Abstract
Mucormycosis, also known as phycomycosis or zygomycosis, is caused by common Zygomycete fungi frequently found in soil and decaying vegetation. These mainly infect immunocompromised patients and cause an acute fulminating fungal disease; mucormycosis rarely affects otherwise healthy people. Mucormycosis is a fatal infection with a poor prognosis. Of the different types of mucormycosis, the rhinocerebral type is the most severe one, and its type 2 subtype, the rhino-orbital-cerebral form is the deadliest variety. Here, we report a case of mucormycosis presenting with extensive necrosis of the maxilla with extension into the retrobulbar and infrabulbar region in an otherwise healthy patient. He underwent extensive debriding surgery followed by amphotericin B first and then oral antifungal therapy, but unfortunately, even after extensive surgery and medical treatment, he did not survive.
Background
Mucormycosis is an opportunistic fungal infection which usually infects immunocompromised individuals. This infection is often fatal. Proper and timely intervention surgically and medically can lead to the cure of the patient. Here, we present a case of rhino-orbital mucormycosis in an otherwise immunocompetent patient, which is seen very rarely.
Case presentation
A 30-year-old man presented with progressive numbness of the left side of the face for 2 years, headache associated with mild pain in the left side of the face for 6 months, proptosis of left eye for 3 months, ptosis on the left side and gradual loss of vision of the left eye since 20 days. The patient's medical history revealed no relevant systemic diseases. On general physical examination, the patient appeared to be healthy. Physical examination revealed swelling of the left side of face with proptosis, ptosis and loss of vision of the left eye. (figure 1) The patient had no perception of light and pupil in his left eye was dilated and non-reacting to light. Cranial nerve examination revealed primary optic atrophy, complete ophthalmoplegia, loss of pain, touch and temperature sensation with lower motor type of facial palsy on the left side. On intraoral examination, the mucosa was seen to be stripped off on the left side of the palate leaving denuded bone (figure 2). The lesion extended from the left buccal gingiva to mid-way of the left half of the palate mediolaterally and from the first premolar to the first molar, anteroposteriorly measuring about 1.5 to 2 2 inches. The lesion had an undermined margin and the base was covered with slough causing loosening of teeth (figure 3).
Figure 1.
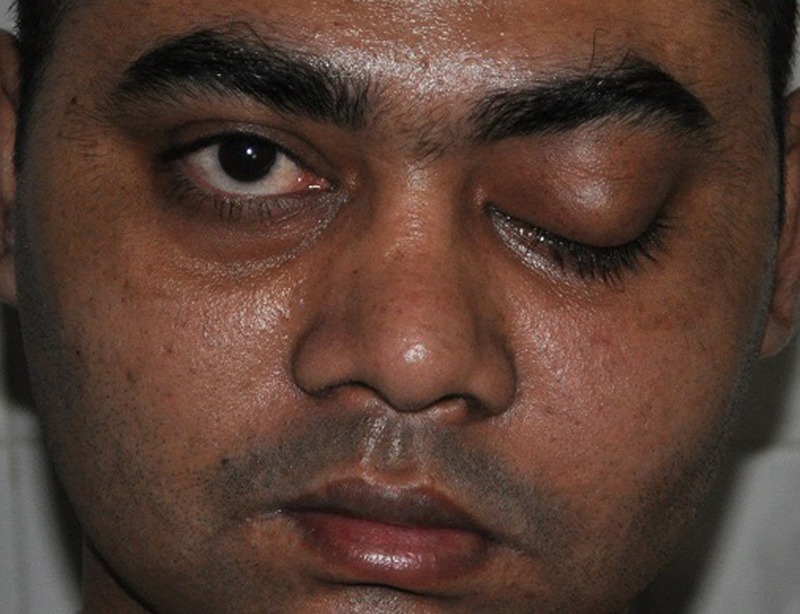
Picture showing ptosis and proptosis of left eye with swelling of left side of face of the patient.
Figure 2.
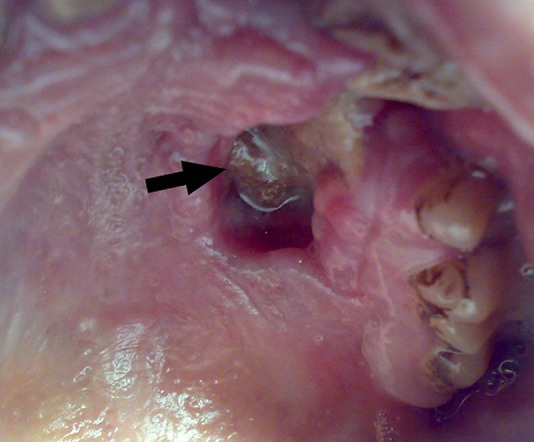
Preoperative intraoral picture showing lesion on the palatal area causing denudation of hard palate on left side (arrow).
Figure 3.
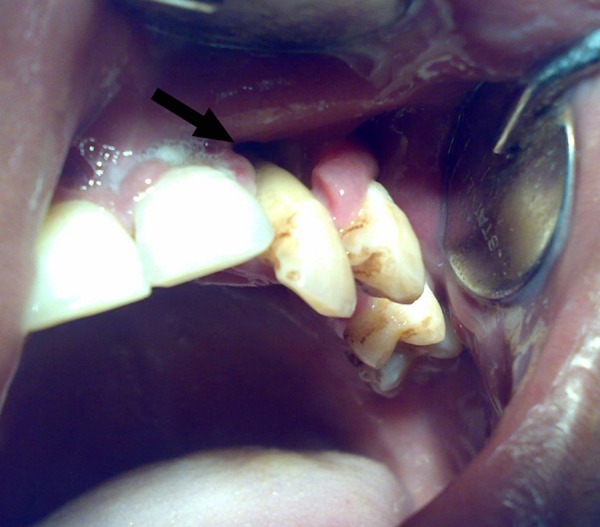
Picture showing lesion involving soft tissue and alveolar bone of the left side of upper jaw causing loosening of teeth (arrow).
Investigations
All the biochemical parameters including blood count and blood sugar levels were within normal limits. He had no evidence of tuberculosis or HIV infection. MRI of the orbit revealed proptosis of the left globe and an irregular soft tissue density in the inferior aspect of the left orbit slightly displacing the optic nerve and compressing the adjacent extraocular muscles and that had extension into the infratemporal fossa as well as into the maxillary antrum (figure 4). CT of the brain revealed no abnormality. CT scan of the facial region revealed soft tissue hyperdense mass with underlying bone erosion along the anterior wall of the maxillary antrum, extending into the orbit from the inferior aspect with mild contrast enhancement (figure 5).
Figure 4.
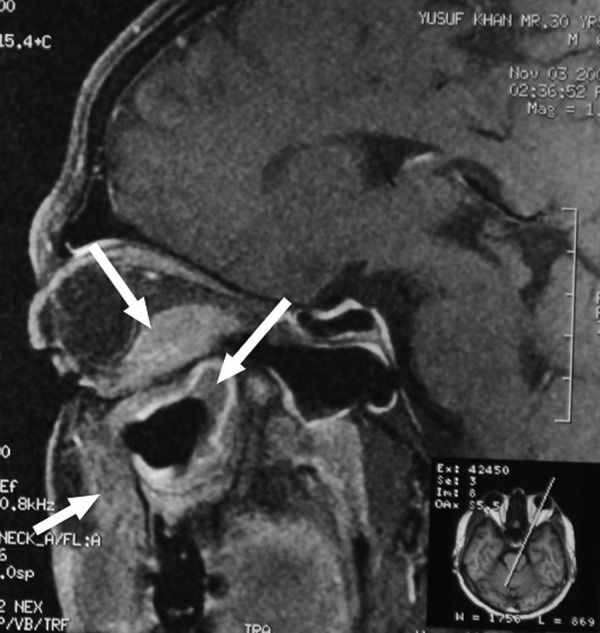
Contrast MRI in sagittal view showing mild contrast enhancement of the lesion in the retrobulbar and infrabulbar, infratemporal and maxillary regions (arrows).
Figure 5.
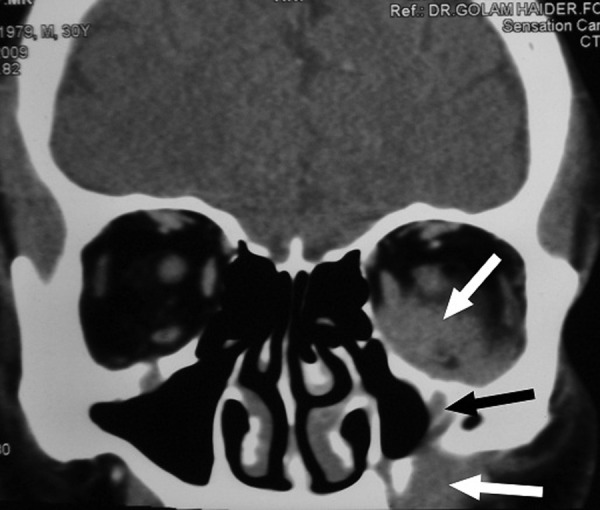
Contrast CT scan, coronal view showing mild contrast enhancement of the lesion in infrabulbar region, maxillary antral wall and infratemporal region (white arrows). Erosion of the maxillary antram is also seen (black arrow).
Differential diagnosis
Aspergillosis.
Treatment
Fine needle aspiration cytology from the left cheek was performed initially, which disclosed fungal granuloma, possibly of mucormycosis. We decided to perform surgery with the goal to debride the lesion as much as possible. Partial maxillectomy and gross total removal of the mass in and around the maxilla as well as the infraorbital part was accomplished with two separate incisions—a Weber-Fergusson incision that was continued intraorally up to the junction of the hard and soft palate and a vestibular incision that was continued posteriorly around the lesion towards the maxillary tuberosity. The orbital floor, which was already eroded to some extent, was removed to have better access to the retrobulbar and infrabulbar portion of the mass. The mass in the orbit, which was in continuation with the maxillary portion of the lesion, was then removed in piecemeal fashion; this mass was tough, white, fibrous and avascular as was that of the lesion in the maxillary region. The lesion was removed gross totally until the periorbital fat was seen (figure 6). Copious irrigation was performed. Antibiotic-soaked ribbon gauze was packed into the defect and the defect was covered with a prefabricated obturator. The patient was put on amphotericin B (plain), but after 3 days, the patient developed hepatic toxicity. Consequently, we discontinued amphotericin, and once the patient's biochemical parameters came back to normal, oral fluconazole was started.
Figure 6.
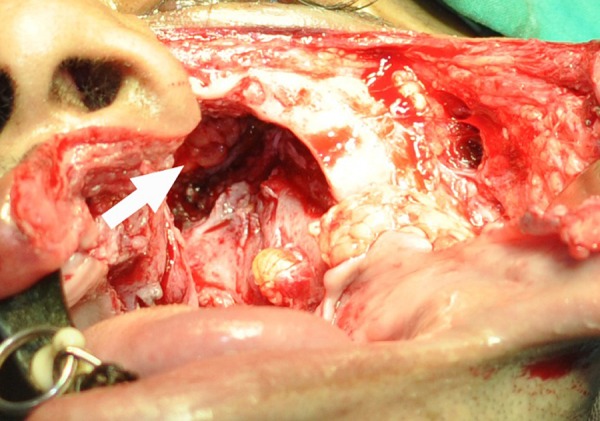
Preoperative picture showing periorbital fat (arrow) after partial maxillectomy and removal of the lesion from the infrabulbar and retrobulbar area.
Outcome and follow-up
The patient was doing relatively well until he died 2 months later, following sudden unconsciousness. Owing to sociolegal constraints we could not have any autopsy to determine the probable cause of death.
Discussion
Mucormycosis also known as phycomycosis or zygomycosis is an acute, fulminating fungal disease caused by common Zygomycete fungi frequently found in soil and decaying vegetation, which when inhaled, produce infection in the sinuses and bronchi. The genera most commonly responsible for mucormycosis are Mucor, Rhizopus and Asbidia.1–6 This is the third most common cause of fungal infection after candidiasis and aspergillosis and mainly infects immunocompromised patients like patients with diabetes, haematological malignant disease, immunosuppressive therapy, thermal burns, renal failure, cirrhosis, surgery, solid organ transplantation, HIV infection, etc. Our patient had no systemic disease like these, which is very uncommon, even though infection in immunocompetent patients has been reported.2 4–9
The hyphae are broad and non-septate. When spores are converted into hyphae, they become invasive and may spread through the paranasal sinuses into the brain and orbitae. Extension along blood vessels is followed by invasion and thrombosis.3–5 Zygomycosis has a high death rate of 20–100%, but some patients may be cured by surgical excision and amphotericin. The most common form of presentation is pulmonary mucormycosis, which accounts for 64% and involves orbital and maxillofacial areas in 24%. For decades, the death rate of mucormycosis has remained high, despite aggressive surgical and polyene antifungal therapy.5 9 10
Chronic invasive rhinocerebral mucormycosis shows slow progression which usually affects one side, followed by rapid spread to the brain and meninges. The common presenting symptoms and signs are headache, fever, acute sinusitis, decreased vision, unilateral periorbital or facial swelling, facial pain, alterations in mental state and necrotic ulcer in the nasal or oral mucosa. Although rare, the orbital apex syndrome which presents with ptosis, proptosis, ophthalmoplegia, sensory deficit and visual loss may be the initial manifestation of the disease.4 5 11 The rhinocerebral type is usually subdivided into types 1 and 2. Type 1 is a highly fatal rhino-orbital-cerebral form, whereas type 2 is a less fatal rhino-maxillary form.1 Our case was of the rhino-orbital type, a type between type 1 and 2 as the infection was limited to the nose, maxilla and orbit, and did not involve the cerebrum. The patient had gradual loss of sensation on left side of face for 2 years and developed very quick proptosis over 3 months and ptosis with complete ophthalmoplegia and loss of vision over a period of 20 days. Most of the reported cases of maxillar mucormycosis presented with a small ulceration on the palate, while our patient had extensive destruction of the maxilla.
It is assumed that the port of entry is colonisation of the nasal mucosa, allowing the fungus to spread via the paranasal sinuses. Once fungal hyphae enter into the blood stream, they can spread to other organs, which can be fatal. Mucor hyphae form thrombi within the blood vessels that reduce vascularity to the tissues and cause necrosis.5 11 12 The exact pathogenesis of rhinocerebral mucormycosis and its pathways of spread are not clearly known. Different sources state that, Mucor initially inoculates the nasal mucosa, then spreads to the pterygopalatine fossa which acts as a reservoir, or to the paranasal sinuses (specially, the ethmoid and maxillary sinuses) from where it spreads to the orbit through the lamina papyracea and finally intracranially.11 13 The origin of the infection could not be ascertained in this case, but since the patient was healthy with no history or findings of immunosuppression, it seems that the fungus invaded the adjacent areas starting from the nasal mucosa and then spread through the paranasal sinuses to the other parts. As multiple sites were involved, a multidisciplinary team, including an ophthalmologist, a neurosurgeon and a maxillofacial surgeon, are required for management of such patients,12 and were also recruited for our patient.
CT findings include nodular mucosal thickening and hyperdense soft tissues in the paranasal sinuses without air-fluid levels. Intracranial extension and orbital involvement may also be visible in contrast-enhanced CT scans; however, T2-weighted and contrast-enhanced T1-weighted MRI are considered superior to CT.5 14 In both the CT and MRIs of our patient, the lesion in the orbit and the maxilla was seen well.
The principle of managing mucormycosis is firstly to remove the necrotic bone and tissue, which act as a nidus of infection and prevent action of systemically administered antifungal drugs (due to thrombosis of blood vessels).5 11 In this patient, the entire necrotic maxilla was removed by partial maxillectomy along with removal of the orbital floor and the infrabulbar and retrobulbar mass (figure 7). Then, amphotericine B was administered parenterally as the drug of choice in the treatment of mucormycosis infection. As the patient developed hepatotoxicity 3 days after starting amphotericine B, we switched him to oral fluconazole after 7 days, following which his hepatic function came back to normal.
Figure 7.
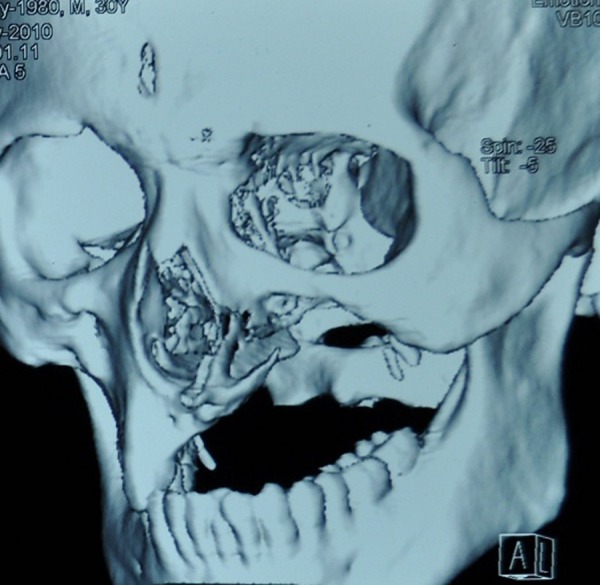
Postoperative three-dimensional CT scan showing extent of removal of maxilla.
A definitive diagnosis of mucormycosis can be made by tissue biopsy that identifies the characteristic hyphae.15 In this case, the fungus was identified by periodic acid-Schiff stain with diastase digestion. The histopathology revealed multiple epitheloid granulomata, including many giant cells. Fungal hyphae were also identified on histopathology confirming the diagnosis of mucormycosis (figure 8).
Figure 8.
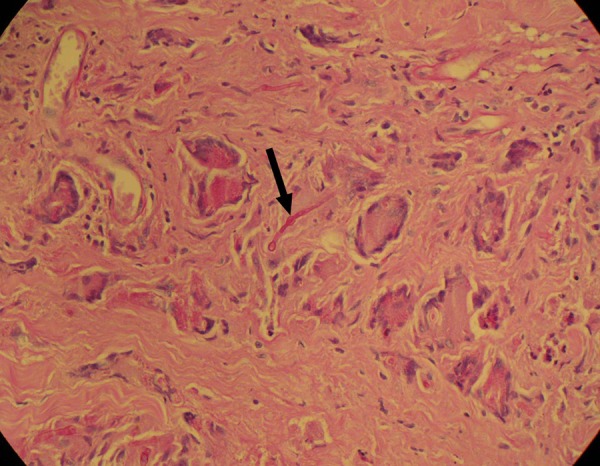
Photomicrograph showing non-septated hyphae (arrow).
Learning points.
Early diagnosis and treatment is essential to manage rhino-orbital mucormycosis.
Combined medical treatment with antifungal agents, preferably parenteral ones and surgical removal of the involved area is essential.
Delay in management may lead to a fatal outcome.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Jindal S, Kulkarni S, Sheikh S, et al. Invasive rhinomaxillary mucormycosis: a case report with a review of the literature. Oral Radiol 2008;24:42–8 [Google Scholar]
- 2.Cagatay AA, Oncu SS, Calangu SS, et al. Rhinocerebral mucormycosis treated with 32 gram liposomal amphotericin B and incomplete surgery: a case report. BMC Infect Dis 2001;1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pastore PN. Mucormycosis of the maxillary sinus and diabetes mellitus: report of a case with recovery. South Med J 1967;60:1164–7 [DOI] [PubMed] [Google Scholar]
- 4.Rumboldt Z, Castillo M. Indolent intracranial mucormycosis: case report. AJNR Am J Neuroradiol 2002;23:932–4 [PMC free article] [PubMed] [Google Scholar]
- 5.Haliloglu NU, Yesilirmak Z, Erden A, et al. Rhino-orbito-cerebral mucormycosis: report of two cases and review of the literature. Dentomaxillofac Radiol 2008;37:161–6 [DOI] [PubMed] [Google Scholar]
- 6.Mane R, Watve J, Mohite A, et al. Rhinocerebral mucormycosis: a deadly disease on the rise. Indian J Otolaryngol Head Neck Surg 2007;59:112–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav S, Singh J, Ranga R, et al. Mucormycosis. Indian J Otolaryngol Head Neck Surg 2003;55:208–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao S, Kumar K, Rokade V, et al. Orbital apex syndrome due to mucormycosis caused by Rhizopus microsporum. Indian J Otolaryngol Head Neck Surg 2006;58:84–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karanth M, Taniere P, Barraclough J, et al. A rare presentation of zygomycosis (mucormycosis) and review of the literature. J Clin Pathol 2005;58:879–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spellberg B, Walsh TJ, Kontoyiannis DP, et al. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis 2009;48:1743–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseini SM, Borghei P. Rhinocerebral mucormycosis: pathways of spread. Eur Arch Otorhinolaryngol 2005;262:932–8 [DOI] [PubMed] [Google Scholar]
- 12.Auluck A. Maxillary necrosis by mucormycosis. a case report and literature review. Med Oral Patol Oral Cir Bucal 2007;12:E360–4 [PubMed] [Google Scholar]
- 13.Kulkarni NS, Bhide AR, Wadia RS. Rhinocerebral mucormycosis: an analysis of probable mode of spread and its implication in an early diagnosis and treatment. Indian J Otolaryngol Head Neck Surg 2005;57:121–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nithyanandam S, Correa M. Rhino-orbital mucormycosis and aspergillosis: differences in outcome, clinical and imaging characteristics. Eur Arch Oto-Rhino-Laryngol 2010;267:161–2 [DOI] [PubMed] [Google Scholar]
- 15.Tugsel Z, Sezer B, Akalin T. Facial swelling and palatal ulceration in a diabetic patient. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004;98:630–6 [DOI] [PubMed] [Google Scholar]


