the above-mentioned criteria.
We were only able to find six previously reported cases of IPTs with cranial nerve involvement using a Pubmed search (table 1).
Table 1.
Case reports of cranial nerve-related intracranial IPTs
| Authors and year | Patient age (years), sex | Involved cranial nerves | Presenting symptoms and signs | Treatment | Result | Follow-up (months) |
|---|---|---|---|---|---|---|
| Le Marc'hadour et al (1985) | 40, M | rt III or rt V | rt blindness, HA | 1. Partial removal 2. Steroid |
1. III paralysis 2. No change in tumour and symptoms |
24 |
| Yanagihara et al (1991) | 41, M | rt VII | rt facial paralysis | 1. Steroid 2. Total removal |
1. Remission of symptoms, recurrence after the treatment 2. VII paralysis, no recurrence |
24 |
| Kodsi et al (1993) | 40, M | lt V | lt optic neuropathy | 1. Biopsy 2. Steroid |
1. No deficit 2. Partial reduction of the tumour, remission of the symptom |
17 |
| McKinney et al (2006) | 50, M | lt II, V, IX | lt blindness, ptosis, facial numbness, dysphonia, dysphagia, hoarseness | 1. Biopsy 2. Steroid |
1. No deficit 2. Remission of tumour and symptoms, recurrence by the reduced dose |
6 |
| Jung et al (2006) | 52, M | lt V | lt facial numbness, HA | 1. Total removal | 1. V paralysis (facial numbness), no recurrence | 18 |
| Seol et al (2009) | 24, F | lt V | lt facial numbness, HA | 1. Biopsy 2. Steroid |
1. No deficit 2. Remission of tumour and symptoms |
8 |
| Present study | 63, M | bil III | bil optic neuropathy | 1. Biopsy 2. Steroid |
1. III paralysis 2. Remission of tumour, no change in symptoms |
9 |
HA, headache.
Only Le Marc'hadour et al5 reported a case with possibility of oculomotor nerve involvement. The patient complained of right blindness, and the CT scan showed a contrast-enhanced tumour in the right cavernous sinus, extending to the superior orbital fissure. The oculomotor nerve was sacrificed at tumour removal, but a detailed intraoperative finding was not described. Yanagihara et al6 reported an IPT case with unique facial nerve involvement. All the other four reported cases involved the trigeminal nerve.7–10 McKinney et al9 reported a case of IPTs involving optic, trigeminal and vagus nerve involvement. Among these, our case is unique because it contained bilateral cranial nerve involvement and was an IgG4-related case.
Tumour removal was performed in three of the cases discussed above, but all these procedures resulted in severe cranial nerve paralysis.5 7 9 Biopsy of the tumour was performed in all the other reported cases, which resulted in no deficits. Unfortunately, biopsy resulted in oculomotor nerve paralysis in our case. Insufficiently small biopsy specimens may lead to misdiagnosis in IgG4-related hypophysitis, since clusters of IgG4-positive plasma cells can also be observed in every case of secondary inflammation.3 A sufficient amount of specimen was imperative for diagnosis. However, we were not able to confirm the histological relationship of the tumour and nerve fibres. Yanagihara et al6 reported that the nerve was surrounded by fibrous granulation tissue of the tumour in their case of IPT of the facial nerve. The tumour surrounded the nerve and seemed to originate from the nerve sheath. If the tumour surrounded the oculomotor nerve fibres, that may be the reason why the oculomotor nerve had not been impaired by the tumour in our case.
A few rare cases of IgG4-related pachymeningitis have been reported.11–14 Our case was also compatible with a pachymeningitis diagnosis based on intraoperative and radiological findings. It is known that the dura propia and arachnoid mater continue to the epineurium and perineurium of the cranial nerves at the dural cave. This is probably the reason for which the intracranial pachymeningitis extends to extracranial regions along the trigeminal nerves in some reported cases, that is, orbit or infratemporal fossa.8–10 One possibility that could account for our findings is that retrograde extension of pachymeningitis along the cranial nerve sheath may have resulted in IPTs along with the bilateral oculomotor nerves.
There is no consensus regarding the ideal treatment course of IgG4-related IPTs. The most well-known treatment is glucocorticoid administration after biopsy, but the optimal dose and schedule is still unclear. In case reports of the cavernous sinus and skull base IPTs, steroid doses varied greatly, with treatment ranging from 1 to 8 months.15 We also administrated oral prednisolone for 6 months. His right oculomotor nerve paralysis gradually improved, visual acuity did not worsen and tumour was reduced. Five of the reported cases of IPTs with cranial nerve involvement were also treated by corticosteroids.5 6 8–10 Tumour regression was observed in four of five treated cases.6 8–10 Tumour regression provides symptomatic improvement, but tumour recurrence and symptomatic deterioration after the administration of steroid were reported in two cases.9 6
Learning points.
The mechanism of intracranial IgG4-related IPTs remains unclear.
In order to facilitate an accumulation of data to elucidate such a mechanism, we recommend that IgG4 levels be evaluated for all intracranial IPT cases.
Our IgG4-related IPTs along the bilateral oculomotor nerves case caused bilateral optic nerve atrophy, but it did not cause oculomotor nerve paralysis.
Pachymeningitis seemed to extend along both the oculomotor nerves in our case.
Tumour regression, symptomatic improvement and serum IgG4 decrease were all achieved by prednisolone administration.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Commissioned; internally peer reviewed.
References
- 1.Ishihara M, Izumoto S, Yoshimine T, et al. Immunohistochemical study of multiple inflammatory pseudotumors with both brain and spinal cord involvement—case report. Neurol Med Chir (Tokyo) 2010;50:246–50 [DOI] [PubMed] [Google Scholar]
- 2.Hamano H, Kawa S, Akamatsu T, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732–8 [DOI] [PubMed] [Google Scholar]
- 3.Nishioka H, Shibuya M, Haraoka J. Immunohistochemical study for IgG4-positive plasmacytes in pituitary inflammatory lesions. Endocr Pathol 2010;21:236–41 [DOI] [PubMed] [Google Scholar]
- 4.Umehara H, Okazaki K, Saeki T, et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol 2012;22:21–30 [DOI] [PubMed] [Google Scholar]
- 5.Le Marc'hadour F, Fransen P, Pasquier B, et al. Intracranial plasma cell granuloma: a report of four cases. Surg Neurol 1994;42:481–8 [DOI] [PubMed] [Google Scholar]
- 6.Yanagihara N, Segoe M, Ueda N, et al. Inflammatory pseudotumor of the facial nerve as a cause of recurrent facial palsy: case report. Am J Otol 1991;12:199–202 [PubMed] [Google Scholar]
- 7.Jung TY, Jung S, Kang SS, et al. Hemorrhagic intracranial inflammatory pseudotumor originating from the trigeminal nerve: a case report. J Neurooncol 2006;76:139–42 [DOI] [PubMed] [Google Scholar]
- 8.Kodsi SR, Younge BR, Scheithauer BW, et al. Intracranial plasma cell granuloma presenting as an optic neuropathy. Surv Ophthalmol 1993;38:70–4 [DOI] [PubMed] [Google Scholar]
- 9.McKinney AM, Short J, Kim Y, et al. Inflammatory myofibroblastic tumor of the orbit with associated enhancement of the meninges and multiple cranial nerves. AJNR Am J Neuroradiol 2006;27:2217–20 [PMC free article] [PubMed] [Google Scholar]
- 10.Seol JG, Loevner LA, Grady MS, et al. Inflammatory pseudotumor of the trigeminal nerve: a neoplastic mimic you do not want to miss. AJNR Am J Neuroradiol 2009;30:1941–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SK, Cheuk W, Chan JK, et al. IgG4-related sclerosing pachymeningitis: a previously unrecognized form of central nervous system involvement in IgG4-related sclerosing disease. Am J Surg Pathol 2009;33:1249–52 [DOI] [PubMed] [Google Scholar]
- 12.Kim EH, Kim SH, Chang JH, et al. Immunoglobulin G4-related hypertrophic pachymeningitis involving cerebral parenchyma. J Neurosurg 2011;115:1242–7 [DOI] [PubMed] [Google Scholar]
- 13.Kosakai A, Ito D, Suzuki N, et al. A case of definite IgG4-related pachymeningitis. Neurology 2010;75:1390–2 [DOI] [PubMed] [Google Scholar]
- 14.Riku S, Hashizume Y, Riku Y, et al. Is hypertrophic pachymeningitis a dural lesion of IgG4-related systemic disease? Rinsho Shinkeigaku 2009;49:594–6 (Jpn). [DOI] [PubMed] [Google Scholar]
- 15.McCall T, Fassett DR, Couldwell WT, et al. Inflammatory pseudotumor of the cavernous sinus and skull base. Neurosurg Rev 2006;29:194–200 [DOI] [PubMed] [Google Scholar]