Abstract
Disseminated cysticercosis is an uncommon presentation of cysticercosis. Less than 10 cases of disseminated cysticercosis have been reported worldwide in children. We report the case of an 8-year-old boy with disseminated cysticercosis, who had presented with a swelling of the body for 1 month and proptosis of the eyeballs for 14 days. On examination, he had bilateral proptosis, subcutaneous nodules and hypertrophy of muscles of the limbs, neck and face. The CT cranium was normal, but the orbit showed bilateral bulky extraocular muscles heterogeneous in their whole length. The MRI cranium and whole body showed multiple non-enhancing vesicular cysts involving the brain, extraocular muscle, heart, trunk and muscles of the extremities and subcutaneous tissues. A Doppler study of the femoral vein showed thrombosis of the right common femoral vein. He was managed with corticosteroid, albendazole, phenytoin sodium, low-molecular-weight heparin followed by warfarin for 6 months and recovered completely.
Background
Neurocysticercosis was not considered as a public health problem until the second half of the 20th century, when British researchers investigated the disease among soldiers returning from India.1 Cysticercosis, the infection caused by the larval stage of the tapeworm, Taenia solium, is a major parasitic disease of the nervous system in humans and the single most common cause of acquired seizures in the developing world, including India, where the prevalence rates of epilepsy are twice as much as in developed countries.2
Cysticercosis usually affects highly vascularised tissues such as the brain, masticatory, extraocular, tongue and skeletal muscles. Central nervous system (CNS) involvement is seen in 60–90% of infested patients. The cerebrum and cerebellum are common sites but may involve the brainstem, basal ganglion, thalamus and lateral sinus. Orbital involvement, particularly of the extraocular muscle, is the second most common presentation. Seizures are the commonest presenting features in a majority of cases, but can manifest with headache, focal neurological deficits, visual loss, ataxia, dystonia, dementia, hydrocephalus and raised intracranial pressure.3 Disseminated cysticercosis is an uncommon presentation of cysticercosis. It is characterised by a massive symptomatic burden that can involve virtually any organ. Less than 50 cases of disseminated cysticercosis have been reported worldwide and in fewer than 10 cases in children.4 5 We report the case of a child who had presented with constellation of manifestations of disseminated cysticercosis with CNS, eye, subcutaneous tissue, skeletal muscles, myocardium and thrombosis of the right common femoral vein.
Case presentation
An 8-year-old boy presented with a swelling of the body for 1 month, intermittent fever, headache, vomiting, prominence of both eyes and constipation for 14 days. There was no history of seizure or diminution of vision. He was the third in the order of seven siblings. There was no history of similar illness in the family. His father is an owner of a pork shop.
On physical examination, the child was irritable and had symmetrical hypertrophy of the lower limbs, most prominent in the calf muscles, but also had affection of the upper limbs, neck and facial muscles. The overlying skin showed venous prominences. There were subcutaneous nodules present on the upper eyelid and the nape of the neck. Bilateral proptosis was present (figure 1). A marked limitation of medial duction could be assessed with a forced duction test in both eyes. Exophthalmometry disclosed a 3 mm left and 4 mm right proptosis. The intraocular pressure was recorded as 28 mm Hg in the right eye and 26 mm Hg in the left eye. Fundus examination did not show a retinal cyst or any abnormality at the time of admission. On motor examination, there was weakness in both the upper and lower limbs (power grade IV) with positive Homan's sign. Sensory and other systemic examinations were unremarkable.
Figure 1.
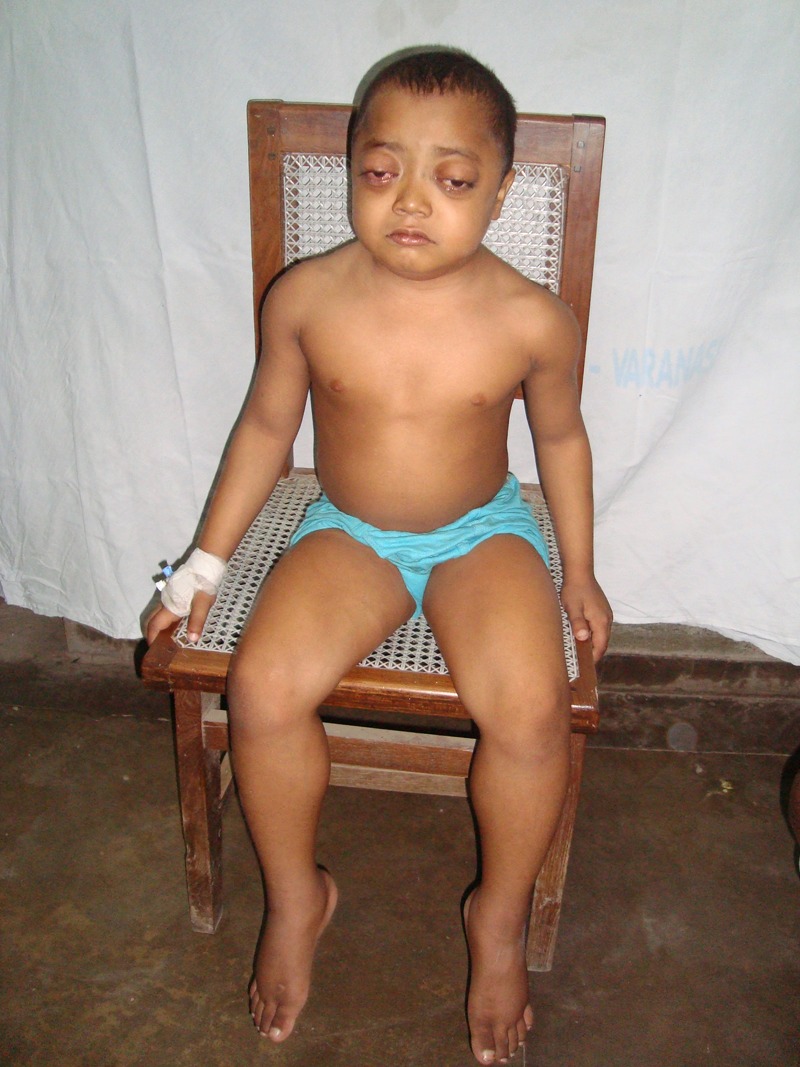
Patient's photograph showing the proptosis of both eyes with hypertrophy of the calf muscles.
On day 5 of admission, the patient developed diminution of vision to 20/20 in the left eye and repeat fundus examination showed an elevated cystic mass over the macula of size 3 D with a central yellowish patch in the left eye (figure 2).
Figure 2.
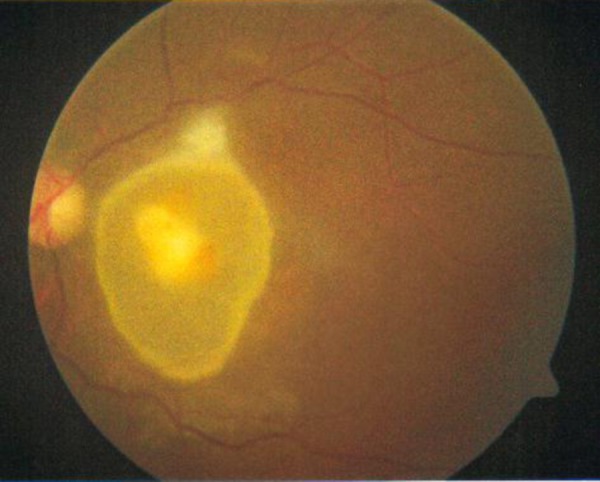
Fundal photograph showing an elevated cystic mass over the macula with a central yellowish patch in the left eye.
Investigations
Laboratory investigation showed haemoglobin of 9 g/dl, a total leucocyte count of 7800/mm3 with 55% polymorphs, 38% lymphocytes, 3% eosinophils and 4% monocytes. Blood urea, serum creatine, creatine kinase and thyroid function tests were normal. Examination of stools (three samples) did not reveal any ova or cyst. Chest x-ray showed cardiomegaly with a cardiothoracic ratio of 0.61 and prominent vascular markings in the right perihilar area. ECG showed right axis deviation with biventricular enlargement. Echocardiography (two-dimensional and color Doppler) revealed concentric hypertrophy of both ventricles, diffuse hypokinesia of the left ventricle with mild pericardial effusion and a left ventricular ejection fraction of 35%.
B-scan ultrasonography of both eyes showed cystic lesions in the extraocular muscles with a few showing echogenic nidus (scolex). The left eye showed a regular thin-walled cystic lesion with echogenic nidus seen in the subretinal area ‘hole with a dot sign’. Ultrasonography of the facial and strap muscles of the neck also showed multiple cystic lesions. A ‘honeycomb pattern’ of densely packed vesicular cysts was present in the thigh and calf muscles (figure 3). A Doppler flow study showed thrombosis of the right common femoral vein (figure 4). There was no evidence of calcification of the muscles in an x-ray of the extremities.
Figure 3.
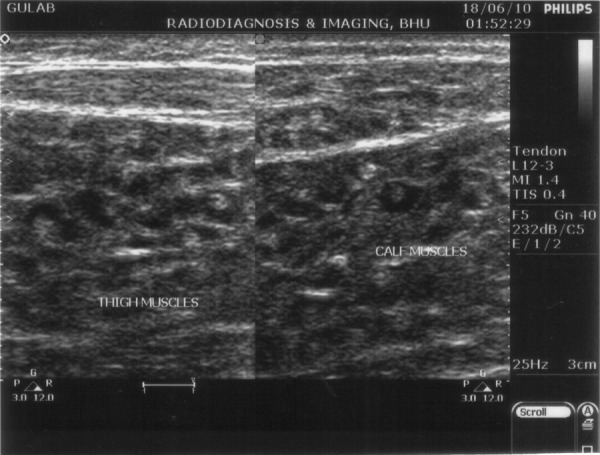
Ultrasonography of the skeletal muscles showing a honeycomb pattern of densely packed cysts.
Figure 4.
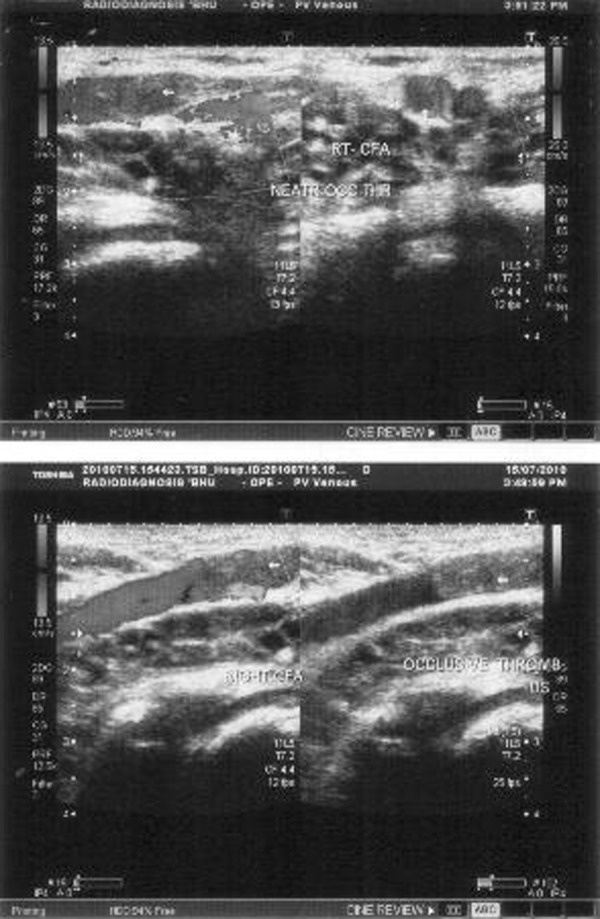
A Doppler flow study showing thrombosis of the right common femoral vein.
CT cranium showed normal morphology, but the orbit showed bilateral bulky extraocular muscles heterogeneous in their whole length (figure 5). MRI of the brain showed multiple cystic, densely packed subcentimeter lesions in the bilateral cerebral hemispheres, cerebellar hemispheres and brainstem, with few showing ring enhancement with no significant perilesional oedema, features suggestive of disseminated neurocysticercosis in vesicular stage ‘starry sky appearance’. MRI of the thigh muscle showed neurocysticerci embedded in muscle mass ‘leopard spots’ and the cardiac muscles showed multiple cystic lesions with increased muscle mass (figure 6A–D).
Figure 5.
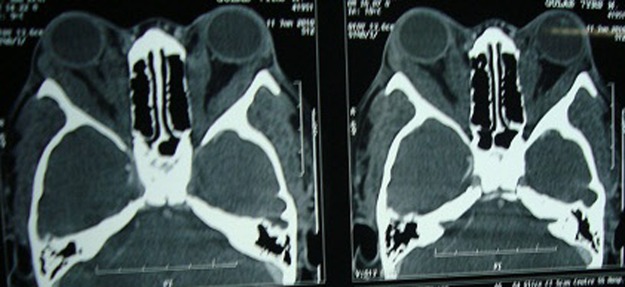
Cranial CT showing a bilaterally bulky medial and lateral rectus muscle.
Figure 6.
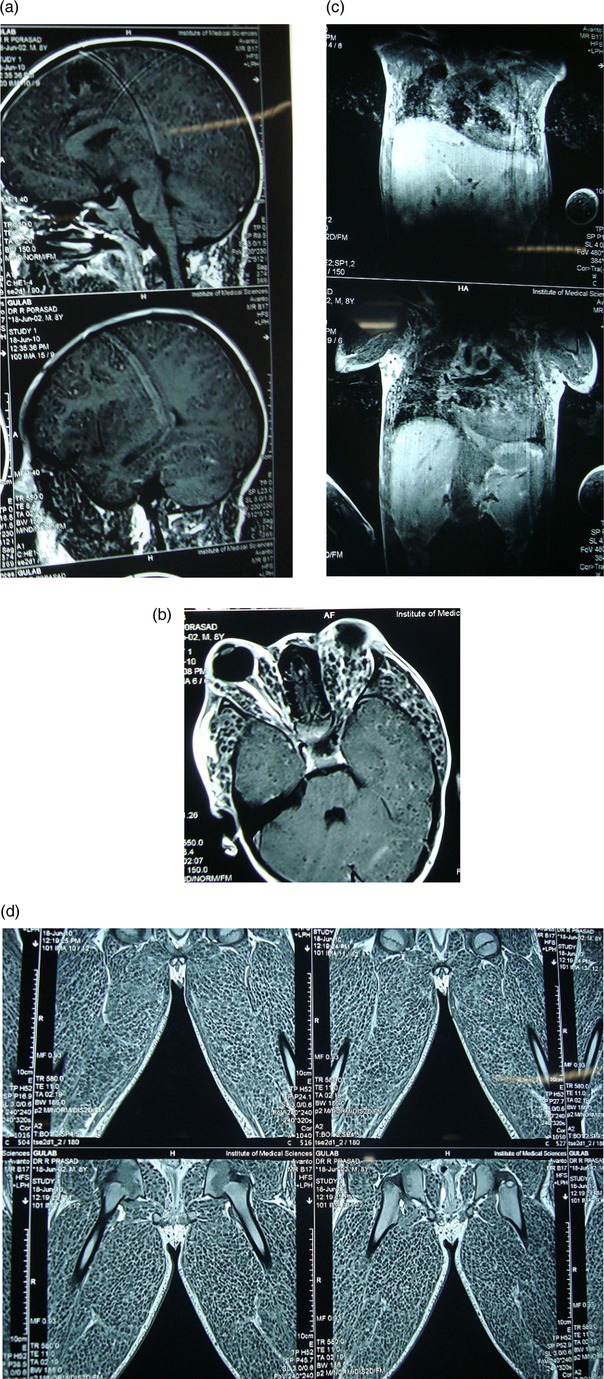
(A–D) Serial MRI showing multiple cystic, densely packed subcentimeter lesions in the brain, extraocular muscle, cardiac muscle and skeletal muscles of the face, neck, trunk, and upper and lower limbs.
Differential diagnosis
The differential diagnoses include acute myeloid leukaemia, neuroblastoma, muscular dystrophy, myotonia congenita, trichinosis, hypothyroidism, amyloidosis and glycogenesis of type I (Pompe disease). The normal peripheral blood smear examination, thyroid profile (T3, T4 and thyroid-stimulating hormone) and serum creatine kinase exclude the diagnoses of acute myeloid leukaemia, hypothyroidism and muscular dystrophy, respectively.
Treatment
The patient was treated with intravenous low–molecular-weight heparin for 7 days followed by warfarin and dexamethasone (0.5 mg/kg/day) for 3 days; thereafter, oral prednisolone 1 mg/kg/day and oral albendazole 15 mg/kg/day were started on day 3 of corticosteroids. On the third day of albendazole therapy, there was deterioration in consciousness and generalised tonic clonic seizure (cysticidal syndrome). The child was managed with intravenous 20% mannitol, anticonvulsants (lorazepam and phenytoin sodium) and other supportive measures. The child improved and was discharged on day 14 of admission. Albendazole and corticosteroids were continued up to 28 days. Warfarin and phenytoin sodium were also needed to be continued for 6 months.
Outcome and follow-up
There was a marked reduction in muscle bulk and recovery of normal vision in the left eye, and the patient is seizure-free at 1 and 3 months of follow-up. A repeat Doppler study at 3 months showed a resolution of the thrombus in the right common femoral vein with normal blood flow, but warfarin and phenytoin sodium were continued up to 6 months. At 3 months of follow-up, MRI of the cranium was also done and it showed complete resolution of the diffuse cystic lesions in the brain parenchyma and extraocular muscles. MRI of the extremities was not done because of financial constraints. At 6 months, the patient was symptom-free and doing well.
Discussion
Disseminated cysticercosis is characterised by pseudomuscular hypertrophy (100%), palpable subcutaneous nodules (87%), seizures (78%) and abnormal mentation (65%).6 Manifestations of disseminated cysticercosis depend on the organ system involved. CNS involvement can be parenchymal, affecting the brain, spinal cord or eye, or extraparenchymal, involving the ventricle or subarachnoid space or a combination of them. Ophthalmic cysticercosis may present with the involvement of cysts in the subretinal space, vitreous humour, subconjunctiva or anterior chamber.3 Orbital involvement can present with proptosis, signs of inflammation, extraocular muscle involvement,7 subconjunctival cysts, lid nodules and optic neuritis.8 Skeletal muscle involvement causes pseudohypertrophy of the affected muscles, which are generally non-tender. These must be differentiated from pseudohypertrophy, muscular dystrophy, myotonia congenita, trichinosis, hypothyroidism, amyloidosis and glycogenesis of type I (Pompe disease) in its juvenile form.9 Cardiac involvement with cysticercosis occurs in about 5% of cases of proven cysticercosis and is known to be asymptomatic.10 Our patient had involvements of the CNS, eye, subcutaneous tissue, skeletal muscles, heartand thrombosis of the right common femoral vein. Deep vein thrombosis in patients with disseminated cysticercosis has not been reported in the literature. The exact mechanism of thrombosis in disseminated cysticercosis is not known but may be due to the release of mediators of inflammation, that is, cytokines.
Clinical diagnosis of disseminated cysticercosis is often delayed due to vague presentation, variable serology results, unyielding physical examination in the majority of cases and the overall rarity of the condition.11 Imaging plays a central role in the diagnosis and management of disseminated cysticercosis. MRI is the most accurate technique to assess the degree of infection, location and the evolutionary stage of parasites.12 Extraparenchymal neurocysticercosis is associated with worse prognosis and needs aggressive management.2 In patients with massive infections (cysticercosis encephalitis), antiparasitic drugs should not be used because they may exacerbate inflammatory reaction in the brain parenchyma. However, there is no consensus on whether to use antiparasitic drugs in these patients after resolution of cerebral oedema.2 Experience in the use of albendazole with methylprednisolone for the treatment of retinal cysticercosis and as a presurgical treatment for intravitreal cysticercosis has been reported13 but not yet replicated.2 Owing to the increased morbidity and mortality associated with such extensive involvement without treatment, we used anticysticidal drugs. The patient responded well, though there was cysticidal syndrome on the third day. Such an extensive involvement of various organs like the heart, eyes, orbits, CNS and muscles is rare in children.
Disseminated cysticercosis with a right common femoral vein thrombosis may be missed initially because of vague presentation. This is to make medical practitioners aware of an uncommon presentation of a common disease. The early recognition and institution of therapy may be lifesaving.
Learning points.
Disseminated cysticercosis is rare in children.
Concomitant orbital, subcutaneous and cardiac involvement is extremely rare.
Cysticercosis as a cause of deep vein thrombosis is not reported in the literature.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.García HH, Del Brutto OH. The cysticercosis Working group in Peru neurocysticercosis: updated concepts about an old disease. Lancet Neurol 2005;4:653–61 [DOI] [PubMed] [Google Scholar]
- 2.García HH, Evans WCA, Nash TE, et al. Current consensus guidelines for treatment of neurocysticercosis. Clin Microbiol Rev 2002;15:747–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singhi P, Ray M, Singhi S, et al. Clinical spectrum of 500 children with neurocysticercosis and response to albendazole. J Child Neurol 2000;15:207–13 [DOI] [PubMed] [Google Scholar]
- 4.Bhalla A, Sood A, Sachdev A, et al. Disseminated cysticercosis: a case report and review of the literature. J Med Case Rep 2008;2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar A, Bhagwani DK, Sharma RK, et al. Disseminated cysticercosis. Indian Pediatr 1996;33:337–9 [PubMed] [Google Scholar]
- 6.Wadia N, Desai S, Bhatt M. Disseminated cysticercosis: new observations, including CT scan findings and experience with treatment by praziquantel. Brain 1988;111:597–614 [DOI] [PubMed] [Google Scholar]
- 7.Prasad R, Bagri N, Mishra OP, et al. Proptosis of eyeball in children with medial rectus cysticercosis: report of two cases. Eur J Ophthal 2010;20:240–2 [DOI] [PubMed] [Google Scholar]
- 8.Sandes AR, Mouzinho A, Valente P. Orbital cysticercosis diagnosis and treatment controversies. Pediatr Infect Dis J 2007;26:180–1 [DOI] [PubMed] [Google Scholar]
- 9.Lana-Piexoto MI, Lana-Piexoto MA, Campos G Belisario. Pseudohypertrophic myopathy caused by cysticercosis: report of a case. Arq Neuropsiquiatr 1985;43:396–402 [DOI] [PubMed] [Google Scholar]
- 10.Rabiela MT, Rivas A, Rodriguz J, et al. Anatomopathological aspects of human brain cysticercosis. In: Flisser A, Willms K, Laclette JP, Larraldle C, Ridaura C, Beltran F, eds. Cysticercosis: present state of knowledge and perspectives. New York: Academic Press, 1982:179–200 [Google Scholar]
- 11.Kumar A, Goenka AH, Choudhary A, et al. Disseminated cysticercosis in a child: whole-body MR diagnosis with the use of parallel imaging. Pediatr Radiol 2010;40:223–7 [DOI] [PubMed] [Google Scholar]
- 12.García HH, Gonzalez AE, Evans CA, et al. Cysticercosis Working Group in Peru. Taenia solium cysticercosis. Lancet 2003;362:547–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berche M, Hayot B, Mokrane M, et al. Ocular cysticercosis, typical forms and treatment. Ophthalmologie 1990;4:377–9 [PubMed] [Google Scholar]


