Abstract
Medical treatment of pulmonary arterial hypertension (PAH) is increasingly common. Prostacyclins were introduced in the early 90s, and treprostinil is one of the most frequently used drugs of this class today, owing to its long half-life and to the possibility to administer the molecule through several routes. Treprostinil is considered a safe drug and is associated with a significant improvement of exercise capacity, especially in patients with idiopathic PAH (iPAH). Systemic sclerosis-associated PAH (sc-PAH) correlates to a worse prognosis compared with that of iPAH. Despite these considerations, safety data on treprostinil are still limited and mainly derived from randomised controlled trials and retrospective studies with relatively small and heterogeneous cohorts of patients with PAH. We report the occurrence of a severe intra-abdominal bleeding during treprostinil infusion in a patient with sc-PAH.
Background
Prostacyclins improve survival in patients with idiopathic pulmonary arterial hypertension (iPAH),1 while improved exercise capacity was demonstrated in other secondary forms of PAH.2–4 Systemic sclerosis associated PAH (sc-PAH) is associated with poor outcome and increased mortality when compared with other subgroups.2 Data on prostacyclin safety are relatively scanty, with a documented risk for gastrointestinal bleedings of 1.3% in patients receiving subcutaneous treprostinil.5
Case presentation
A 43-year-old man with sc-PAH, receiving full-dose treatment with bosentan, sidenalfil and long-term warfarin, was electively admitted to our ward to initiate continuous subcutaneous infusion of treprostinil. Owing to a history of ventricular tachycardia, a cardioverter-defibrillator had been implanted 10 years back.
Over the previous 6 months he was admitted to the cardiac intensive care unit three times owing to worsening of the right heart failure, and suffered severe functional impairment with New York Heart Association (NYHA) functional class IV. Four months back he also experienced a gastrointestinal bleeding owing to erosive gastritis, and was since then treated with full-dose omeprazol.
On the first day of treprostinil infusion with a dose of 3.5 ng/kg/min, the patient experienced some pain limited to the site of the subcutaneous access. The dose was hence increased to 10 ng/kg/min over the following 2 days. On day 4, pronounced scrotal oedema arose, and the site of the subcutaneous infusion canula was changed, accompanied with local pain also at the second site, for 2 more days.
Owing to the pain at the site of administration, subcutaneous infusion was switched to intravenous treprostinil on day 6. However, within a couple of hours after starting with the intravenous route, the patient experienced diffuse abdominal pain and demonstrated signs of increased right heart failure with peripheral oedema and low blood pressure; treprostinil administration was therefore immediately discontinued.
The following day, symptoms persisted and increasing peripheral oedema was observed. The abdomen was distended, with skin stretched tightly, protruding navel and dull sound at percussion.
Investigations
Blood tests showed a drop in haemoglobin from 115 g/l, prior to treprostinil administration, to 75 g/l with no signs of haemolysis.
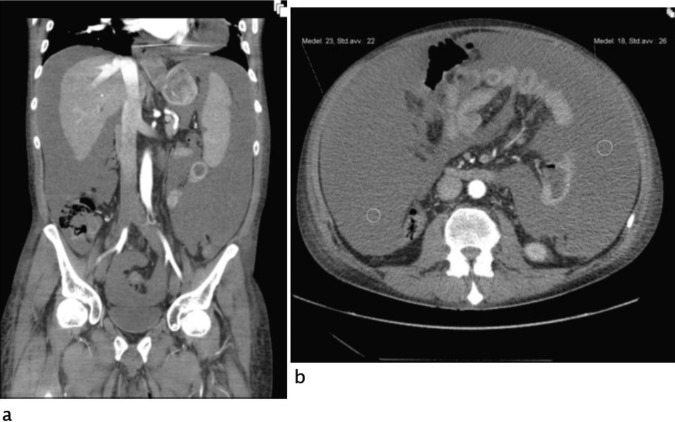
An ultrasound of the abdomen demonstrated the presence of large amounts of ascites with high echogenicity but no signs of any lesion of intra-abdominal organs (figure 1). A CT angiography could not confirm the suspicion of active bleeding and haemoperitoneum (figure 2). Acute gastroscopy and a sigmoidoscopy, up to 40 cm, excluded gastrointestinal bleeding.
Figure 1.
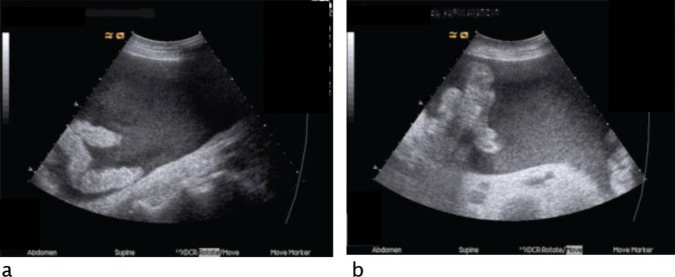
Abdominal ultrasound: hipoechogenic free peritoneal fluid with echogenic debris at right lower quadrant (A) and in the upper abdomen (B).
Figure 2.
Abdominal CT angiography, arterial phase, coronal multiplanar reconstruction (A) and axial image at middle abdomen (B): free peritoneal fluid; mean fluid attenuation: 20 HU.
Subsequently, an ultrasound-guided paracentesis demonstrated the presence of blood in the abdomen. Cytological and microbiological tests on the fluid did not show signs of malignancy or infection.
Treatment
During the next 48 h, the patient required 8, 2 and 2 units of erythrocytes, plasma and platelets, respectively, as well as intravenous tranexamic acid and phytometadione to maintain a haemoglobin concentration above 100 g/l. Three days after the onset of symptoms, the haemoglobin concentration stabilised with no further need of transfusions.
Outcome and follow-up
The patient survived the bleeding and the problem did not recur; however, this adverse event precluded any further attempts to treat his PAH with prostacyclins. A heart–lung transplantation was excluded, owing to the course of systemic sclerosis and the involvement of other organs. The patient eventually died of heart failure 4 months after the intra-abdominal bleeding.
Discussion
This case highlights the risk of potential severe adverse events during treprostinil infusion, especially in patients with severe pulmonary hypertension and advanced NYHA functional class.
We report here, for the first time, a severe, life-threatening, intra-abdominal bleeding during treprostinil infusion. Treprostinil is considered a safe drug, but data on safety are mainly derived from randomised controlled trials and retrospective studies with relatively small and heterogeneous cohorts of patients.2–8
Our patient had a severe form of rapidly progressive systemic sclerosis, was in NYHA functional class IV and had an increased risk for bleeding because of ongoing treatments and previous history. However, the occurrence and the features of this life-threatening event during prostacyclin infusion are peculiar and deserve further discussion: the anaemisation occurred during treprostinil infusion concomitantly with abdominal pain; no other cause of bleeding could be identified by radiological and laboratory tests; in particular, the CT-angiography failed to demonstrate any focal source of bleeding in the abdomen; the bleeding resolved after discontinuation of treprostinil, like previously described in literature for gastrointestinal bleedings.5
Perhaps treprostinil infusion contributed to the congestion of visceral organs by vasodilation, contributing eventually to cause a diffuse intraperitoneal haemorrhage from small blood vessels. Epoprostenol-induced hypersplenism has recently been described in patients with portopulmonary hypertension.9
A recent survey reported that technical errors in the administration of treprostinil are very common, and can contribute to a fatal outcome10; however, a thorough review of our files did exclude any mistakes in the administration procedures.
Our case highlights the possibility of a life-threatening adverse event in the treatment of PAH with treprostinil infusion not described earlier. Awareness of haemorrhagic risks and high degree of clinical suspicion are of paramount importance for detecting similar events in this particular group of patients.
Learning points.
Safety data on drugs for uncommon or rare clinical conditions are mostly derived from clinical trials with a limited sample size and often with heterogeneous cohorts.
Patients with secondary pulmonary hypertension might have an increased risk of adverse events not described earlier, owing to the characteristics of the underlying disease, to the low incidence of these conditions and to the complexity of ongoing treatments.
High degree of clinical suspicion is necessary to detect similar adverse events among these patients.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Paramothayan NS, Lasserson TJ, Wells AU, et al. Prostacyclin for pulmonary hypertension in adults. Cochrane Database Syst Rev 2005;(2):CD002994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benza RL, Gomberg-Maitland M, Naeije R, et al. Prognostic factors associated with increased survival in patients with pulmonary arterial hypertension treated with subcutaneous treprostinil in randomized, placebo-controlled trials. J Heart Lung Transplant 2011;30:982–9 [DOI] [PubMed] [Google Scholar]
- 3.Barst RJ, Galie N, Naeije R, et al. Long-term outcome in pulmonary arterial hypertension patients treated with subcutaneous treprostinil. Eur Respir J 2006;28:1195–203 [DOI] [PubMed] [Google Scholar]
- 4.Oudiz RJ, Schilz RJ, Barst RJ, et al. Treprostinil, a prostacyclin analogue, in pulmonary arterial hypertension associated with connective tissue disease. Chest 2004;126:420–7 [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Barst RJ, Galie N, et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 2002;165:800–4 [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin VV, Gaine SP, Barst RJ, et al. Efficacy and safety of treprostinil: an epoprostenol analog for primary pulmonary hypertension. J Cardiovasc Pharmacol 2003;41:293–9 [DOI] [PubMed] [Google Scholar]
- 7.Tapson VF, McLaughlin VV, Gomberg-Maitland M, et al. Delivery of intravenous treprostinil at low infusion rates using a miniaturized infusion pump in patients with pulmonary arterial hypertension. J Vasc Access 2006;7:112–17 [DOI] [PubMed] [Google Scholar]
- 8.Tapson VF, Gomberg-Maitland M, McLaughlin VV, et al. Safety and efficacy of IV treprostinil for pulmonary arterial hypertension: a prospective, multicenter, open-label, 12-week trial. Chest 2006;129:683–8 [DOI] [PubMed] [Google Scholar]
- 9.Touma W, Nayak RP, Hussain Z, et al. Epoprostenol-induced hypersplenism in portopulmonary hypertension. Am J Med Sci 2012;344:345–9 [DOI] [PubMed] [Google Scholar]
- 10.Kingman MS, Tankersley MA, Lombardi S, et al. Prostacyclin administration errors in pulmonary arterial hypertension patients admitted to hospitals in the United States: a national survey. J Heart Lung Transplant 2010;29:841–6 [DOI] [PMC free article] [PubMed] [Google Scholar]