Abstract
Hereditary haemorrhagic telangiectasia (HHT) is a rare inherited autosomal-dominant vascular dysplasia involving multiple organs. Brain abscess is an uncommon and potential fatal complication. We report a case of HHT caused by a novel ENG mutation who initially presented as brain abscess. The patient, with a family history of epistaxis, presented with fever, headache and right-sided haemiparesis. Upon examination, brain MRI showed a contrast-enhanced abscess on the left fronto-parietal region. Open brain drainage was performed and pus culture yielded Actinomyces meyeri. The chest image revealed multiple pulmonary arterio-venous fistulas. HHT was diagnosed according to Curacao criteria. Genetic analysis revealed a novel duplication on exon 6 of ENG gene, which segregates with symptomatic subjects in her family. Clinicians should be cautiously aware of HHT as a differential diagnosis if patients presented with an unknown entry source of intracerebral infections.
Background
Hereditary haemorrhagic telangiectasia (HHT), also called as Rendu–Osler–Weber syndrome, is a rare autosomal-dominant hereditary disease with a prevalence rate suggested to be 1 in 50 000–100 000.1 Although this disease has been reported in all races, it is rare in Asian populations.1 2 Mutations in two genes have been shown to be associated with HHT, including genes encoding endoglin (ENG) and activin A receptor type II-like 1 (ACVRL1).3 4 Both gene products related to the function of transforming growth factor-related angiogenesis.4 5 HHT usually manifested with nose bleeding, telangiectasia over mucosa or skin, and multiple recurrent arterio-venous malformations (AVMs), especially in the gastrointestinal tract, liver, lung or brain.6 7 Most of the HHT patients lived without major bleeding episodes except recurrent epistaxis. However, brain abscess is a rare and potentially fatal complication.8–11 Here we report a HHT patient who initially presented with brain abscess and confirmed to have a novel gene mutation of ENG.
Case presentation
A 39-year-old lady without a systemic disease before had a history of recurrent nose bleeding since she was a teenager. She presented to the neurology clinic with progression of right-side limbs weakness for several days. Upon admission, the patient was alert and oriented. Her physical examination was unremarkable except for mild fever. There were no obvious skin telangiectasia lesions. There was no neck stiffness and the fundoscopy was normal. Neurological examination revealed right central-type facial palsy, slurred speech and right limbs weakness. During hospitalizstion, there were several episodes of fever along with intermittent headache.
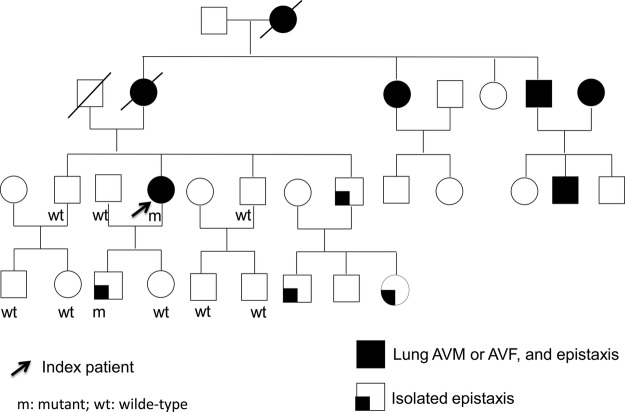
Tracking back her family history, her mother, grandmother, uncle and one of her aunts had been diagnosed with lung AVMs. Besides, there was a strong family history of epistaxis over most of her family members (figure 1). The son of the proband, who is 21 years old now, has frequent nosebleeds since he was in elementary school. The images of the brain and lung did not reveal any evidence of AVMs.
Figure 1.
Family pedigree of our index patient. The proband we described is marked with an arrow. M denotes mutated alleles and wt denotes normal alleles.
Investigations
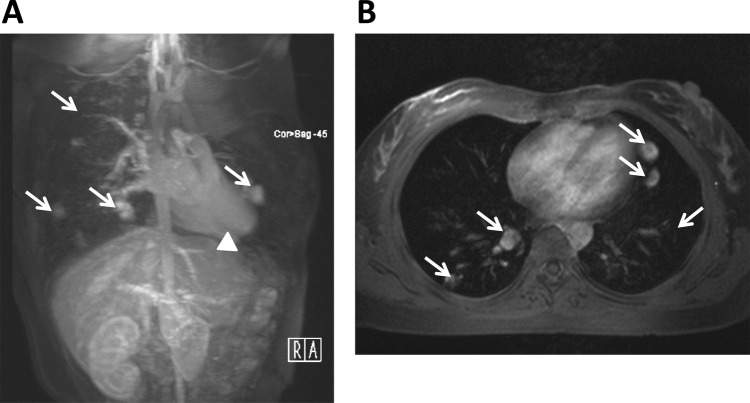
All her biochemical data and cell count of the blood sampling were within normal limits, including elevated whole blood count and C reactive protein. The patient did not have immuocompromised status. MRI of the brain revealed a 2 cm hypodense lesion with perifocal oedema and contrast enhancement on the left fronto-parietal region. Based on clinical history, a brain abscess was strongly suspected and she received open craniotomy with abscess drainage on the third day after admission. Culture of the pus drained yielded Actinomyces meyeri, while blood cultures were sterile. Cardiac and abdominal ultrasonography showed no site of potential microbial entry. Chest x-ray and MRI revealed multiple well-defined small nodules at bilateral lungs, which were pulmonary arterio-venous fistulas (AVFs) (figure 2). In addition, some tiny vascular tufts were also noted over the right lobe of liver, suggesting intrahepatic AVMs.
Figure 2.
MRI findings of our index patient. MR angiography of the lung and liver shows prominent pulmonary arteries, pulmonary arterio-venous malformations AVMs and AVFs (arrows) with multiple aberrant collateral vessels in both sagittal (A) and coronal (B) views, and abnormal dilation of the hepatic artery (arrowhead) in the liver (A). AVM, arterio-venous malformations; AVF, arterio-venous fistulas.
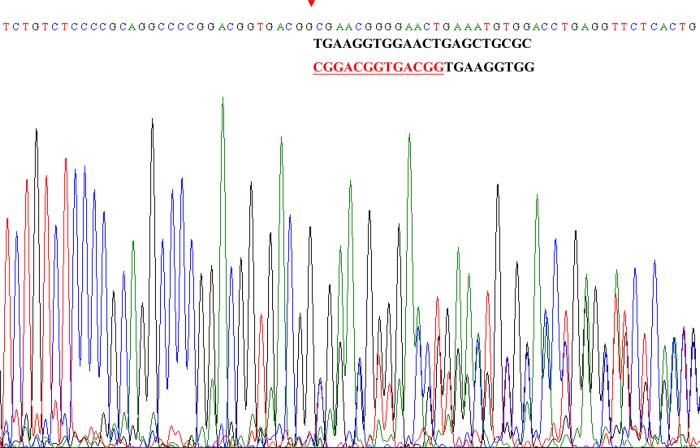
According to the clinical presentation of our index patient and radiological evidences of multiple AVMs in lung and liver associated with a strong family history of vascular dysplasia, she was clinically diagnosed as definite HHT according to Curacao criteria.12 After getting the informed consent forms, DNA was extracted from 6 ml of peripheral venous blood from the subject and her family members, using standard protocols. All exons with intron—exon boundaries of ENG gene and ACVRL1 gene were amplified by PCR using the primers reported in the GDB database (Human Genome Database) and were sequenced by TagMan ABI 3730 (Applied Biosystems Inc). Each mutation was confirmed at least by a second reverse sequence. Base variants, deletions or duplications are labelled according to the longest mRNA transcript (GenBank accession number NM_001114753.1). The genetic analysis of the index patient shows normal findings of ACVRL1 gene but a novel duplication on exon 6 of ENG gene, 975–988 dupl CGGACGGTGACGG, segregating symptomatic subjects in her family (figures 1 and 3).
Figure 3.
Genetic findings in the index patient with ENG mutation. Chromatograms of direct sequencing of the ENG genomic sequence of our patient. The start position of the genetic duplication identified in this study is indicated by red arrowheads and the duplicated nucleotides are marked in underlined red characters.
Differential diagnosis
A clinically diagnosed HHT patient, presented with brain abscess as an initial presentation, was confirmed by genetic analysis to have a novel ENG gene mutation.
Treatment
The patient was treated with ceftriaxone and metronidazole intravenously for 5 weeks. The treatment course was uneventful and she was discharged 5 weeks after admission.
Outcome and follow-up
She remained independent with only mild right foot weakness after follow-up for 10 years. All the family members were suggested to be aware of frequent nosebleeds, which are a common sign of HHT. The affected carriers receive long-term follow-up and regular image studies.
Discussion
We report a family with clinically and genetically confirmed HHT, in whom the proband presented initial cerebral symptoms as brain abscess. We identified a novel ENG mutation contributing to the clinical phenotypes of this family.
HHT is a rare disease marked by a familial pattern of telangiectasia on mucocutaneous surfaces and larger AVMs in other organ systems.2 6 As compared with the incidence of brain abscess in normal population that is 1.3/100 000,13 previous studies have shown that brain abscess is estimated to occur in 1% of patients with HHT and carries as high as 40% death rate.9 Many reported cases are associated with pulmonary AVMs or AVFs. Several possible mechanisms of brain abscess formation in patients with HHT have been proposed. Pulmonary vascular malformations make the pulmonary artery flow into the venous return directly and skip the capillary circulation. This would not only lead to blood acidosis in artery circulation, but also allow micro-metre-level particles like bacteria break through the capillary bed barrier, resulting in the catastrophic cerebral abscess.8 14 In addition, secondary bacterial seeding of an ischaemic portion of the brain after paradoxical sterile emboli also contributes to the brain abscess formation.15
A meyeri is an infrequent causative agent of brain abscess. It is usually an oral saprophyte pathogen that particularly affects periodontal lesions and carious teeth.16 It is rarely present as a pathogen of central nervous system infection. Rarely if ever as a cause of brain abscess, it has been associated with previous dentogingival disease, chronic alcoholism or immunodeficiency.16 Given that our patient did not have drinking history or was not under immunocompromised state, we speculated the possible entry focus of our patient was dentogingival infection, albeit there was no preceding dental surgery.
In the recent decade, dysfunction of transforming growth factor-β owing to mutations of ENG and ACVRL-1 genes which represented HHT-1 and HHT-2, respectively, delineated the pathophysiology of HHT.3 4 Patients carrying ENG mutations are more frequently from Northern Europe and the Americas, while Mediterranean populations have a majority of ACVRL1 mutations.17–19 As compared with the mutations of ACVRL1 gene clustered in exons 3, 7, 8, ENG mutations are almost evenly distributed in the various exons coding for the extracellular domain of endoglin protein.19 In line with these previous studies, the present index patient has a genetic duplication on the exon six of ENG gene, which is on the extracellular domain of endoglin protein. In addition to point mutations, frameshift mutations and duplication of ENG gene were frequently reported in patients with HHT.19
In conclusion, this case clearly demonstrates that brain abscess could be the first symptom of HHT, a potentially devastating disease. HHT should be considered in patients with intracranial infection without an obvious entry source of infection. Screening family members of patients with HHT by means of chest radiography and neuroimages has been recommended. Genetic testing could be provided as a diagnostic tool for susceptible family members with only mild symptoms and genetic counselling is recommended for those who know the disease runs in their families.
Learning points.
Hereditary haemorrhagic telangiectasia (HHT) should be considered in patients with an intracranial infection without an obvious entry source of infection.
Screening family members of patients with HHT by means of chest radiography and neuroimages are suggested.
Genetic testing could be provided as a diagnostic tool for susceptible family members with only mild symptoms of epistaxis.
Acknowledgments
We thank all the patients participating in this study and are grateful to the staff of the Second Core Lab in Department of Medical Research at National Taiwan University Hospital for its technical support.
Footnotes
Contributors: C-HL: study concept and design; K-HC and C-HL: acquisition of data; K-HC, C-HL: analysis and interpretation of data; K-HC: drafting of the manuscript; C-HL: critical revision of the manuscript for important intellectual content; C-HL: study supervision.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Grand'Maison A. Hereditary hemorrhagic telangiectasia. CMAJ 2009;180:833–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med 1996;156:714–19 [PubMed] [Google Scholar]
- 3.Llister KA, Grogg KM, Johnson DW, et al. Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet 1994;8:345–51 [DOI] [PubMed] [Google Scholar]
- 4.Johnson DW, Berg JN, Baldwin MA, et al. Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 1996;13:189–95 [DOI] [PubMed] [Google Scholar]
- 5.Yamashita H, Ichijo H, Grimsby S, et al. Endoglin forms a heteromeric complex with the signaling receptors for transforming growth factor-beta. J Biol Chem 1994;269:1995–2001 [PubMed] [Google Scholar]
- 6.Guttmacher AE, Marchuk DA, White RI., Jr Hereditary hemorrhagic telangiectasia. N Engl J Med 1995;333:918–24 [DOI] [PubMed] [Google Scholar]
- 7.Román G, Fisher M, Perl DP, et al. Neurological manifestations of hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease): report of 2 cases and review of the literature. Ann Neurol 1978;4:130–44 [DOI] [PubMed] [Google Scholar]
- 8.Dong SL, Reynolds SF, Steiner IP. Brain abscess in patients with hereditary hemorrhagic telangiectasia: case report and literature review. J Emerg Med 2001;20:247–51 [DOI] [PubMed] [Google Scholar]
- 9.Hall WA. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) presenting with polymicrobial brain abscess. Case report. J Neurosurg 1994;81:294–6 [DOI] [PubMed] [Google Scholar]
- 10.Mylona E, Vadala C, Papastamopoulos V, et al. Brain abscess caused by enterococcus faecalis following dental procedure in a patient with hereditary hemorrhagic telangiectasia. J Clin Microbiol 2012;50:1807–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shovlin CL, Jackson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008;63:259–66 [DOI] [PubMed] [Google Scholar]
- 12.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000;91:66–7 [DOI] [PubMed] [Google Scholar]
- 13.Nicolosi A, Hauser WA, Musicco M, et al. Incidence and prognosis of brain abscess in a defined population: Olmsted county, Minnesota, 1935–1981. Neuroepidemiology 1991;10:122–31 [DOI] [PubMed] [Google Scholar]
- 14.Stern WE, Naffziger HC. Brain abscess associated with pulmonary angiomatous malformation. Ann Surg 1953;138:521–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svanteson B, Nordstrom CH, Rausing A. Non-traumatic brain abscess. Epidemiology, clinical symptoms and therapeutic results. Acta Neurochir 1988;94:57–65 [DOI] [PubMed] [Google Scholar]
- 16.Apotheloz C, Regamey C. Disseminated infection due to Actinomyces meyeri: case report and review. Clin Infect Dis 1996;22:621–5 [DOI] [PubMed] [Google Scholar]
- 17.Bossler AD, Richards J, George C, et al. Novel mutations in ENG and ACVRL1 identified in a series of 200 individuals undergoing clinical genetic testing for hereditary hemorrhagic telangiectasia (HHT): correlation of genotype with phenotype. Hum Mutat 2006;27:667–75 [DOI] [PubMed] [Google Scholar]
- 18.Lesca G, Olivieri C, Burnichon N, et al. Genotype-phenotype correlations in hereditary hemorrhagic telangiectasia: data from the French-Italian HHT network. Genet Med 2007;9:14–22 [DOI] [PubMed] [Google Scholar]
- 19.Olivieri C, Pagella F, Semino L, et al. Analysis of ENG and ACVRL1 genes in 137 HHT italian families identifies 76 different mutations (24 novel). comparison with other European studies. J Hum Genet 2007;52:820–9 [DOI] [PubMed] [Google Scholar]