Abstract
Objectives
This experimental study investigated the possible protective effect of beta glucans on amikacin ototoxicity.
Methods
Thirty-eight rats with normal distortion product otoacoustic emissions (DPOAEs) were divided into four groups. Group K was the control group. Group A was injected intramuscularly (i.m.) with amikacin 600 mg/kg/day between days 1-15. Group AB was given beta glucan gavage 1 mg/kg/day on days 0-15 and given amikacin 600 mg/kg/day i.m. on days 1-15. Group B was administered only beta glucan gavage, 1 mg/kg/day, on days 0-15. The DPOAEs were elicited in different frequency regions between 2,003 and 9,515 Hz, as distortion product diagrams (DPgrams), before and after the medication was administered, in all groups, on days 1, 5, 10, and 15.
Results
No significant changes in the DPgrams were observed in group K. In group A, significant deterioration was observed at the 8,003 and 9,515 Hz frequencies on day 10, and at the 3,991, 4,557, 5,660, 6,726, 8,003, and 9,515 Hz frequencies on day 15. For group AB, statistically significant deterioration was observed at the 2,824, 8,003, and 9,515 Hz frequencies on day 15. The results for group B showed a significant improvement of hearing at the 2,378, 2,824, 3,363, and 3,991 Hz frequencies on day 1, at the 3,363, 3,991, and 8,003 Hz frequencies on day 10, and at the 8,003 Hz frequency on day 15.
Conclusion
This study suggests that amikacin-induced hearing loss in rats may be limited to some extent by concomitant use of beta glucan.
Keywords: Beta glucan, Amikacin, Otoacoustic emission measurement
INTRODUCTION
Aminoglycoside antibiotics have been used worldwide, especially in the treatment of tuberculosis and certain other infections. They have a bactericidal effect by inhibiting protein synthesis. However, the toxic effects, especially for nephrotoxicity and ototoxicity, limit the use of aminoglycosides. The ototoxicity ratios of aminoglycosides are in the range of 2%-25% [1,2].
Aminoglycosides are polycationic and highly polar molecules that are insoluble in lipids [3], which means they are poorly absorbed in the gastrointestinal tract. Because of this, they are generally administered parenterally or topically [3]. After injection to the bloodstream, all cells appear to take up aminoglycosides; however, kidney proximal tubule cells and cochlear sensory hair cells are more sensitive [4]. After rapidly crossing the inner ear fluids [5], the drug stays within the inner ear cells for many months, whereas it is cleared from blood circulation within hours [6]. Aminoglycosides initially enter into the hair cells in the inner ear via the mechanoelectrical transducer canals at the tips of their stereocilia or through endocytosis at the apical surface [7,8]. In the inner ear, the target cells of aminoglycosides are the outer hair cells, one of the components of the sensory epithelium along the different turns of the cochlea, especially the basal one. Once taken up into the hair cells, aminoglycosides have a wide variety of effects, such as intracellular calcium level elevation [9] and the generation of toxic levels of reactive oxygen species (ROS) [10]. In particular, drug vesicles bind with iron and produce chelated metal complexes. These complexes form an aminoglycoside-iron complex that potentiates the formation of free oxygen radicals and mediates the ROS' induced cell damage (apoptosis) in the inner ear [11-13].
Beta glucans are glucose polymer groups that form a fibrotic extracellular matrix in the cell walls of yeast [14], plants [15], and some bacteria [16]. Currently, different types of beta glucans serve as immunomodulators that activate cellular and humoral components of the host immune system [17]. Several studies indicate further possible effects: activation of macrophages [18]; precipitation of wound healing by increasing wound-growth factors [14]; increased defense mechanisms against bacterial, viral, fungal, and parasitic infections [19]; and protective effects against oxidative damage in DNA through an effective free-radical scavenger function [20,21]. In light of these acknowledged properties, especially the free-radical scavenger function, our aim was to determine whether beta glucans could limit the ototoxic effect of amikacin in the inner ear.
MATERIALS AND METHODS
Animals and procedures
This experimental study was performed after receiving permission from the Inonu University Experimental Animal Ethics Committee (2009/20).
Thirty-eight adult female Wistar albino rats, initially weighing from 200-280 g at three months of age, were registered for the study. All the animals were harbored in a room with a cycle of 12 hours of light and 12 hours of darkness; the room temperature was 21℃ and they were fed ad-libitum. Animals with a normal Preyer's reflex were accepted for the study. The anesthetic was a ketamine hydrochloride (40 mg/kg) and xylazine (5 mg/kg) combination administered intramuscularly (i.m.) before the distortion product otoacoustic emissions (DPOAEs) were recorded.
Animals were randomly assigned to four groups. The control group (group K) was consisted of eight rats, which received no medication. The amikacin group (group A) was consisted of twelve rats, which were injected i.m. with amikacin 600 mg/kg/day between days 1-15. The ten rats in amikacin and beta glucan group (group AB) were given amikacin 600 mg/kg/day i.m. on days 1-15 and they were given beta glucan 1 mg/kg/day as a gavage on days 0-15. The eight rats in beta glucan group (group B) were administered beta glucan 1 mg/kg/day as a gavage on days 0-15. For all groups, the measurement of the DPOAEs was performed before and after drug administration on days 1, 5, 10, and 15.
OAE measurements
Rats that had normal hearing in the DPOAE measurements taken before drug administration were included in the study. The DPOAE recordings were performed in a quiet room and a special quiet cabin (Fig. 1). The DPOAE measurements were performed using a standard commercial GSI Audera DPOAE (Grason Stadler, Madison, USA). Both the control and experimental rats had their right ears measured. After administering anesthesia, an earphone was inserted into the external ear canal with a plastic adapter and the primary tones were given. The DPOAEs were recorded as distortion product diagrams (DPgrams). The intensity of the primary tones was constant and the DPOAE data were recorded at different frequencies, ranging from 2,003 to 9,515 Hz (2,003, 2,378, 3,363, 3,996, 4,757, 5,660, 6,726, 8,003, and 9,515 Hz), and planned as a function of f2. For the DPOAEs, the intensity of the primary stimuli were set equilevel (L1=L2) at 65 dB. The frequencies (f1 and f2) were adjusted to f2/f1=1.21. The DPgram resolution was set at four points per octave. The noise floor level was measured at a frequency 50 Hz above the DPOAE frequency using similar averaging techniques. The DPOAE results at the 2f1-f2 amplitude, which were 3 dB above the noise floor level at the 2f1-f2+50 Hz frequency, were adopted as a viable response.
Fig. 1.

Special quiet cabin used for distortion product otoacoustic emission measurements.
Statistical analyses
In the statistical evaluation, SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA) was used. Measured variables were expressed as the mean±standard error (SE). Results were analyzed statistically using the Mann-Whitney U-test to determine differences in the amplitudes of the DPOAEs and the corresponding noise floor differences in each of the groups. Additionally, the non-parametric Wilcoxon signed rank test was used to compare the domestic differences in the groups.
RESULTS
Group K (control group)
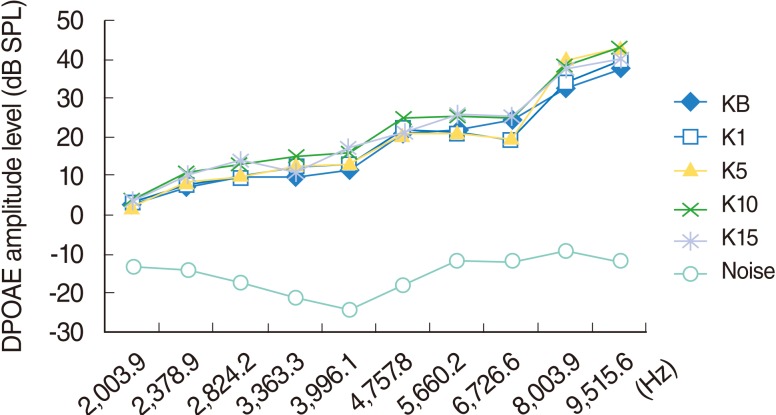
The DPOAEs measured at baseline and on day 1, 5, 10, and 15 of the study indicated the responses arising from 2,003.9, 2,378.9, 2,824.2, 3,363.3, 3,991.1, 4,757.8, 5,660.2, 6,726.6, 8,003.9, and 9,515.6 Hz areas. Over time, two of the rats (one on day 5 and one on day 10) did not wake up after anesthesia. No significant change was perceived in this group during the study (P>0.05) (Fig. 2).
Fig. 2.
Variations in amplitudes of distortion products otoacoustic emissions (DPOAEs) with frequency (F2) for different time points in control group. KB, baseline; K1, 1st day; K5, 5th day; K10, 10th day; K15, 15th day measurements.
Group A (amikacin group)
Over time, five of the 12 rats died. One did not wake up from anesthesia on day 1, while the other four rats died from anorexia or weight loss, caused by the toxic effects of amikacin, during different stages of the study. The seven rats that completed the study were used for statistical evaluation.
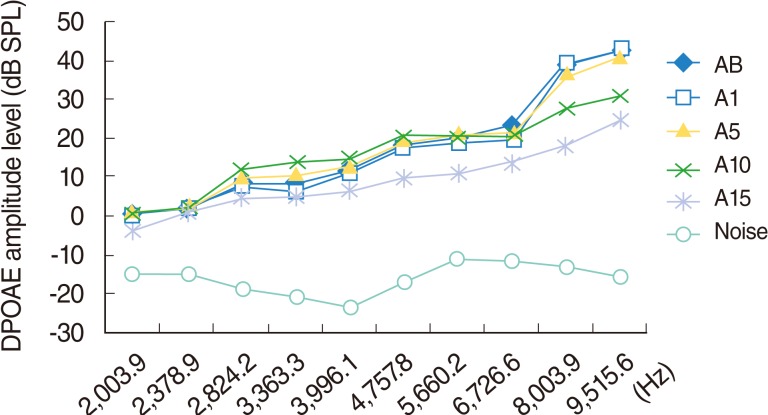
Significant deterioration was observed at the 8,003 and 9,515 Hz frequencies on day 10, and at the 3,991, 4,557, 5,660, 6,726, 8,003, and 9,515 Hz frequencies on day 15 (P<0.05) (Table 1, Fig. 3).
Table 1.
Standard error of mean values of amikacin group
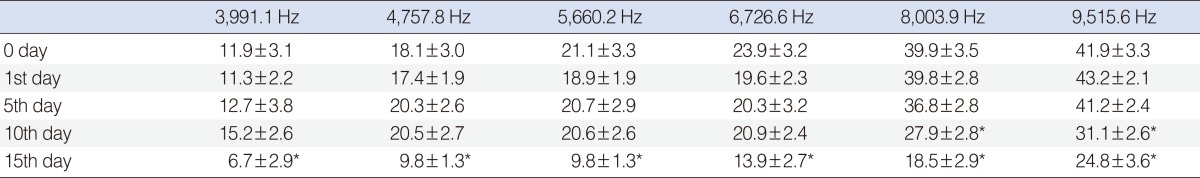
*Shows the significant decrease, when compared with basal (0 day) measurement (P<0.05; frequencies if there was statistical significant differences).
Fig. 3.
Variations in amplitudes of distortion products otoacoustic emissions (DPOAEs) with frequency (f2) for different time points in amikacin used group. AB, baseline; A1, 1st day; A5, 5th day; A10, 10th day; A15, 15th day measurements.
Group AB (amikacin-beta glucan group)
In this group, four of the ten rats died during the study; two of them did not wake up from anesthesia, and the other two died as a result of anorexia or weight loss, due to the toxic effects of amikacin, during different stages of the study. The six rats that completed the study were used for the statistical evaluation.
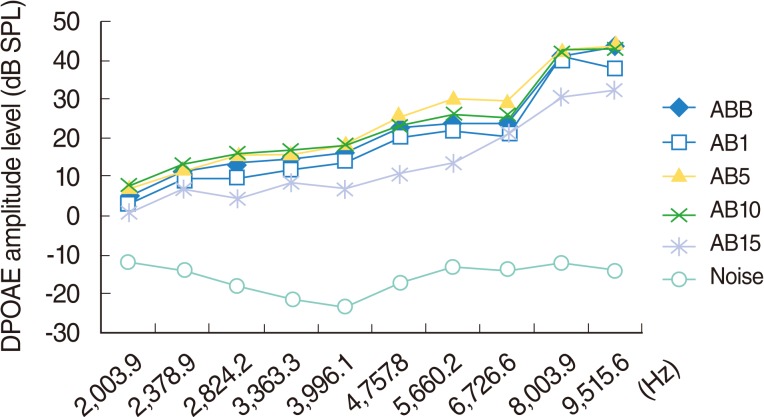
Statistically significant deterioration was observed at the 2,824, 8,003, and 9,515 Hz frequencies on day 15 (P<0.05) (Table 2, Fig. 4).
Table 2.
Standard error of mean values of amikacin+beta glucan group
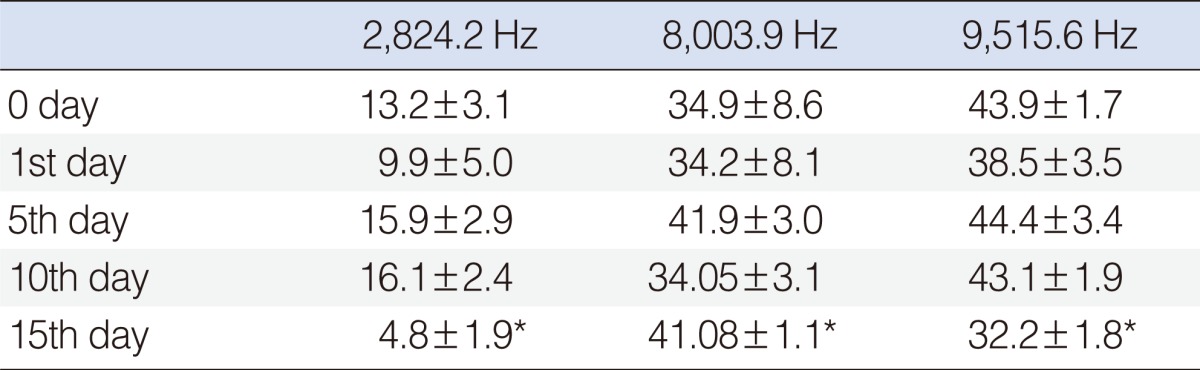
*Shows the significant decrease, when compared with basal (0 day) measurement (P<0.05; frequencies if there was statistical significant differences).
Fig. 4.
Variations in amplitudes of distortion products otoacoustic emissions (DPOAEs) with frequency (f2) for different time points in amikacin and beta glucan used group. ABB, baseline; AB1, 1st day; AB5, 5th day; AB10, 10th day; AB15, 15th day measurements.
Group B (beta glucan group)
One of the eight rats in this group died on the fifth day of the study, due to the anesthetic. The other seven rats completed the study.
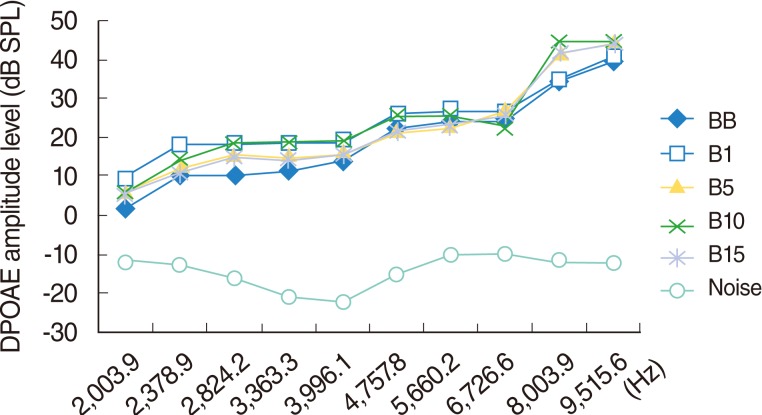
The results for this group showed a significant hearing improvement at the 2,378, 2,824, 3,363, and 3,991 Hz frequencies on day 1, at the 3,363, 3,991, and 8,003 Hz frequencies on day 10, and at the 8,003 Hz frequency on day 15 (P<0.05) (Table 3, Fig. 5).
Table 3.
Standard error of mean values of beta glucan group
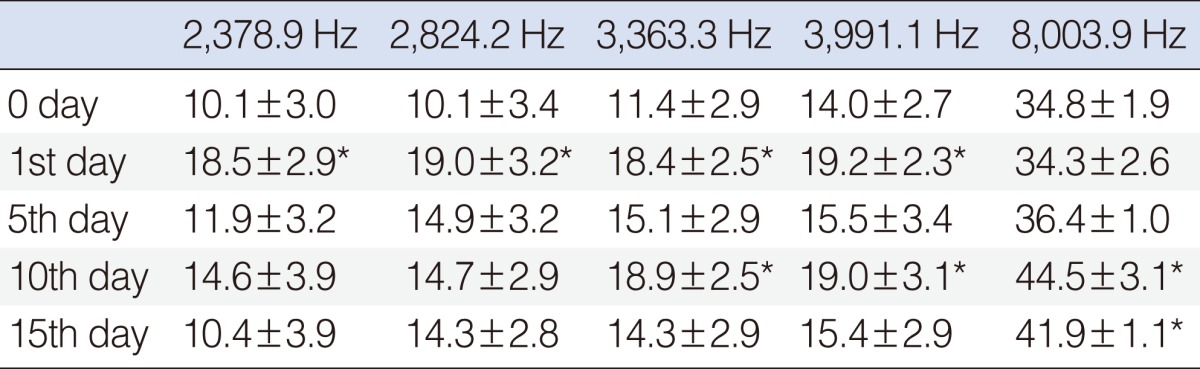
*Shows the significant increase, when compared with basal (0 day) measurement (P<0.05; frequencies if there was statistical significant differences).
Fig. 5.
Variations in amplitudes of distortion products otoacoustic emissions (DPOAEs) with frequency (f2) for different time points in beta glucan used group. BB, baseline; B1, 1st day; B5, 5th day; B10, 10th day; B15, 15th day measurements.
DISCUSSION
The clinically cochleotoxic effect of aminoglycoside characterized by permanent sensorineural hearing loss, initially affects the high frequencies, according to the specific damage to the outer hair cells in the basal turn [1]. Early damage cannot be revealed unless high frequency (8-20 Hz) pure tone audiometry is employed. DPOAEs or auditory brainstem responses may be evaluated in this stage [3]. When damage progresses to the apex of the cochlea, hearing loss becomes detectable at the lower frequencies inside the speech range [3].
Free radicals are very effective and unstable elements, with a low molecular weight and a short life, and they have one or more unmapped electrons in their outer orbit [22]. Because these free radicals have a tendency to become stable, they react with other molecules of the cell to match its unmapped electron and they have the ability to change the structure of other molecules thereby destroying the cell. This sequence of events continues unless being broken by an antioxidant agent. Free radicals can be formed in various ways, radiation and medicines being the commonest exogenous sources [22].
Free oxygen radicals have the capacity to oxidize certain cellular components, such as lipids, proteins, and nucleic acids. In particular, when unsaturated fatty acids in the cell membrane encounter free radicals, a diffuse peroxidation occurs. As a result of this reaction, the wholeness of the cell membrane is disrupted, resulting in apoptosis [21]. Under normal conditions, there are no free oxygen radicals in the circulatory system because the harmful effects of these radicals are hindered by a host of antioxidant defense systems. However, the over-production of free oxygen radicals, or weakened antioxidant defense systems, can result in toxic effects [21]. Therefore, the oxidant stress caused by free oxygen radicals is thought to contribute a part in the pathogenesis of diabetes mellitus, cancer, and atherosclerosis, as well as in the ototoxicity and nephrotoxicity caused by certain medicines [21].
It has been suggested that aminoglycosides cause cell death by acting as free oxygen radicals. While the sites of the inner ear damage (and the clinical and pathological results) have been established, absolute agreement on the mechanism of ototoxicity at the molecular level has not been declared. The most accepted ototoxicity mechanism is based on mechanoelectrical transducer channels [8]. According to this mechanism, aminoglycosides enter into the hair cells through the mechanoelectrical transducer channels and form a complex with iron. This aminoglycoside-iron complex acts as a free oxygen radical, and it forms during arachidonic acid metabolism and reacts with electron donors. This process results in cell death via oxygen radicals or enzyme activation [23].
In light of this basic, it can be suggested that the aminoglycoside group of antibiotics causes ototoxicity due to free radicals. Antioxidants protect cells against the adverse effects of drugs, carcinogens, and toxic radical reactions [24,25]. They inhibit the peroxidation chain reaction and collect ROS to inhibit lipid peroxidation [26]. Physiologically, oxidant stress is balanced by the existing antioxidants. When this balance is disrupted, tissue injury or disease can develop. In order to eliminate or minimize oxidant damage, remove the oxidant-enhancing effects, avert the triggered biochemical events, or neutralize the oxidant-secreting cells antioxidant agents can be used. Using antioxidants, which inactivate the oxidants directly, is the most commonly adopted method. Nowadays, different types of antioxidants, such as recombinant superoxide dismutase (SOD), iron chelators (desferrioxamine), allopurinol, E, C, and A vitamins, beta-carotene, methionine, glutathione, probukal, and angiotensin converting enzyme inhibitors, are used for therapeutic purposes [25]. Also, the use of beta glucans, which are known as effective antioxidants, is supported by some studies in literature as treatment modalities [27].
Beta glucans are heterogeneous polymers of glucose that exist as a structural element in the cell walls of yeasts, fungi, and cereals. As beta glucans have no known side effects or toxicity, and they have strong immunomodulatory, anti-infective, antitumor, and radiation-protective effects, regulation of the body's health can be considered among its important biological activities. Aside from these efficacious activities, beta glucans are also known for their potent antioxidant activity [28].
There are studies in the literature oriented toward preventing aminoglycoside ototoxicity [29]. They have proved aminoglycoside ototoxicity, with the aminoglycosides acting as free radicals, by doing iron chelation. One study shows, histopathologically and audiologically, that neomycin toxicity can be partially prevented by the iron chelating agent deferoxamine, which affects the aminoglycoside-iron complex formation [30]. Also, dihydrochloride benzoate and salicylates, known to be iron-chelating and antioxidant agents, were found effective in protecting against aminoglycoside ototoxicity [31-33].
It has been shown that another mechanism of ototoxicity, free radical formation, can be prevented with glutathione and glutathione-related enzymatic reactions, as these are known to protect organisms from free radical damage [34].
These studies, carried out with gentamicin and amikacin, show that D-methionine protects against ototoxicity through different antioxidant mechanisms, and that it is more effective than other agents, such as glutathione, histidine, and ebselen [34,35]. Also, various studies show that potential free radical scavengers, such as α-tocopherol (vitamin E) and α-lipoic acid, inhibit gentamicin ototoxicity by neutralizing the free radicals [30,36]. The antioxidant agent, edaravone, which is used particularly in the treatment of acute myocardial infarction, was found effective at inhibiting streptomycin and tobramycin ototoxicity [37,38]. In addition, protection from gentamicin ototoxicity was found in agent M40403, which has a similar efficacy as SOD [39].
In our study, we found that beta glucan, a potent antioxidant agent, reduces amikacin ototoxicity. The collected data shows statistically significant differences at the 3,991.1, 4,757.8, 5,660.2, 6,726.6, 8,003.9, and 9,515.6 Hz frequencies on days 0-15, and in the 8,003.9 and 9,515.6 Hz frequencies on days 0-10 (for the group that received only amikacin), whereas in the group that received both amikacin and beta glucan, hearing loss and statistically significant differences were not observed at many frequencies. These results suggest that beta glucan can prevent amikacin-induced ototoxicity. In this study, beta glucans were used in 1 mg/kg/day doses. Using higher doses of beta glucan should increase protection against amikacin. Further studies are needed for investigating the possible dosage levels for beta glucan.
Furthermore, in the group that was administered beta glucan, statistically significant results were found at the 2,378.9, 2,824.2, 3,363.3, and 3,991.1 Hz frequencies on days 0-1, at the 8,003.9 and 9,515.6 Hz frequencies on days 0-5, at the 3,363.3, 3,991.1, and 8,003.9 Hz frequencies on days 0-10, and at the 8,003.9 Hz frequency on days 0-15, and these are interpreted as hearing improvement. These results suggest that beta glucan may have a positive effect on hearing improvement when used alone. In the literature, it was discussed that beta glucan does not directly affect cancer cells or infectious microorganisms but shows its biological activities through activation of host immune systems [40]. Long-term follow-up studies, and studies using different doses of beta glucan, are necessary to clarify this effect.
This study suggests that amikacin-induced hearing loss in rats may be limited to some extent by concomitant use of beta glucan. Dose-dependent changes in the possible effects of beta glucan need further investigation. Future morphologic studies may contribute to expose clearly the protective effect of beta glucan.
ACKNOWLEDGMENTS
The authors would like to thank Professor Saim Yologlu, MS, MD, from Department of Biostatistics, Inonu University Medical Faculty, for contributing to the statistical evaluation.
Footnotes
No potential conflict of interest relevant to this article was reported.
This article was presented at the 32nd Congress of Turkish Otorhinolaryngology and Head and Neck Surgery, Antalya, Turkey, October 27-31, 2010.
References
- 1.Priuska EM, Schacht J. Mechanism and prevention of aminoglycoside ototoxicity: outer hair cells as targets and tools. Ear Nose Throat J. 1997 Mar;76(3):164–171. [PubMed] [Google Scholar]
- 2.Roland PS. Characteristics of systemic and topical agents implicated in toxicity of the middle and inner ear. Ear Nose Throat J. 2003 Jan;82(Suppl 1):3–8. [PubMed] [Google Scholar]
- 3.Rizzi MD, Hirose K. Aminoglycoside ototoxicity. Curr Opin Otolaryngol Head Neck Surg. 2007 Oct;15(5):352–357. doi: 10.1097/MOO.0b013e3282ef772d. [DOI] [PubMed] [Google Scholar]
- 4.Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res. 2006 Mar;213(1-2):64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tran Ba Huy P, Bernard P, Schacht J. Kinetics of gentamicin uptake and release in the rat: comparison of inner ear tissues and fluids with other organs. J Clin Invest. 1986 May;77(5):1492–1500. doi: 10.1172/JCI112463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulon D, Hiel H, Aurousseau C, Erre JP, Aran JM. Pharmacokinetics of gentamicin in the sensory hair cells of the organ of Corti: rapid uptake and long term persistence. C R Acad Sci III. 1993 Jul;316(7):682–687. [PubMed] [Google Scholar]
- 7.Hashino E, Shero M. Endocytosis of aminoglycoside antibiotics in sensory hair cells. Brain Res. 1995 Dec;704(1):135–140. doi: 10.1016/0006-8993(95)01198-6. [DOI] [PubMed] [Google Scholar]
- 8.Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005 Sep;567(Pt 2):505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirose K, Westrum LE, Stone JS, Zirpel L, Rubel EW. Dynamic studies of ototoxicity in mature avian auditory epithelium. Ann N Y Acad Sci. 1999 Nov;884:389–409. doi: 10.1111/j.1749-6632.1999.tb08657.x. [DOI] [PubMed] [Google Scholar]
- 10.Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res. 1997 Feb;104(1-2):1–14. doi: 10.1016/s0378-5955(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Gonzalez MA, Delgado F, Lucas M. Aminoglycosides activate oxygen metabolites production in the cochlea of mature and developing rats. Hear Res. 1999 Oct;136(1-2):165–168. doi: 10.1016/s0378-5955(99)00122-7. [DOI] [PubMed] [Google Scholar]
- 12.Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995 Nov;50(11):1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- 13.Wu WJ, Sha SH, Schacht J. Recent advances in understanding aminoglycoside ototoxicity and its prevention. Audiol Neurootol. 2002 May-Jun;7(3):171–174. doi: 10.1159/000058305. [DOI] [PubMed] [Google Scholar]
- 14.Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999 May;42(1-3):61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 15.Lee KY, Lee MH, Chang IY, Yoon SP, Lim DY, Jeon YJ. Macrophage activation by polysaccharide fraction isolated from Salicornia herbacea. J Ethnopharmacol. 2006 Feb;103(3):372–378. doi: 10.1016/j.jep.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Kim MK, Lee IY, Ko JH, Rhee YH, Park YH. Higher intracellular levels of uridinemonophosphate under nitrogen-limited conditions enhance metabolic flux of curdlan synthesis in Agrobacterium species. Biotechnol Bioeng. 1999 Feb;62(3):317–323. doi: 10.1002/(sici)1097-0290(19990205)62:3<317::aid-bit8>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 17.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 18.Rao KM. MAP kinase activation in macrophages. J Leukoc Biol. 2001 Jan;69(1):3–10. [PubMed] [Google Scholar]
- 19.Brown GD, Gordon S. Immune recognition: a new receptor for beta-glucans. Nature. 2001 Sep;413(6851):36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 20.Tsiapali E, Whaley S, Kalbfleisch J, Ensley HE, Browder IW, Williams DL. Glucans exhibit weak antioxidant activity, but stimulate macrophage free radical activity. Free Radic Biol Med. 2001 Feb;30(4):393–402. doi: 10.1016/s0891-5849(00)00485-8. [DOI] [PubMed] [Google Scholar]
- 21.Patchen ML, D'Alesandro MM, Brook I, Blakely WF, MacVittie TJ. Glucan: mechanisms involved in its "radioprotective" effect. J Leukoc Biol. 1987 Aug;42(2):95–105. doi: 10.1002/jlb.42.2.95. [DOI] [PubMed] [Google Scholar]
- 22.Abdollahi M, Bahreini-Moghadam A, Emami B, Fooladian F, Zafari K. Increasing intracellular cAMP and cGMP inhibits cadmium-induced oxidative stress in rat submandibular saliva. Comp Biochem Physiol C Toxicol Pharmacol. 2003 Jul;135C(3):331–336. doi: 10.1016/s1532-0456(03)00120-0. [DOI] [PubMed] [Google Scholar]
- 23.Feldman L, Efrati S, Eviatar E, Abramsohn R, Yarovoy I, Gersch E, et al. Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int. 2007 Aug;72(3):359–363. doi: 10.1038/sj.ki.5002295. [DOI] [PubMed] [Google Scholar]
- 24.Young SG, Parthasarathy S. Why are low-density lipoproteins atherogenic? West J Med. 1994 Feb;160(2):153–164. [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa I, Kiyama S, Yoshioka T. Renal antioxidant enzymes: their regulation and function. Kidney Int. 1994 Jan;45(1):1–9. doi: 10.1038/ki.1994.1. [DOI] [PubMed] [Google Scholar]
- 26.Cheeseman KH, Slater TF. An introduction to free radical biochemistry. Br Med Bull. 1993 Jul;49(3):481–493. doi: 10.1093/oxfordjournals.bmb.a072625. [DOI] [PubMed] [Google Scholar]
- 27.Karaduman D, Eren B, Keles ON. The protective effect of beta-1,3-D-glucan on taxol-induced hepatotoxicity: a histopathological and stereological study. Drug Chem Toxicol. 2010;33(1):8–16. doi: 10.3109/01480540903380472. [DOI] [PubMed] [Google Scholar]
- 28.Cleary JA, Kelly GE, Husband AJ. The effect of molecular weight and beta-1,6-linkages on priming of macrophage function in mice by (1,3)-beta-D-glucan. Immunol Cell Biol. 1999 Oct;77(5):395–403. doi: 10.1046/j.1440-1711.1999.00848.x. [DOI] [PubMed] [Google Scholar]
- 29.Aquino TJ, Oliveira JA, Rossato M. Ototoxicity and otoprotection in the inner ear of guinea pigs using gentamicin and amikacin: ultrastructural and functional aspects. Braz J Otorhinolaryngol. 2008 Nov-Dec;74(6):843–852. doi: 10.1016/S1808-8694(15)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conlon BJ, Smith DW. Supplemental iron exacerbates aminoglycoside ototoxicity in vivo. Hear Res. 1998 Jan;115(1-2):1–5. doi: 10.1016/s0378-5955(97)00171-8. [DOI] [PubMed] [Google Scholar]
- 31.Sinswat P, Wu WJ, Sha SH, Schacht J. Protection from ototoxicity of intraperitoneal gentamicin in guinea pig. Kidney Int. 2000 Dec;58(6):2525–2532. doi: 10.1046/j.1523-1755.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- 32.Song BB, Sha SH, Schacht J. Iron chelators protect from aminoglycoside-induced cochleo- and vestibulo-toxicity. Free Radic Biol Med. 1998 Jul;25(2):189–195. doi: 10.1016/s0891-5849(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen Y, Huang WG, Zha DJ, Qiu JH, Wang JL, Sha SH, et al. Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic. Hear Res. 2007 Apr;226(1-2):178–182. doi: 10.1016/j.heares.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Campbell KC, Meech RP, Klemens JJ, Gerberi MT, Dyrstad SS, Larsen DL, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res. 2007 Apr;226(1-2):92–103. doi: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Kopke RD, Liu W, Gabaizadeh R, Jacono A, Feghali J, Spray D, et al. Use of organotypic cultures of Corti's organ to study the protective effects of antioxidant molecules on cisplatin-induced damage of auditory hair cells. Am J Otol. 1997 Sep;18(5):559–571. [PubMed] [Google Scholar]
- 36.Fetoni AR, Sergi B, Scarano E, Paludetti G, Ferraresi A, Troiani D. Protective effects of alpha-tocopherol against gentamicin-induced oto-vestibulo toxicity: an experimental study. Acta Otolaryngol. 2003 Jan;123(2):192–197. doi: 10.1080/00016480310001484. [DOI] [PubMed] [Google Scholar]
- 37.Horiike O, Shimogori H, Yamashita H. Effect of edaravone on streptomycin-induced vestibulotoxicity in the guinea pig. Laryngoscope. 2004 Sep;114(9):1630–1632. doi: 10.1097/00005537-200409000-00023. [DOI] [PubMed] [Google Scholar]
- 38.Asplund MS, Lidian A, Linder B, Takumida M, Anniko M. Protective effect of edaravone against tobramycin-induced ototoxicity. Acta Otolaryngol. 2009 Jan;129(1):8–13. doi: 10.1080/00016480802008199. [DOI] [PubMed] [Google Scholar]
- 39.McFadden SL, Ding D, Salvemini D, Salvi RJ. M40403, a superoxide dismutase mimetic, protects cochlear hair cells from gentamicin, but not cisplatin toxicity. Toxicol Appl Pharmacol. 2003 Jan;186(1):46–54. doi: 10.1016/s0041-008x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- 40.Kim HS, Hong JT, Kim Y, Han SB. Stimulatory effect of β-glucans on immune cells. Immune Netw. 2011 Aug;11(4):191–195. doi: 10.4110/in.2011.11.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]