Abstract
Among the metastases to thyroid gland, metastases from renal cell carcinoma (RCC) are not rare and their frequent macroscopic looks are similar to primary thyroid tumours. We report an unusual case of thyroid metastases from renal carcinoma in a 72 -year-old man presented with a 1-year history of choking spells, stridor and dyspnoea. Patient underwent right nephrectomy for RCC, 24 years ago. In the present case, a right hemithyroidectomy was performed for a suspected anaplastic thyroid carcinoma. Histological examination showed a metastases of a clear cell renal carcinoma. Although the RCC showed an indolent biological behaviour, the late thyroid metastases have concurred with a poor prognosis and the patient died 5 months after surgery. The interest of this case lies in the long progression-free survival of the RCC preceded by the diagnosis of the thyroid nodule and the discrepancy between the clinical–radiological and the histological assessment.
Background
Unusual presentation of disease with problems of differential diagnosis and therapeutic management.
Case presentation
We report the case of a 72-year-old man presented with dysfonia at our endocrinology ambulatory. His medical history includes right radical nephrectomy performed in another hospital, 24 years ago, owing to renal cell carcinoma (RCC) classified as pT2a. Recently, the patient noted a decline of voice, choking spells, stridor and dyspnoea associated with a mass of the neck.
Investigations
Physical examination of the neck region detected a thyroid enlargement; dosage of thyroid hormones, thyroglobulin, anti-TG antibodies and calcitonin levels was in the normal range. Thyroid ultrasonography (USG) showed an enlarged thyroid gland with multiple solid nodules and nodal involvement. Combining USG with Doppler can result in a better malignant potential estimation. In short, the following USG patterns suggested presence of malignancy lesion: irregular shape, ill-defined borders, hypoechogenecity, solidity, heterogeneous internal echoes and the presence of suspicious regional lymph nodes. The patient also performed a flexibile laryngoscopy that revealed right vocal-cord palsy. Neck MRI showed an increased thyroid volume, especially at right lobe (maximum transverse diameter 7 cm) and oesophageal compression. There were no tracheal wall or cricoid and thyroid cartilages involvements. MRI also showed enlarged cervical and submandibular lymph nodes (the largest lymph node measuring 2.3 cm). The patient was submitted to fine needle aspiration biopsy (FNAB) of a thyroid nodule and submandibular lymph node for a preoperative diagnosis. Both the FNABs demonstrated the presence of pleomorphic cells, clear cells and clusters of epithelial cells with eosinophilic cytoplasms in a haemorrhagic background (figure 1A, 1B); a diagnosis of poorly differentiated or anaplastic thyroid carcinoma was made. A right hemithyroidectomy with nodal dissection was performed: the surgical specimen showed a white-grayish, solid and partially encapsulated mass (figure 2). Histologically thyroid tumor (figure 2, inset) was composed of clear cells forming pseudo papille (figure 3A) and glandular spaces containing erythrocytes (figure 3B). Immunohistochemistry showed positivity for CD10 (figure 3C), but not for TTF-1 (figure 3D). A diagnosis of metastases of a clear cell renal carcinoma was made.
Differential diagnosis
Primary thyroid tumour or metastases from renal carcinoma.
Outcome and follow-up
Although the RCC showed an indolent biological behaviour, the late thyroid metastases have concurred with a poor prognosis and the patient died 5 months after surgery.
Discussion
Metastases to the thyroid gland are not as rare; most cases have been diagnosed in autopsy series and the overall incidence varies from 1.25% to 24%.1 2 Tumours that more frequently metastasise to the thyroid gland are pulmonary squamous cell carcinoma (11%), RCC (12%), breast carcinoma (21%), oesophageal squamous cell carcinoma, oropharynx squamous cell carcinoma.3–7 Rarely, a thyroid metastasis may be the first sign of a primary carcinoma of other sites, or it can present many years after the primary tumour removal of except that for RCC.8–10 In fact, the clear cell variant of RCC show a very unpredictable behaviour. Its metastases are found in atypical sites such as the gums and nasal cavities; they may occasionally be discovered before the primary renal tumour, or arise many years or decades after its surgical removal.11–14 RCC spreads both through the lymphatic system, to regional and sometimes to retroperitoneal, periaortic and mediastinal lymph nodes, or the lymph-blood system via the thoracic duct, but above all through the blood, via the renal vein and the inferior vena cava, often the site of a neoplastic thrombosis.10 Furthermore, thyroid metastases from RCC could be considered rare compared with the most common sites of visceral metastases. Thyroid metastases usually are not symptomatic and patients generally complain of a palpable nodule. More rarely, it may present with breathing difficulty, hoarseness, dysphonia, dysphagia and pain.2 10 In these cases, diagnosis of primary thyroid tumour should be always excluded, also in patients with a history of RCC. A preoperative FNAB may be useful in problematic cases. The peculiarity of our case is the long latency between the primary tumour and metastases. Rarely in the literature there have been reported cases with as long an interval time between primary tumour and metastatic disease with an exceptional case described by Bradham et al15 in 1973 that had a 25-year interval between the detection of pulmonary metastases. The evidence of a thyroid nodule many years or decades after the treatment of a primary renal cancer represents always a diagnostic dilemma and causes problems for the therapeutic management of the patient.16 17 In fact, the detection of metastases to the thyroid often indicates a poor prognosis also in ‘silent’ cases or in patients in good general conditions. A diagnosis of metastases from clear cell RCC is easy in patients with a history of nephrectomy or of recent discovery of renal mass. In these cases, a preoperative FNAB may be useful for the diagnosis and correct management of thyroid nodule. Instead, the diagnosis may be very difficult in cases without a history of RCC and in patients with a solitary and well-circumscribed nodule. Moreover, primary thyroid tumours are composed mainly of clear cells that are relatively common and clear cell RCC may have a papillary architecture. In difficult cases, a small immunohistochemical panel with CD10, TTF1, CK7 and thyroglobulin may be necessary for a correct diagnosis. In conclusion, although rare, thyroid metastases may be a diagnostic problem. A thyroid metastasis from an RCC could be considered in any patient presenting with a thyroid nodule and a positive clinical history for RCC. For isolated metastatic cancer to the thyroid, surgical treatment should be considered in order to avoid potential morbidity of tumour recurrence in the neck, even if the prognosis remain poor. Although therapy of metastatic malignancies is often considered to be palliative, aggressive surgical treatment in isolated cases may be curative and of clear survival benefit. In conclusion, we recommend that in all patients with a history of RCC, the appearance of a new thyroid nodule could be investigated.
Figure 2.

Grossly, the right lobe of thyroid showed a white-grayish solid, partially encapsulated mass (inset). Histologically, thyroid tumour showed a prevalent solid pattern: clear cells were evident (H&E ×10).
Figure 1.
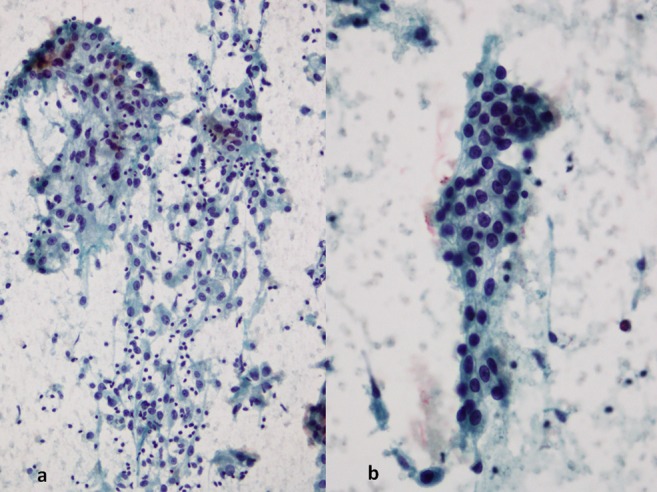
(A) Cell cluster formed by atypical epithelial cells with pleomorphic nuclei and prominent nucleoli. The background contains numerous polymorphous nuclear neutrophils and red blood cells (Papanicolau ×10). (B) A monolayer of cells with clear cytoplasms and well-defined cell borders (Papanicolau ×20).
Figure 3.
(A) A pseudopapillary architecture resembling a primary thyroid tumour was present (H&E ×10). (B) Presence of erythrocytes in the centre of pseudogland simulated renal cell carcinoma (H&E ×20). (C) Tumour cells were positive for CD10 1 (immunoperoxidase ×20). (D) Tumour cells showed no reactivity with TTF-1 (immunoperoxi8dase ×40).
Learning points.
Thyroid metastases from renal carcinoma may cause problems in differential diagnosis with primary thyroid tumours.
They may occasionally be discovered before the primary renal tumour, or arise many years or decades after its surgical removal.
Fine needle aspiration biopsy may be useful for the diagnosis and correct management of thyroid nodule.
Therapeutic management of thyroid metastases from renal carcinoma represents a challenge for the clinician.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Berge T, Lundburg S. Cancer in Malmo 1958–1969. An autopsy study. Acta Pathol Microbiol Scand Suppl 1977;260:1–235 Quoted from Poon D. Toh HC, Sim CS. Two case reports of metastates from colon carcinoma to the thyroid. Ann Acad Med Singapore 2004;33:100–2 [PubMed] [Google Scholar]
- 2.Haugen BR, Nawaz S, Cohn A, et al. Secondary malignancy of the thyroid gland: a case report and review of the literature. Thyroid 1994;4:297–300 [DOI] [PubMed] [Google Scholar]
- 3.Menegaux F, Chigot JP. Thyroid metastases. Ann Chir 2001;126:981–4 [DOI] [PubMed] [Google Scholar]
- 4.Nakhjavani M, Gharib H, Goellner JR, et al. Metastases to the thyroid gland, a report of 43 cases. Cancer 1997;79:574–8 [DOI] [PubMed] [Google Scholar]
- 5.Duggal NM, Horattas MC. Metastatic renal cell carcinoma to the thyroid gland. Endocr Pract 2008;14:1040–6 [DOI] [PubMed] [Google Scholar]
- 6.Dequanter D, Lothaire P, Larsimont D, et al. Intrathyroid metastasis: 11 cases. Ann Endocrinol (Paris) 2004;65:205–8 [DOI] [PubMed] [Google Scholar]
- 7.Shimaoka K, Sokal JE, Pickren JW. Metastatic neoplasms in the thyroid gland. Pathological and clinical findings. Cancer 1962;15:557–65 [DOI] [PubMed] [Google Scholar]
- 8.Testini M, Lissidini G, Gurrado A, et al. Acute air way failure secondary to the thyroid metastasis from renal carcinoma. World J Surg Oncol 2008;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iesalnieks I, Trupka A, Raab M, et al. Renal cell carcinoma metastases to the thyroid gland-8 cases reported. Thyroid 2007;17:49–52 [DOI] [PubMed] [Google Scholar]
- 10.Chung AY, Tran TB, Brumund KT, et al. Metastases to the thyroid: a review of the literature from the last decade. Thyroid 2012;22:258–68 [DOI] [PubMed] [Google Scholar]
- 11.Chin CJ, Franklin JH, Moussa M, et al. Metastasis from renal cell carcinoma to the thyroid 12 years after nephrectomy. CMAJ 2011;183:1398–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miah MS, White SJ, Oommen G, et al. Late simultaneous metastasis of renal cell carcinoma to the submandibular and thyroid glands seven years after radical nephrectomy. Int J Otolaryngol 2010;25:2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kihara M, Yokomise H, Yamauchi A. Metastasis of renal cell carcinoma to the thyroid gland 19 years after nephrectomy: a case report. Auris Nasus Larynx 2004;31:95–100 [DOI] [PubMed] [Google Scholar]
- 14.Innocente R, Bortolus R, De Paoli A, et al. Solitary thyroid metastasis from a renal cell carcinoma diagnosed and treated 12 years before: a case report. Tumori 1995;81:450–3 [DOI] [PubMed] [Google Scholar]
- 15.Bradham RR, Wannamaker CC, Pratt-Thomas HR. Renal cell carcinoma metastases 25 years after nephrectomy. JAMA 1973;223:921–2 [DOI] [PubMed] [Google Scholar]
- 16.Gelb AB. “Renal cell carcinoma: current prognostic factors.” Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 1997;80:981–6 [PubMed] [Google Scholar]
- 17.Zisman A, Pantuck AJ, Dorey F, et al. Mathematical model to predict individual survival for patients with renal cell carcinoma. J Clin Oncol 2002;20:1368–74 [DOI] [PubMed] [Google Scholar]



