Abstract
This is the third in a series of case studies on an individual with normal coronaries who sustained an idiopathic acute myocardial infarction . Bilateral pulmonary emboli almost 2 years post-myocardial infarction (MI) revealed coagulopathy as the cause. The original MI resulted in 16% myocardial scar tissue. An increasing number of patients are surviving MI, hence the burden for healthcare often shifts to heart failure. Accumulating evidence suggests high-intensity aerobic interval exercise (AHIT) is efficacious in improving cardiac function in health and disease. However, its impact on MI scar has never been assessed. Accordingly, the 50-year-old subject of this case study undertook 60 weeks of regular AHIT. Successive cardiac MRI results demonstrate, for the first time, a decrease in MI scar with exercise and, alongside mounting evidence of high efficacy and low risk, suggests AHIT may be increasingly important in future prevention and reversing of disease and or amelioration of symptoms.
Background
Myocardial infarction (MI) is usually the consequence of acute cardiac muscle ischaemia downstream of a blockage in the coronary vasculature. The resulting myocardial scar tissue has traditionally been considered to be inert and permanent, perhaps a logical assumption for a postmitotic organ such as the heart. That view is increasingly being challenged however, and it has been suggested that the heart of patients surviving MI may demonstrate some potential for regeneration.1
Cardiomyocytes of the adult mammalian myocardium are terminally differentiated cells that are permanently withdrawn from the cell cycle.2 3 Thus, the adult heart is composed of a large mass of postmitotic cells, but, nevertheless, it has a remarkable capacity for regeneration, both under normal conditions4 and in response to diverse pathological5 6 and physiological stimuli, including aerobic exercise training.7–9 The potential for regeneration is ensured by the presence of endogenous tissue-specific cardiac stem-progenitor cells.10 11 Elucidation of the molecular signalling pathways that govern myocardial regeneration and repair, and a better understanding of endogenous cardiac stem cell biology will allow the design of optimal therapeutic interventions.
During the last decade, a growing body of evidence has demonstrated the efficacy of high-intensity interval training (HIT) in general, and of aerobic HIT (AHIT) specifically, in the treatment and prevention of a number of pathologies.12 HIT (ie, maximal anaerobic and aerobic interval training at greater than 85% of maximum heart rate, HRM) has been demonstrated to be more effective than moderate or low-intensity exercise for improving health status. Several parameters have seen positive change with the implementation of HIT including: improved insulin sensitivity in patients with type II diabetes,13 improved lipid profiles,14 and improved VO2max, the generic marker of aerobic conditioning which is inversely related to all-cause morbidity and mortality.15 16
A number of studies have demonstrated the benefits of starting exercise rehabilitation soon after MI in order to prevent remodelling rather than having to ‘reverse-remodel’ following a delay in the onset of a cardiac rehabilitation programme.17–19 Accordingly, exercise has been demonstrated as being not only positive, but also essential to the recovery process and the prevention of further disease or deterioration in function.
Recent experimental studies have used a rodent model of MI to evaluate the effects of AHIT typically involving repeated intervals of 8 min of exercise at an intensity that elicits >90% of HRM with 3 min of active recovery between each work interval. In a sample of eight studies that have been carried out on rodents, no coronary events or deaths have resulted from AHIT (U Wisloff, O Kemi, J Helgerud, J Talanian and O Ellingsen, personal communication, March 2011). Human participants have in general performed AHIT comprising 4 min intervals with 3 min recoveries. Using this paradigm, a recent study of 4846 coronary heart disease patients, in Norway, with a range of diagnoses including coronary artery disease, congestive heart failure, metabolic syndrome, hypertension, obesity and older age, were exercised either at a high-intensity or moderate-intensity. Of a total of 175 820 exercise training hours, where all patients performed both types of training, there was one fatal cardiac arrest during moderate-intensity exercise (129 456 exercise hours) and two non-fatal cardiac arrests during high-intensity interval exercise (46 364 exercise hours). Overall, there were no MIs, concluding that the risk of a cardiovascular event is low after both high-intensity and moderate-intensity exercise in a cardiovascular rehabilitation setting.20 Moreover, Herman et al21 examined the effect of interval training for 8 weeks with daily intervals of greater than 85% of HRM in long-term heart transplant recipients and reported ‘no serious adverse events’.
In practice, AHIT involves exercise utilising a number of short duration efforts (1–4 min) at a relatively high exercise intensity (ie, >90% of HRM) and with short rest intervals of 30 s–3 min. The use of this type of exercise training in patients with a prior MI, in the presence of ventricular damage, is uncommon particularly in the UK. This is, presumably, because of the perception of risk; however, there is no empirical evidence that AHIT is more risky than moderate-intensity or low-intensity exercise. Furthermore, many clinicians are simply unaware of the positive benefits to health to be accrued through regular AHIT.
Therefore, the aim of this work was to implement a 12-month AHIT intervention in a single individual who had previously sustained an MI and, using cardiac MRI (cMRI), assess any changes in the extent of MI scar, left ventricular (LV) systolic function, viability and diastolic compliance. If AHIT provides a stimulus for improved LV remodelling then this will be the first time that the efficacy of high-intensity exercise will have been demonstrated for myocardial repair and may provide a platform for the investigation of AHIT as a therapeutic intervention in MI patients.
Case presentation
The patient is a 50-year-old man of mixed race (Caucasian mother, Indian father) with a 35-year history of extensive and intensive physical activity. In September 2007, the patient suffered an MI at the age of 45 years.22 The underlying cause of the original MI remained unknown for just under 2 years until, following almost 3 months of breathlessness and dizziness, the patient was admitted to A&E with a deep vein thrombosis and bilateral pulmonary emboli (July 2009). Follow-up investigation resulted in a consensus, non-definitive, diagnosis of coagulopathy.23 cMRI 14 months post-acute myocardial infarction (AMI) demonstrated ischaemic scar involving the mid and basal segments of the inferior wall, amounting to 16.3% of total LV myocardial mass.
Investigations
Three separated cMRI studies: study 1, performed 14 months after AMI (when spontaneous LV remodelling can be considered complete); study 2, obtained after 14 weeks of AHIT and study 3, acquired after a total of 60 weeks of AHIT. The cMRI protocol included a full set of cine images to assess LV function and delayed postcontrast images for scar evaluation.
In May and August 2011 and March 2012, maximal aerobic power (VO2max) was assessed on a cycle ergometer (Excalibur Sport, Lode Gronigen, the Netherlands) with ergospirometry (Quark b2, Cosmed, Rome, Italy).
Treatment
Between January 2011 and March 2012, the patient voluntarily undertook AHIT which included sets of up to 20×(1 min hard followed by 1 min easy); 6×(2 min hard followed by 2 min easy); 4×(4 min hard followed by 2 min easy) on an exercise bike 2–4 times/week. All sessions elicited a heart rate >90% HRM in each interval and, for several intervals per session, a heart rate >95% of maximum was elicited. The number and volume of sessions of monthly AHIT for the 14-month intervention period is given in table 1.
Table 1.
Number and volume of sessions of aerobic high-intensity interval training(AHIT) per month
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Dec | Feb | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHIT (no) | 12 | 8 | 15 | 13 | 6 | 8 | 14 | 9 | 6 | 10 | 6 | 7 | 6 | 9 |
| AHIT (min) | 410 | 285 | 410 | 474 | 220 | 254 | 563 | 280 | 185 | 285 | 245 | 272 | 240 | 366 |
In addition to the intervention described above, it should be noted that, throughout this period, the patient was subject to the following daily medication: 75 mg aspirin, 20 mg simvastatin and 5 mg warfarin.
Outcome and follow-up
The initial cMRI scan demonstrated an ischaemic scar involving the mid and basal segments of the inferior wall, amounting to 16.3% of total LV myocardial mass. A progressive decrease in scar tissue was noted from successive cMRIs: 9.6% in study 2 and 8.5% of LV myocardial mass in study 3. This represents almost a 50% scar reduction following long-term AHIT (figure 1). The relative reduction in scar size is, at least in part, related to an increase in LV mass after AHIT (from 152 to 153 g, and then 162 g from studies 1 to 3, respectively). However, the greatest reduction in scar size (∼40%) occurred between studies 1 and 2 (after 14 weeks of AHIT) when the LV mass remained unchanged. Cine images from repeated cMRI did not demonstrate clinically meaningful changes in LV systolic and diastolic function, ejection fraction or peak filling rate (table 2).
Figure 1.
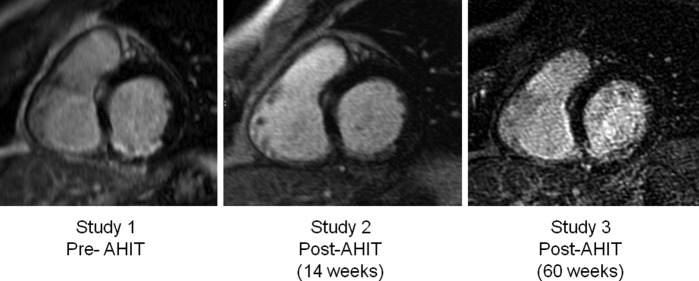
Representative cardiac MRI images of the evolution of the myocardial scar, involving the basal segment of the inferior left ventricular wall, from study 1 (14 months after acute myocardial infarction) to study 2 (14 weeks after aerobic high-intensity interval training (AHIT) to study 3 (60 weeks after AHIT).
Table 2.
LV volumes, mass and function
| EDV (ml) | ESV (ml) | EF (%) | Mass (g) | Peak filling rate (ml/s) | |
|---|---|---|---|---|---|
| Study 1 (Nov 2008) | 151 | 43 | 72 | 152 | 974 |
| Study 2 (May 2011) | 161 | 60 | 63 | 153 | 823 |
| Study 3 (Mar 2012) | 160 | 56 | 65 | 162 | 888 |
EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricular.
In May, VO2max was 34 ml/kg/min; in August, it was 38 ml/kg/min and in March 2012, it was 43 ml/kg/min. This demonstrates a 26% increase in aerobic power over the course of a year of AHIT.
Discussion
The results of this single individual case study show that 14 weeks of AHIT resulted in ∼40% reduction of the MI scar, which was improved as evidenced by ∼50% reduction following 60 weeks of AHIT. To date a reduction in MI scar as a result of physical training has never been reported in humans or indeed any animal model. This case study is the first to demonstrate a reduction in MI scar tissue in response to high-intensity exercise and so may act as a valuable tool in treatment of the post-MI patient.
Exercise training activates circulating stem-progenitor cells 24 as well as the resident tissue-specific cardiac stem-progenitor cells.8 We have previously reported an increased proliferation, number and cardiogenic differentiation of the resident endogenous cardiac stem-progenitor cells with physiological overload in the form of swimming and intensity-controlled treadmill endurance exercise training.8 9 This, in turn, leads to the accumulation of new myocardial cells, namely, new myocytes and microvasculature.7 9 These cellular modifications are dependent on exercise duration and intensity and result in increased contractile muscle mass and enhanced cardiac function, which reduces myocardial wall stress.9 Moreover, we have shown that intracoronary administration of the growth factors, IGF-1 and HGF, which are typically upregulated with physiological overload,8 9 to the infarcted pig heart can reduce pathological remodelling and fibrosis and trigger a regenerative response of the resident endogenous cardiac stem-progenitor cells, leading to decreased scar size and improved ventricular function.25
Whether this individual's three-and-a-half decade history of intensive and extensive exercise is relevant to the outcome is unknown but that it may have contributed cannot be ruled out. In addition, AHIT requires significant determination to complete even a single session as it represents a significant challenge both physically and psychologically. Whether it would be possible to achieve such a level of physical activity with the previously sedentary, or even those who currently take part in moderate-intensity exercise, is also unknown.
Limitations of this study include: findings are from only a single individual, and cMRI was not used immediately before start of exercise intervention and, hence, spontaneous resolution cannot be discounted. However, this has never been recorded before and so, seems unlikely. Accordingly, the finding in this current case study, coupled with the very low incidence of adverse events culled from previous studies, suggests the need for a prospective study of a population of MI patients undertaking AHIT to confirm any effect on MI scar and myocardial compliance. Future research should aim to provide further evidence for the efficacy of AHIT in improving cardiac performance and to examine the safety of such training.
Learning points.
High-intensity aerobic interval training may reduce scar post-acute myocardial infarction and may reduce the risks of subsequent development of heart failure.
High-intensity aerobic interval training has been demonstrated to improve cardiac function in the healthy but also for many with disease, including heart disease.
There is no empirical evidence to suggest that high-intensity interval exercise represents a greater risk to cardiac health than low-intensity or moderate-intensity exercise.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beltrami AP, Urbanek K, Kajstura J, et al. Evidence that human cardiac myocytes divide after myocardial infarction. N Engl J Med 2001;344:1750–7 [DOI] [PubMed] [Google Scholar]
- 2.Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell 1978;15:855–64 [DOI] [PubMed] [Google Scholar]
- 3.Nadal-Ginard B, Kajstura J, Leri A, et al. Myocyte death, growth, and regeneration in cardiac hypertrophy and failure. Circ Res 2003;92:139–50 [DOI] [PubMed] [Google Scholar]
- 4.Bergmann O, Bhardwaj RD, Bernard S, et al. Evidence for cardiomyocyte renewal in humans. Science 2009;324:98–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urbanek K, Quaini F, Tasca G, et al. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci USA 2003;100:10440–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urbanek K, Torella D, Sheikh F, et al. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci USA 2005;102:8692–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boström P, Mann N, Wu J, et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 2010;143:1072–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellison GM, Waring CD, Vicinanza C, et al. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart 2012;98:5–10 [DOI] [PubMed] [Google Scholar]
- 9.Waring CD, Vicinanza C, Paplamprou A, et al. The adult heart responds to increased workload with physiologic hypertrophy, cardiac stem cell activation, and new myocyte formation. Eur Heart J 2012. doi:10.1093/eurheartj/ehs338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003;114:763–76 [DOI] [PubMed] [Google Scholar]
- 11.Torella D, Ellison GM, Karakikes I, et al. Resident cardiac stem cells. Cell Mol Life Sci 2007;64:661–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibabla MJ, Little JP, MacDonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol 2012;590.5:1077–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Babraj JA, Vollaard NBJ, Keast C, et al. Extremely short duration high intensity interval training substantially improves insulin action in young males. BMC Endocr Disord 2009;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Musa DI, Adeniran SA, Dikko AU, et al. The effect of a high-intensity interval training program on high-density lipoprotein cholesterol in young men. J Strength Cond Res 2009;23:587–92 [DOI] [PubMed] [Google Scholar]
- 15.Blair SN, Kohl HW, Paffenbarger RS, et al. Physical fitness and all-cause mortality: a prospective study of healthy men and women. J Am Med Assoc 1989;262:2395–401 [DOI] [PubMed] [Google Scholar]
- 16.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. J Am Med Assoc 2009;301:2024–35 [DOI] [PubMed] [Google Scholar]
- 17.De Waard MC, Van der Velden J, Bito V, et al. Early exercise training normalizes myofilament and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res 2007;100:1079–88 [DOI] [PubMed] [Google Scholar]
- 18.Van der Velden J, Merkus D, Klarenbeek BR, et al. (2004). Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Cir Res 2004;95:e85–95 [DOI] [PubMed] [Google Scholar]
- 19.Otsuka Y, Takaki H, Okano Y, et al. Exercise training without ventricular remodelling in patients with moderate to severe left ventricular dysfunction early after acute myocardial infarction. Int J Cardiol 2003;87:237–44 [DOI] [PubMed] [Google Scholar]
- 20.Rognmo O, Moholdt T, Bakken H, et al. Cardiovascular risk of high- versus moderate-intensity aerobic exercise in coronary heart disease patients. Circulation 2012;126:1436–40. [DOI] [PubMed] [Google Scholar]
- 21.Hermann TS, Dall CH, Christensen SB, et al. Effect of high intensity exercise on peak oxygen uptake and endothelial function in long-term heart transplant recipients. Am J Transplant 2011;11:536–41 [DOI] [PubMed] [Google Scholar]
- 22.Whyte G, Godfrey R, O'Hanlon R, et al. Acute myocardial infarction in the presence of normal coronaries and the absence of risk factors in a young, life-long exerciser. BMJ Case Rep 2009;May 25.1. doi:10.1136/bcr.072008.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Godfrey R, O'Hanlon R, Wilson M, et al. Underlying cause discovered for a prior idiopathic AMI. BMJ Case Rep 2011;Mar 29. 10.1136/bcr.02.2011.3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thijssen DH, Torella D, Hopman MT, et al. and The role of endothelial progenitor and cardiac stem cells in the cardiovascular adaptations to age and exercise. Front Biosci 2009;14:4685–702 [DOI] [PubMed] [Google Scholar]
- 25.Ellison GM, Torella D, Dellegrottaglie S, et al. Endogenous cardiac stem cell activation by insulin-like growth factor-1/hepatocyte growth factor intracoronary injection fosters survival and regeneration of the infarcted pig heart. J Am Coll Cardiol 2011;58:977–86 [DOI] [PubMed] [Google Scholar]


