Abstract
This is the first case reporting the results of using an extract of Hypericum flowers (Hypericum perforatum) and neem oil (Azadirachta indica) in foot wounds with exposed bone in a patient with bilateral advanced diabetic ulcers. The effective use of this cheap treatment in patients with diabetic lesions on the feet, if confirmed in a wide controlled study, might allow the caregivers to take care of patients at home.
Background
Diabetes and its complications are becoming one of the major concerns for healthcare systems.1
As diabetic patients with lower extremity ulcers have a poorer clinical outcome and more difficult management, the home care of diabetic ulcers should allow a significant improvement in the management of diabetic complications.2
Case presentation
A man aged 72, with type 2 diabetes from 40 years, came to our angiological centre on 6 April 2011 with diabetic feet ulcers in both legs.
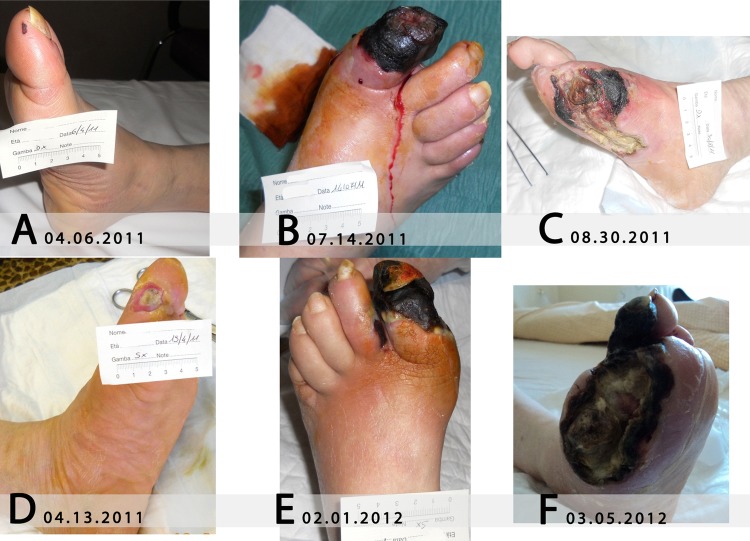
An ischaemic cutaneous lesion was observed on the right foot, at the top of the big toe, as a small ischaemic skin deficit (less than 0.5 cm large) (figure 1A).
Figure 1.
Ulcers evolved at the right foot (A–C) and the left foot (D–F) before the use of Holoil.
The patient declared that he had had a myocardial infarct in March 1997, followed by aortocoronary bypass in June 1999; he had a cardiac pacemaker implanted, since February 2007, for ischaemic dilated cardiomyopathy (ejection fraction (EF), 20%).
The patient was treated with statins (simvastatin 20 mg/die), diuretics (furosemide 25 mg/die and canrenone 50 mg/die), antihypertensives (carvedilol 12.5 mg/die and irbesartan 150 mg/die), cardiac glycosides (metildigoxin 0.1 mg/die), antiaggregant (ticlopidine hydrochloride 500 mg/die) and insulin. As the heart surgery procedure was performed in a different hospital, no further details about the patient's intrasurgical therapies are available to the author.
On 2 April 2011, when the last control was performed, glycated haemoglobin A1c (HbA1c) was 13.2%, fasting glycaemia was 166 mg/dl, and therapy for diabetes was 12 IU of rapid acting insulin three times a day and 10 IU of basal insulin at bedtime.
The patient was able to walk with claudicatio intermittens.
Investigations
During the assessment visit on 6 April 2011, echo-color Doppler showed a sub-genicolate arteriopathy to both the feet, which resulted more serious on the left foot from supine/depend transcutaneous oxygen and carbon dioxide partial pressure, performed according to Melillo et al3. Nevertheless, this exam highlighted microvascular parameters indicating a good metabolic compensation of the tissue on both the legs (table 1).
Table 1.
Microvascular parameters with supine/depend foot; trends through
| Date | Left foot (mm Hg [O2]) | Left foot (mm Hg [CO2]) | Right foot (mm Hg [O2]) | Right foot (mm Hg [CO2]) |
|---|---|---|---|---|
| 6 April 2011 | 48/53 | 37/36 | 50/60 | 37/35 |
| 3 August 2011 | 46/52 | 37/36 | 34/46 | 40/36 |
| 27 December 2011 | 34/43 | 40/36 | 51/63 | 37/35 |
| 1 February 2012 | 38/44 | 37/36 | 51/63 | 37/35 |
| 28 July 2012 | 45/53 | 37/36 | 49/59 | 37/35 |
In August 2011, anticoagulant therapy with warfarin (2 mg/die) was started because of cardiac uncompensation owing to the previously described cardiomyopathy and the presence of left cardiac intraventricular thrombosis. Microvascular parameters worsened at the right foot and were stable at the left foot (table 1); glycaemic control was rapidly improving (HbA1c=8.0%). In November 2011, a pacemaker was substituted because of various cardiac uncompensation episodes (EF: 20%). On 27 December 2011, the cutaneous metabolic parameters at the left foot showed supine hypoxia with tissue acidosis and positive postural recovery, whereas the tissue metabolic measurements were relatively stable at the right foot (table 1).
At the same time, glycaemic control was stable from previous control (HbA1c=7.6%) and anticoagulant therapy was stable and well monitored.
Right foot
The little ischaemic lesion at the right foot worsened dramatically in the next month, and a cyanotic tissue area was observed on 14 July 2011 (figure 1B). Therefore, a first amputation of the two distal phalanges of the big toe was needed. As the tissue was vital, the remaining part of the foot was covered using homologue tissue. Five days after amputation of the big toe, an extension of the lesion was observed, and thus, another intervention removing the distal part of the first metatarsal bone was performed. The necrosis continued to enlarge until the exposition of the first metatarsal bone occurred on 30 August 2011 (figure 1C). Because of this, all the metatarsal bones were removed in another surgical procedure which was carried out in September 2011, followed by further bone remodelling on 15 December 2011.
In February 2012, another surgical toilet was needed, with the implantation of homologue epidermal tissue, because of ulcer worsening.
Left foot
A worsening chronic skin ulcer on the left big toe (referred as appeared since 1 year in April 2010) had skin graft transplantation with homologue tissue, performed in our structure, on 7 April 2011. This was documented by a photo on 13 April 2011 (figure 1D), when it was about 1 cm large and 0.5 cm deep, with an exposed interphalanx ligament, even though the surgical procedure was performed a few days before.
The ulcer at the big toe of the left foot had initial previous treatment with a mixture of clostridiopeptidase and cloramfenicole (Iruxol 30G),4 applied daily in a different structure; the lesion continuously worsened until ligament exposure.
On 15 December 2011, another surgical toilet, with skin graft transplantation, was performed. In the next month, a wide acral sclerosis of the big toe, similar to the one observed a few months before at the right foot, occurred, which then extended to the second finger (figure 1E). The cause of this sclerosis was supposed to be a distal thrombosis, as documented by cutaneous metabolic parameters collected on 27 December 2011.
On 1 February 2012, both the first and second fingers of the left foot were cut and another transplantation of homologue tissue was performed. After this intervention, the necrosis extended to the third finger and to the amputation borders; the lesion was treated with patches of silver hydrofiber to reduce oedema and the possibility of having an infection. However, at this time, the microvascular parameters evaluation (table 1) showed a relatively stable cutaneous tissue oximetry and capnometry.
Both feet
On 5 March 2012, gangrene had affected the third left finger, and the cutaneous border necrosis exposed the first residual metatarsal of the big toe (figure 1F).
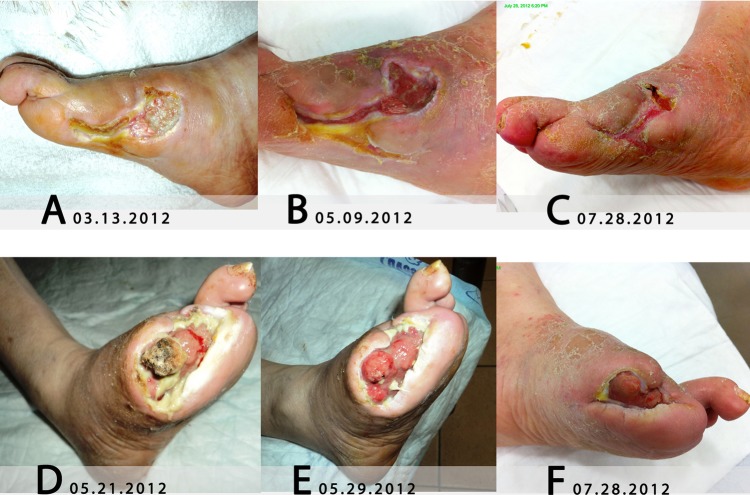
The lesion at the right foot showed adhered fibrin with hyperkeratotic borders and clear bone exposition on 13 March 2012 (figure 2A); these abnormalities indicate that the lesion clearly did not proceed to recovery.
Figure 2.
Ulcers evolved before Holoil treatment on the right foot (A), and after starting the use of Holoil at the right foot (B and C) and the left foot (D–F).
Thus, another surgical debridement and cutaneous transplantation of both lesions could be needed.
Treatment
Because of transportation difficulties of the patient, the relatives and the medical staff agreed to begin the use of Holoil gel to treat the left foot ulcer on 5 March 2012 and Holoil-garze for the right foot ulcer on 13 March 2012, instead of other surgical skin graft reimplantations.
Holoil gel is a mixture of Hypericum flower extract (Hypericum perforatum) and neem oil (Azadirachta indica) produced by RIMOS S.R.L. Mirandola (MO)—Italy (Medical Device Class IIB CE0476). The efficacy of this treatment on an ulcerated vascular leg was described in a previous report.5
The patient's relatives were instructed to clean the lesions with Holoil two times a week and to perform an appropriate bandage to protect the lesion from infections and dust.
Outcome and follow-up
In the next 4 months, the ulcers at both the feet continuously improved.
The right foot ulcer showed granulation tissue and wound healing epithelisation and reduced in size (figure 2B, C).
The left foot ulcer reduced in size and the granulation tissue continuously enlarged (figure 2D). The periosteal was reformed at the exposed metatarsal bone (figure 2E) and a continuous reformation of the cutaneous tissue on the lesion was observed (figure 2F).
During this period of the home wound care, the patient received a cleaning of the lesions with Holoil, only two times a week, performed by relatives without any other surgical or clinical intervention. There were no changes in any other treatment of the patient or significant improvements in glycaemic control (HbA1c was constantly between 7.6% and 7.7%) or clinically relevant changes observed in microvascular parameters (table 1).
Discussion
The use of H perforatum on depressed patients is well known and described.6H perforatum was also proposed as an anticancer drug that induces apoptosis in tumour cells7 8 and inhibits metastasis in vivo.9 An inhibitory effect of hyperforin on the neovascularisation of an experimental murine tumour model has also been suggested,4 as the in vivo inhibition of angiogenesis and the in vitro inhibition of several key steps of angiogenesis, including endothelial cell proliferation, differentiation and invasion, as well as an extracellular matrix degradation by matrix metalloproteinase-2 and urokinase.10
The anti-inflammatory effects of H perforatum extracts were recently demonstrated,11 thus providing the rationale for using these extracts in lower leg wounds, together with neem oil extract.12
Neem extracts have been used for centuries in traditional Indian medicine; its oil, obtained with cold extraction from its berries, is also included in the Ayurvedic Pharmacopoeia of India.13 Neem oil has shown to have cicatrising,14 bacteriostatic15 and anti-inflammatory properties. Its anti-inflammatory activity seems to be due to the presence of a limonoid (epoxyazadiradione) showing its effects on several inflammation markers of the macrophage migration inhibitory factors family.16
Holoil is a mixture of Hypericum and neem extracts: its original formulation could explain the observed effects of fibrin reduction and the improvement of granulation and cutaneous tissue. This is the first case where the positive use of Holoil, together with improved diabetes control, has clearly reversed the worsening of diabetic foot ulcers in a patient having severe diabetic and cardiovascular diseases. The use of Holoil was started when the patient had significantly improved his glycaemic control, and thus, we cannot evaluate if this improvement was due to the patient's improved glycaemic control or this medication or both. For sure, the patient's management had improved when the use of Holoil was started by his caregivers (relatives) at their home, thus avoiding the patient's travels to the hospital for continuously needed surgical toilets of the ulcers. Furthermore, the use of Holoil could have reduced the possibility of local reinfection, supporting the maintenance of diabetes control, as recurrent infections contribute to uncontrolled diabetes.
Learning points.
As both the feet showed an improvement in ulcer lesions when cleaning with Holoil was started, we will have to start its use earlier to understand how and when the use of Holoil could have reduced the worsening of diabetic feet ulcers.
For future patients attending our angiological centre, we suggest patients start using Holoil at home, as a starting treatment for diabetic foot lesions, so they better understand how its use can improve wound healing and thus improve their outcome.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Unwin N, Guariguata L, Whiting D, et al. Complementary approaches to estimation of the global burden of diabetes. Lancet 2012;379:1487–8 [DOI] [PubMed] [Google Scholar]
- 2.Kimball Z, Patil S, Mansour H, et al. Clinical outcomes of isolated lower extremity or foot burns in diabetic versus non-diabetic patients: a 10-year retrospective analysis. Burns 2012. (Epub ahead of print ) [DOI] [PubMed] [Google Scholar]
- 3.Melillo E, Iabichella L, Berchiolli R, et al. Transcutaneus oxygen and corbon dioxide during treatment of critical limb ischemia with iloprost, a prostacyclin derivative. Int J Microcirc 1995;15:60–4 [DOI] [PubMed] [Google Scholar]
- 4.http://www.mister-x.it/salute/foglietto_illustrativo.asp?medicinale=IRUXOL (accessed 21 July 2013).
- 5.Iabichella ML, Dominici P, Mosti G, et al. Mix oil: a new treatment for complicated vascular leg ulcers.Glasgow: EWMA, 02–04, maggio2007 [Google Scholar]
- 6.Greeson JM, Sanford B, Monti DA. St. John's wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology 2001;153:402–14 [DOI] [PubMed] [Google Scholar]
- 7.Schempp CM, Kirkin V, Simon-Haarhaus B, et al. Inhibition of tumour cell growth by hyperforin, a novel anticancer drug from St. John's wort that acts by induction of apoptosis. Oncogene 2002;21:1242–50 [DOI] [PubMed] [Google Scholar]
- 8.Hostanska K, Reichling J, Bommer S, et al. Hyperforin a constituent of St. John's wort (Hypericum perforatum L.) extract induces apoptosis by triggering activation of caspases and with hypericin synergistically exerts cytotoxicity towards human malignant cell lines. Eur J Pharm Biopharm 2003;56:121–32 [DOI] [PubMed] [Google Scholar]
- 9.Dona M, Dell'Aica I, Pezzato E, et al. Hyperforin inhibits cancer invasion and metastasis. Cancer Res 2004;64:6225–32 [DOI] [PubMed] [Google Scholar]
- 10.Martınez-Poveda B, Quesada AR, Medina MA. Hyperforin, a bio-active compound of St. John's Wort, is a new inhibitor of angiogenesis targeting several key steps of the process. Int J Cancer 2005;117:775–80 [DOI] [PubMed] [Google Scholar]
- 11.Koeberle A, Rossi A, Bauer J, et al. Hyperforin, an anti-inflammatory constituent from St. john's wort, inhibits microsomal prostaglandin E(2) synthase-1 and suppresses prostaglandin E(2) formation in vivo. Front Pharmacol 2011;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Läuchli S, Hafner J, Wehrmann C, et al. Post-surgical scalp wounds with exposed bone treated with a plant-derived wound therapeutic. J Wound Care 2012;21:228–33 [DOI] [PubMed] [Google Scholar]
- 13. The Ayurvedic Pharmacopoeia of India, part-I, vol V, 119.
- 14.Dos Santos ACG, Rodrigues OG, De Araujo LVC, et al. Use of neem extract in the control of acariasis by Myobia musculi Schranck (Acari: Miobidae) and Myocoptes musculinus Koch (Acari: Listrophoridae) in mice (Mus musculus var. albina L.). Neotrop Entomol 2006;35:269–72 [PubMed] [Google Scholar]
- 15.Narayanan AS, Raja SSS, Ponmurugan K, et al. Antibacterial activity of selected medicinal plants against multiple antibiotic resistant uropathogens: a study from Kolli Hills, Tamil Nadu, India. Beneficial Microbes 2011;2:235–43 [DOI] [PubMed] [Google Scholar]
- 16.Athar A, Saikat H, Hirekodathakallu T, et al. Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor. J Biol Chem 2012;287:24844–61 [DOI] [PMC free article] [PubMed] [Google Scholar]