Abstract
Diabetic amyotrophy is a distinctive form of diabetic neuropathy. It is characterised by a weakness and wasting of proximal muscles of the lower limbs with associated pain. We report a case of an elderly patient with unusual presentation of diabetic amyotrophy. He presented with myoclonic jerks and recurrent falls. Examination findings and electrophysiological studies were consistent with a diagnosis of diabetic amyotrophy. He responded well to steroids with marked improvement in strength of the lower limb muscles and complete resolution of myoclonic jerks. Diabetic amyotrophy presenting as myoclonic jerks has been rarely reported before.
Background
Diabetic amyotrophy or diabetic proximal neuropathy is a rare complication which affects approximately 1% of the diabetic population. Symptoms commonly reported are asymmetrical pain in the hip or thighs, weakness and wasting of the quadriceps muscles with concomitant weight loss. Myoclonus is a rare manifestation of diabetic amyotrophy. Myoclonus refers to the brief, sudden, shock-like involuntary movements caused by muscular contractions (positive myoclonus) or inhibitions (negative myoclonus). This case report emphasises the importance of early recognition of unusual presentation of diabetic amyotrophy as the condition is potentially treatable.
Case presentation
An 86-year-old right-handed gentleman presented with a 2-year history of gradual onset proximal weakness and pain in both the lower limbs associated with violent jerks in the legs with subsequent falls. His symptoms deteriorated gradually to the point where he had become bed bound. His mobility was restricted significantly by frequent involuntary jerks and weakness in his legs. His medical history was significant for type 2 diabetes mellitus. He was on metformin for diabetes control.
Clinical examination demonstrated involuntary, asynchronous, high amplitude myoclonic jerks in both lower limbs, which were stimulus-insensitive and were present at rest. Both quadriceps muscles were wasted asymmetrically, with the right side worse than the left. Tone was normal. Power in the lower limbs on the Medical Research Council-grading scale was 3/5 proximally and 4/5 distally. He was areflexic in the lower limbs. Plantars were flexor. Motor examination was normal in the upper limbs. Sensory examination demonstrated reduced pinprick sensations in glove and stocking distribution; vibration and joint position sense was reduced bilaterally at the big toes. The rest of the neurological examination was unremarkable.
Investigations
MR scan of the whole spine showed multilevel degenerative changes with no neural or cord compression. MR scan of the brain was consistent with age-related involutional changes. Cerebrospinal fluid (CSF) analysis showed significantly elevated protein at 4.74 g/l (normal value <0.5 g/l); the rest of the CSF constituents were within normal limits. Electrophysiological studies revealed features suggestive of extensive polyradiculoneuropathy with background axonal sensory and motor polyneuropathy (figure 1). The demyelinating changes with evidence of markedly prolonged F wave latencies were localised in the region of the nerve roots (figure 2). Concentric needle electromyography (EMG) sampling showed long duration high amplitude re-innervation motor units in the proximal muscles of the lower limbs. EMG of the thoracic paraspinal muscles showed increased insertional activity, low-amplitude-positive sharp waves and fibrillation potentials.
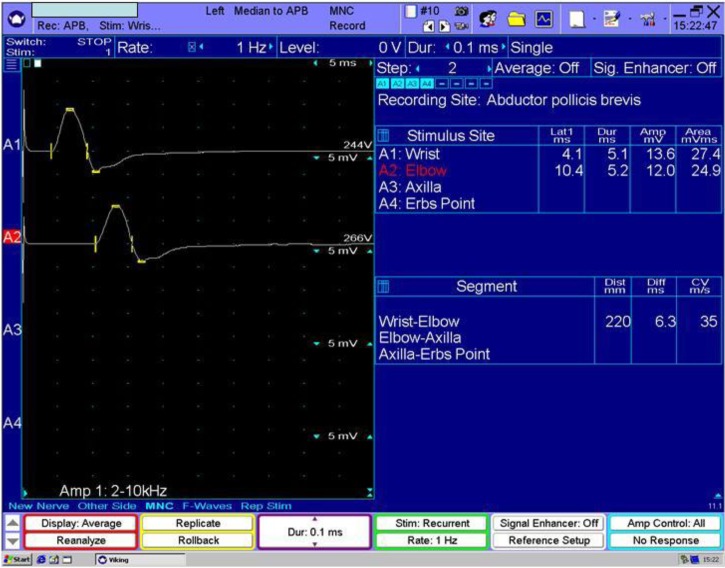
Figure 1.
Mixed demyelinating and axonal neuropathy. Left median motor conduction velocity of 35 m/s.
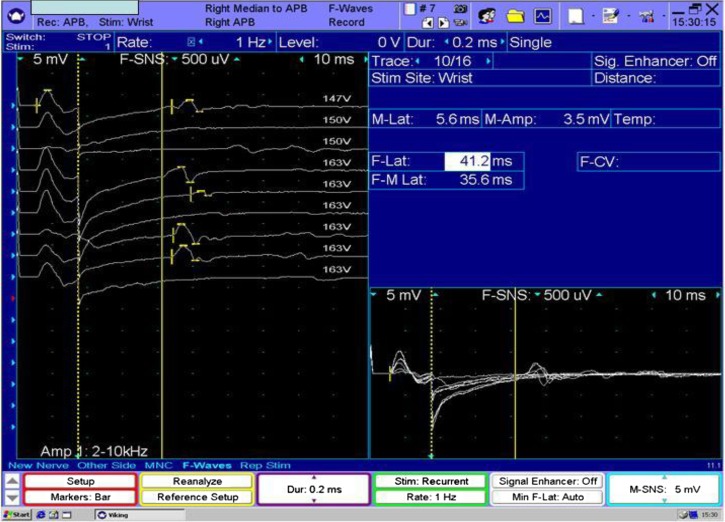
Figure 2.
Mixed demyelinating and axonal neuropathy. Note the prolonged F-wave latency (41.2 ms), which is in the demyelinating range in the right median nerve.
The following investigations were either normal or negative: routine blood count, erythrocyte sedimentation rate, renal, thyroid and liver function tests, serum electrophoresis, vitamin B12, folate, autoimmune, vasculitis and paraneoplastic screen.
Differential diagnosis
Differential diagnosis considered at presentation includes infiltrative, compressive and infective causes of polyradiculopathy, structural disc diseases and chronic demyelinating neuropathies.
Treatment
Clinical suspicion and electrophysiological findings were consistent with a diagnosis of diabetic amyotrophy. He was treated with oral levetiracetam and a 3-day course of intravenous steroids (methylprednisolone) followed by a reducing dose of oral steroids. He also underwent physiotherapy.
Outcome and follow-up
There was dramatic improvement within a few weeks of starting steroids, which was gradually tapered over a 3-month period. Repeat CSF examination at 4 weeks showed protein of 0.75 g/l. At a 2 -month follow-up appointment, he was independently ambulant and his myoclonic jerks had resolved completely.
Discussion
Diabetic amyotrophy, also known as diabetic proximal neuropathy or diabetic lumbosacral radiculoplexus neuropathy, is distinctive and among the most disabling forms of diabetic neuropathy.1 It affects approximately 1% of the diabetic population.2 The affected patients are usually middle-to-old aged with the mean age being 62 years.3 In contrast to other forms of polyradiculoneuropathies like chronic inflammatory demyelinating neuropathy, diabetic amyotrophy has more restricted distribution and is usually considered as a self-limiting illness.4 In our case, symptoms and signs were predominantly limited to the lower limbs.
The characteristic presentation is of subacute onset of asymmetrical pain, weakness and wasting of the proximal lower extremity muscles with associated weight loss.5 However, proximal muscles of the upper limbs may also be involved.6 Autonomic symptoms like orthostatic hypotension, diarrhoea, constipation and sexual dysfunction are not uncommon.7 Although our patient had pain, weakness and wasting of the proximal lower limbs, he was significantly disabled by myoclonic jerks, which was an unusual presentation. Myoclonus can be classified based on the site of origin and may be cortical, subcortical, spinal or peripheral.8 A spectrum of neurological and systemic disorders can manifest with myoclonus, which may be positive or negative. Negative myoclonus is characterised by the sudden interruption of muscle contraction, clinically manifested as shock like jerks, which causes postural lapses and subsequent falls. In our case, the difficulty in ambulation was secondary to a combination of weakness and negative myoclonus.
The diagnosis of diabetic amyotrophy relies on clinical suspicion and characteristic electrophysiological findings. Recent studies have shown that the underlying pathogenesis is microvasculitis, which targets the nerve roots, plexus or peripheral nerves and causes ischaemic injury to the neural tissues.7 9 10
Radiological studies of the spine and plexus like MRI are helpful in excluding other possible structural aetiologies such as compression of the plexus by a tumour, abscess or haematoma, infiltration of the plexus or compressive polyradiculopathy.11
CSF analysis may show abnormally high protein without pleocytosis. A median value of 0.89 g/l (range 0.44–2.14 g/l) is reported in various studies.7 12
Electrophysiological findings may suggest axonal degeneration and secondary demyelination.13 EMG of the affected muscles may show acute denervation with positive sharp waves and fibrillation potentials, especially in the thoracic and lumbar paraspinal muscles.5 Electrophysiological changes are mostly seen in the quadricep and adductor muscles of the thigh.5 However, these abnormalities may also be observed in the clinically unaffected segments like the cervical or lumbar segment.14
The histological findings on nerve biopsies are diagnostic of ischaemic injury from microvasculitis.7
Although diabetic amyotrophy is a self-limiting condition and spontaneous recovery has been reported in a majority of patients,3 10 the evolving evidence for an immune mediated aetiology has led to the investigation of immunomodulatory therapies like steroids, immunoglobulins and plasma exchange.7 10 15–18 The earlier institution of intravenous steroids has shown significant improvement in clinical symptoms.15–17 Patients who do not respond to steroids may get benefit from intravenous immunoglobulins.18 Recurrence or relapse of the illness can occur but is uncommon.
Treatment of myoclonus usually focuses on correction of the underlying disorder. Antiepileptic drugs like levetiracetam help in improving myoclonic jerks.19
In our case, strong clinical suspicion, findings on electrodiagnostic studies and dramatic response to immunosuppressive treatment confirmed the diagnosis of diabetic amyotrophy. Given its potentially treatable nature, an awareness of this condition with unusual presentation is important and should be considered in the correct clinical context.
Learning points.
Diabetic amyotrophy is a rare but important differential of diabetic neuropathies.
Diagnosis is mostly based on a clinical suspicion and characteristic findings on electrophysiological studies.
Recognition of unusual presentations like myoclonus is important as the disease is potentially treatable.
Earlier institution of immunomodulatory therapies like steroids and intravenous immunoglobulins improves the clinical symptoms and hastens the recovery.
Antiepileptic drugs like levetiracetam are effective in treating myoclonus.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Liano C. Diabetic proximal neuropathy: getting at the root of the problem: new insights into diagnosis and treatment. Neurol Today 2004;4:52–3, 56–57 [Google Scholar]
- 2.Dyck PJ, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester diabetic neuropathy study. Neurology 1993;43:817–24 [DOI] [PubMed] [Google Scholar]
- 3.Coppack SW, Watkins PJ. The natural history of diabetic femoral neuropathy. Q J Med 1991;79:307–13 [PubMed] [Google Scholar]
- 4.Pascoe MK, Low PA, Windebank AJ, et al. Subacute diabetic proximal neuropathy. Mayo Clin Proc 1997;72:1123–32 [DOI] [PubMed] [Google Scholar]
- 5.Subramony SH, Wilbourn AJ. Diabetic proximal neuropathy. Clinical and electromyographic studies. J Neurol Sci 1982;53:293–304 [DOI] [PubMed] [Google Scholar]
- 6.Katz JS, Saperstein DS, Wolfe G, et al. Cervicobrachial involvement in diabetic radiculoplexopathy. Muscle Nerve 2001;24:794–8 [DOI] [PubMed] [Google Scholar]
- 7.Tracy JA, Engelstad JK, Dyck PJ. Microvasculitis in diabetic lumbosacral radiculoplexus neuropathy. J Clin Neuromuscul Dis 2009;11:44–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caviness JN. Pathophysiology and treatment of myoclonus. Neurol Clin 2009;27:757–77 [DOI] [PubMed] [Google Scholar]
- 9.Dyck PJ, Norell JE. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 1999;53:2113–21 [DOI] [PubMed] [Google Scholar]
- 10.Thaisetthawatkul P, Dyck JB. Treatment of diabetic and nondiabetic lumbosacral radiculoplexus neuropathy. Curr TreatOptions Neurol 2010;12:95–9 [DOI] [PubMed] [Google Scholar]
- 11.Ishii K, Tamaoka A, Shoji S. MRI of idiopathic lumbosacral plexopathy. Neurology 2004;63:E6. [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002;25:477–91 [DOI] [PubMed] [Google Scholar]
- 13.Tataroglu C, Bicerol B. Proximal femoral conductions in patients with lumbosacral radiculoplexus neuropathy. Clinical Neurol Neurosurg 2007;109:654–60 [DOI] [PubMed] [Google Scholar]
- 14.Dyck PJ, Norell JE. Non-diabetic lumbosacral radiculoplexus neuropathy: natural history, outcome and comparison with the diabetic variety. Brain 2001;124:1197–207 [DOI] [PubMed] [Google Scholar]
- 15.Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol 1995;52:1053.– [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Engelstad J, Norell J. Microvasculitis in non-diabetic lumbosacral radiculoplexus neuropathy (LSRPN): similarity to the diabetic variety (DLSRPN). J Neuropathol Exp Neurol 2000;59:525–38 [DOI] [PubMed] [Google Scholar]
- 17.Kilfoyle DM, Kelkar P. Pulsed methylprednisolone is a safe and effective treatment for diabetic amyotrophy. J Clin Neuromuscul Dis 2003;4:168–70 [DOI] [PubMed] [Google Scholar]
- 18.Tamburin S, Zanette G. Intravenous immunoglobulin for the treatment of diabetic lumbosacral radiculoplexus neuropathy. Pain Med 2009;10:1476–80 [DOI] [PubMed] [Google Scholar]
- 19.Keswani S, Kossoff E. Amelioration of spinal myoclonus with levetiracetam. J Neurol Neurosurg Psychiatry 2002;73:457–8 [DOI] [PMC free article] [PubMed] [Google Scholar]