Abstract
Our patient was a 35-year-old woman, who had undergone right radically modified mastectomy and axillary-lymph-nodes dissection in June 2004. The stage of the patient was T2N0M0. She was treated with six cycles of FAC (5-fluorouracil, doxorubicin, cyclofosfamid), tamoxifen after chemotherapy. In June 2008, right axillary lymphadenopathy progressed and was treated with docetaxel and capecitabine after a hand–foot reaction was observed in the patient as an adverse reaction to capacitabine; the treatment was continued with only trastuzmab between February 2009 and April 2010. The addition of vinoralbine was needed due to the newly developed right paratracheal lymphadenopthy in April 2010. After six cycles of chemotherapy we achieved stable disease; she received only trastuzumab; later on, in control thorax CT, multiple metastatic models were observed in April 2011 and thus lapatinip plus capecitabine treatment was started. After one cycle of chemotherapy her psoriatic lesions were aggravated and we had to stop the treatment.
Background
We know that the epidermal growth factor receptor inhibitor (EGFR1) class of targeted therapies have less systemic toxicities than traditional cytotoxic agents, but that they cause significantly more dermatological side effects in the majority of patients.
It is clear that optimising the management of dermatological toxicities from targeted chemotherapies will continue to gain importance in this new era of targeted agents. Most importantly, it will allow patients to remain on life-altering therapy.
Case presentation
Over the last decade, the development of new systemic cancer therapies has shifted away from the traditional chemotherapy agents towards targeting the specific pathways involved in carcinogenesis. The EGFRIs are one of the best known examples of targeted therapy that are used both as first-line and adjuvant chemotherapy for solid organ cancers. Since the approval of the first targeted therapy by the Food and Drug Administration in 2003, several targeted agents have been tested and approved for the management of non-small cell lung, colon, pancreatic, head and neck squamous cell, and breast cancers. These agents include erlotinib, cetuximab, lapatinib and panitumumab. These agents are less likely than traditional cytotoxic chemotherapeutics to cause myelosuppression, infection, nausea, vomiting and diarrhoea. However, dermatological adverse events from EGFR1s are significantly more common than those with cytotoxic agents which are symptomatic and manifest in cosmetically sensitive areas. Incidence of dermatological toxicities is approximately 30–80% of the patients affected while receiving the targeted therapy treatments. Severe dermatological adverse effects may result in 36% of patients receiving dose modifications or 72% of patients who discontinued treatment. We are only beginning to understand the significant adverse effect of these cutaneous reactions on the quality of life (QoL) of these patients.1 As yet, there are no standardised treatments for these skin side effects.
The EGFR, also referred to as Erb1/human epidermal growth factor receptor 1, is a thyroxin kinase receptor composed of an extracellular ligand-binding domain, a transmembrane segment and an intracellular protein kinase. Where activated, EGFR subsequently regulates cell division apoptosis, motility and DNA repair. On the basis of its binding site, EGFRI's can be divided into two classes: monoclonal antibodies such as cetuximab and panitumumab that bind extracellular and the second class small molecule tyrosine kinase inhibitors that competitively inhibit ATP binding. EGFR is expressed in immature keratinocytes in the skin epidermis (basal layer keratinocytes), hair follicles and sweat glands.
Lapatinib is a dual tyrosine kinase inhibitor which targets both HER2 and EGFR tyrosine kinases.2 It has the advantage of being administered orally. Lapatinib showed promising results both in trastuzumab naive and in pretreated HER2-positive advanced breast cancer.3–6 It has been shown that lapatinib plus capecitabine is superior to capecitabine alone in HER2-positive advanced breast cancer patients previously treated with anthracycline, taxane and trastuzumab.7
Psoriasis is a chronic, immune-mediated, inflammatory condition, seen frequently in clinical practice with a reported prevalence of 0.6–4.8% in the general population.8 9 Some factors known to trigger psoriasis include smoking, alcohol consumption, body mass index, trauma, infection, endocrine disorders, drugs and acute withdrawal of systemic or potent topical corticosteroids.8 Drugs reported to be associated with exacerbation/induction of psoriasis are based mostly on case reports, with no definitive ‘cause-and-effect’ links between these drugs and onset of psoriasis. Drugs have several ways in which they can affect the diathesis of psoriasis, including (1) precipitation of psoriasis de novo in predisposed and nonpredisposed individuals; (2) exacerbation of pre-existing psoriatic lesions; (3) induction of lesions in clinically normal skin in patients with psoriasis; and (4) development of treatment-resistant psoriasis.10 The clinical presentation of drug-provoked psoriasis spans the spectrum of generalised plaque psoriasis, palmoplantar pustulosis and erythroderma.10 The nails and scalp can also be involved, thus making the distinction of drug-associated psoriasis a clinically difficult diagnosis.10 In addition, the mechanism of action can also involve both immunological and nonimmunological pathways.11 Therapeutic agents can also be categorised as having a causal relationship with psoriasis, with considerable but insufficient data supporting induction of psoriasis, or occasionally an association with psoriasis.10
Our patient was a 35-year-old woman who had right radically modified mastectomy and axillary lymph nodes dissection in June 2004. She was premenopausal, had one child and had psoriasis. Immunohistochemical staining of oestrogen receptor (ER) was positive and that of progesterone receptor (PR) was negative; c-erb-B2 scored 3+ and was grade III. She was classified as T2N0M0 according to the tumour size lymphadenopathy distant metastases (TNM) classification. She was treated with six cycles of FAC (5-fluorouracil, doxorubicin, cyclofosfamid) and tamoxifen after chemotherapy. She had a TAH+BSO operation in November 2007. In June 2008 right axillary and supraclaviculary lymphadenopathies progressed. There was 3×3 cm mass at her right supraclavicula and 10 cm lymphadenopathy again at the right axilla. Biopsy was performed and the result was carcinoma metastasis. ER (−), PR (+) and CerbB2 (++) we began the treatment with docetaxel plus capecitabine and cancel fluorescent in situ hybridisation (FISH) was performed. After treatment with four cycles of a chemotherapy combination, we discontinued capecitabine because of the grade IV hand–foot syndrome. We continued treatment with docetaxel plus trastuzumab because FISH was positive. After three cycles of chemotherapy, her lymphadenopathies regressed. She was treated with three more cycles of combination chemotherapy; since she had no other new metastasis, her lymphadenopathies (LAP) regressed and we continued the therapy with only trastuzumab. She was treated with only trastuzumab between February 2009 and April 2010.
While she was on trastuzumab, in April 2010 she had a newly developed right paratracheal LAP at her thorax CT. We added vinorelbine to trastuzumab after four cycles of chemotherapy and control CT were performed. We found a stable response. After six cycles of vinorelbine plus trastuzumab, she received only trastuzumab between August 2010 and April 2011.
In April 2011, thorax CT was performed and multiple metastases were diagnosed and the biggest was 3×4 cm, so we suggested her to undergo biopsy but she did not agree. So we decided to treat her for metastases of breast cancer. She began to take lapatinib plus capecitabine. After one cycle of chemotherapy her psoriasis aggravated and we had to stop the treatment (figure 1). She underwent treatment with methotrexate for her psoriasis. We again treated her with trastuzumab plus vinorelbine. She is still receiving this regimen. Her psoriasis symptoms regressed after treatment (figure 2).
Figure 1.
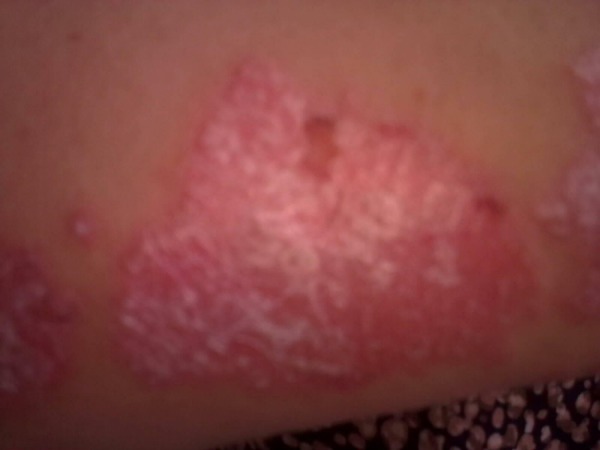
During treatment.
Figure 2.
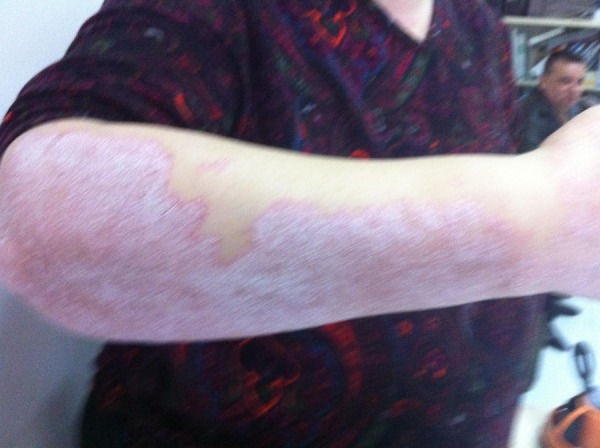
After stopping the treatment.
Outcome and follow-up
She is being treated with trastuzumab plus vinorelbine. She is still receiving this regimen. Her psoriasis symptoms regressed after treatment.
Discussion
Although the EGFR1 class of targeted therapies has less systemic toxicities than traditional cytotoxic agents, they cause considerably more dermatological side effects in the majority of patients. This causes measurable patient discomfort and can lead to attenuation or discontinuation of potentially life-prolonging therapy. Our patient was diagnosed of psoriasis before breast cancer. She was treated with different chemotherapies and there was no problem with her psoriasis. We did not have to discontinue any treatment because of psoriasis aggravation. She was treated with capecitabine before and no problem was diagnosed with her psoriasis but we had to discontinue capecitabine because of the hand–foot syndrome. When she was treated with lapatinib plus capecitabine, after one cycle her psoriasis was aggravated, so we had to discontinue the treatment. Because she had been treated with capecitabine before, we think that this aggravation was due to lapatinib. We know that the EGFR1 class of targeted therapies has less systemic toxicities than traditional cytotoxic agents but they cause considerably more dermatological side effects in the majority of patients. After the treatment her lesions regressed but we do not know it was due to methotrexate or trastuzumab plus vinorelbine.
It is clear that optimising the management of dermatological toxicities from targeted chemotherapies will continue to gain importance in this new era of targeted agents. Most importantly, it will allow patients to remain on life-altering therapy.
Learning point.
Importance of tyrosine kinase inhibitors (TKI) treatments, side effects of the treatment, and the importance of quality of life.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Joshi SS, Ortiz S, Witherspoon JN, et al. Effects of epidermal growth factor receptor inhibitor-induced dermatologic toxicities on quality of life. Cancer 2010;116:3916–23 [DOI] [PubMed] [Google Scholar]
- 2.Murphy CG, Modi S. Her2 breast cancer therapies: a review. Biologics 2009;3:289–301 [PMC free article] [PubMed] [Google Scholar]
- 3.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for her2-overexpressing advanced or metastatic breast cancer that progressed on Wrst- or second-line trastuzumab-containing regimens. Ann Oncol 2009;20:1026–31 [DOI] [PubMed] [Google Scholar]
- 4.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory her2-positive and her2-negative advanced or metastatic breast cancer. Ann Oncol 2008;19:1068–74 [DOI] [PubMed] [Google Scholar]
- 5.Gomez HL, Doval DC, Chavez MA, et al. EYcacy and safety of lapatinib as Wrst-line therapy for erbb2-ampliWed locally advanced or metastatic breast cancer. J Clin Oncol 2008;26:2999–3005 [DOI] [PubMed] [Google Scholar]
- 6.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated eYcacy and biomarker analyses. Breast Cancer Res Treat 2008;112:533–43 [DOI] [PubMed] [Google Scholar]
- 7.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733–43 [DOI] [PubMed] [Google Scholar]
- 8.Naldi L. Epidermiology of psoriasis. Curr Drug Targets Inflamm Allergy 2004;3:121–8 [DOI] [PubMed] [Google Scholar]
- 9.Raychaudhuri SP, Farber EM. The prevalence of psoriasis in the world. J Eur Acad Dermatol Venereol 2001;15:16–17 [DOI] [PubMed] [Google Scholar]
- 10.Tsankov N, Irena A, Kasandjieva J. Drug-induced psoriasis: recognition and management. Am J Clin Dermatol 2000;1:159–65 [DOI] [PubMed] [Google Scholar]
- 11.O'Brian M, Koo J. The mechanism of lithium and beta-blocker agents in inducing and exacerbating psoriasis. J Drugs Dermatol 2006;5:426–33 [PubMed] [Google Scholar]


