Abstract
Although rare, pseudomembranous colitis may be a cause of perineal necrotising fasciitis in a context of immunosuppression, as in the case we report. This origin must be quickly identified because the therapeutic management, especially surgery, is unlikely to be the same as usual. Similarly, antibiotic treatment is also a matter of discussion due to the potential deleterious role of antibiotics in pseudomembranous colitis.
Background
Treatment of perineal necrotising fasciitis usually associates surgery, antibiotics and supportive measures. In the rare case we report here, the perineal necrotising fasciitis found its origin in pseudomembranous colitis. This led us to criticise our usual management.
Case presentation
A 63-year-old woman treated for sideroblastic anaemia with a pyrimidine analogue (antimetabolite), lenograstim and repeated red blood cell transfusions, received a 7-day course of β-lactam antibiotics for pharyngitis. Three weeks later, her condition deteriorated, with fever and diarrhoea, followed a week later by areas of skin necrosis on the labia majora and the left buttock, suggestive of perineal necrotising fasciitis. CT scan of the abdomen and pelvis showed pancolitis and extensive perineal fasciitis with gas in the labia majora reaching to the left buttock (figure 1). Surgical treatment consisted of sigmoid colostomy and large debridement of the vulva, perineum and left thigh. During surgery, the patient went into septic shock. The next day, the necrotising lesions had spread, requiring further surgery to achieve a new level of debridement in the left buttock and drainage with corrugated sheet drains. Intraoperative perineal bacteriological samples found Gram-positive and Gram-negative bacilli. She was then transferred to our intensive care unit (ICU) for further treatment. On arrival in our ICU, the patient was intubated, ventilated, sedated and was receiving norepinephrine for haemodynamic instability. The abdomen was distended without guarding or rigidity. Laboratory tests showed hypoalbuminaemia at 22 g/l, inflammatory syndrome, pancytopenia and acute renal failure. Septic shock was treated as recommended by the Surviving Sepsis Campaign. The patient received piperacillin/tazobactam and amikacin. Associated treatments included fluconazole, lenograstim and hyperbaric oxygen therapy. We then looked for an origin of this perineal necrotising fasciitis. CT scan already carried out showed pancolitis, initial stool cultures identified Clostridium difficile with A and B toxins and colonoscopy through the colostomy found an erythematous mucosa with pseudomembranes. The biopsies revealed acute colitis without specific histopathological data. Cytomegalovirus polymerase chain reaction was negative on colon biopsies. All viral serology performed were negative: Hepatitis B and C, Human immunodeficiency virus, Herpes simplex virus, Varicella-zoster virus, Cytomegalovirus, Epstein barr virus, Parvovirus B19 and Human Herpes virus 6. No specific enteropathogens (Yersinia, Campylobacter, Salmonella and Shigella) were detected in the stools. We concluded that the origin of the perineal necrotising fasciitis was the pseudomembranous colitis which increased intestinal wall permeability allowing a passage of bacteria from intestinal lumen to neighbouring soft tissue. This explains the polymicrobial infectious flora as usually found in perineal necrotising fasciitis. Thus, we add vancomycin orally and as enema in the colostomy for treat the origin of the perineal necrotising fasciitis. Respiratory and haemodynamic parameters rapidly improved and the patient was weaned from norepinephrine and extubated. In addition, renal function gradually improved. However, abundant diarrhoea persisted. The stool cultures performed after initiation of treatment were sterile. CT scan performed on day 21 found an unchanged aspect of colitis with persistent diffuse thickening of the colon wall and increased ascites (figure 2). In the absence of improvement in pancolitis observed on CT scan, it was decided to complete the treatment of pseudomembranous colitis with metronidazole in combination with vancomycin. Locally, the lesions healed satisfactorily but significant removal of necrotic tissues and fibrin was required during daily dressings (figure 3). Pancytopenia worsened, in the context of the severe haematological disease with a poor prognosis. Repeated transfusions of red blood cells and platelets were required, with continued treatment with lenograstim. General and local improvement allowed the patient to be discharged from the ICU on day 35. However, long-term outcome was unfavourable with persisting pancytopenia, and recurring colitis on cessation of antibiotics and the patient died of haematological failure on day 45.
Figure 1.
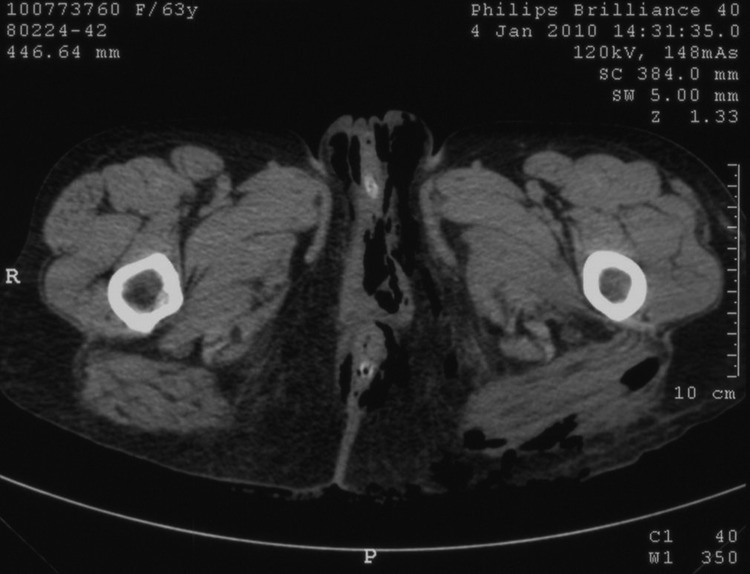
CT scan of pelvis performed on admission showed extensive perineal fasciitis.
Figure 2.
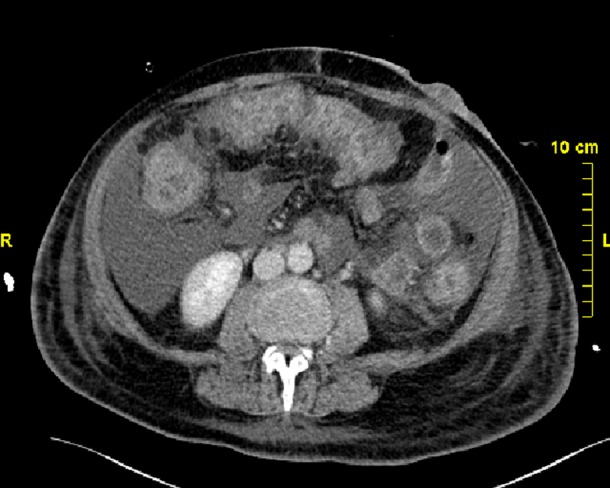
CT scan performed on day 21 found an unchanged aspect of colitis.
Figure 3.
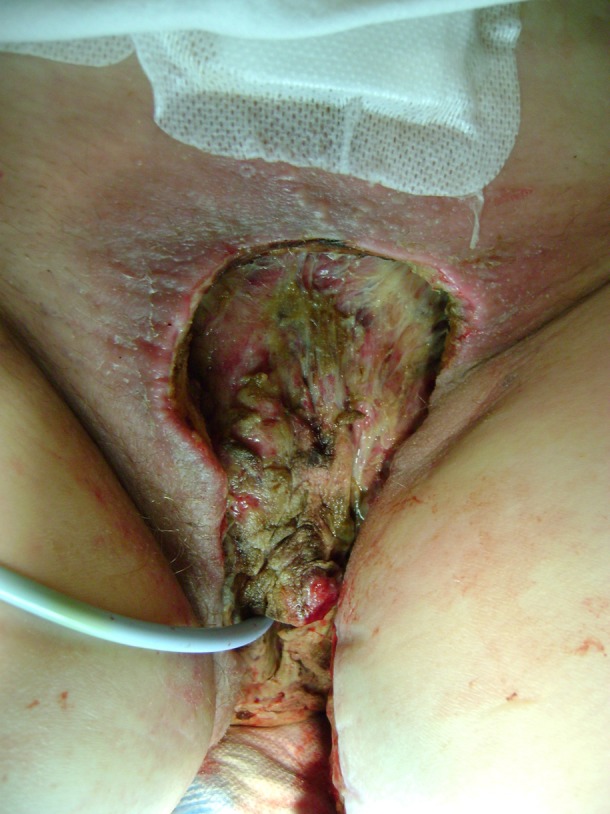
Local aspect on day 21.
Discussion
Necrotising fasciitis associated with C difficile pseudomembranous colitis has never been reported in the literature. The suspected pathophysiological mechanisms of this unusual cause of perineal necrotising fasciitis are bacterial translocation or microperforation secondary to pseudomembranous colitis. The infection then progresses as a spreading inflammatory reaction resulting in obliterative endarteritis causing cutaneous and subcutaneous vascular thrombosis and necrosis of skin and subcutaneous tissues. Tissue necrosis is secondary to local ischaemia and to pathogenic action of bacteria. In turn, tissue ischaemia favours bacterial growth. In the case we report the cause of necrotising fasciitis was definitely pseudomembranous colitis. However, C difficile was never identified on the successive sets of blood and tissue cultures. C difficile is a non-invasive bacterium and rarely induces systemic and serious forms of soft tissue infection unlike other species of clostridium.1 2 Thus, we may conclude C difficile is not the pathogen responsible of the necrotising fasciitis but most probably, the intestinal wall alterations induced by the pseudomenbranous colitis allow translocation of pathogen intestinal bacteria which cause the fasciitis.
In our case, the initial evolution was favourable with renal improvement and weaning of vasopressors. Perineal necrotising fasciitis was stabilised and a third surgical procedure was not necessary. However, we observed a resistance to antibiotic treatment of C difficile pseudomembranous colitis in this context of immunosuppression.
Apart from initial antibiotic therapy and hospitalisation, immune deficiency is identified as a risk factor for C difficile colitis and for increased severity of infection.3 4 Our treatment was based on surgery, β-lactam antibiotics (with a broad anti-anaerobic spectrum combined with an aminoglycoside as it is now well established in the literature)5 and hyperbaric oxygen therapy.5–9
The resistance of pseudomembranous colitis in our case report raises three points: With hindsight, the surgical management may have been inadequate. The colostomy may have been inappropriate in this context of pseudomembranous colitis. Because of the impossibility of controlling colitis, it might have been preferable to perform an ileostomy with or without a total colectomy. The role of surgery remains unclear in the treatment of C difficile colitis. It is indicated in multiple organ failure secondary to severe colitis, in perforation, in peritonitis or in toxic megacolon.10 The term fulminant colitis defines severe acute colitis with signs of systemic toxicity such as ulcerative colitis.11 Fulminant colitis develops in up to 8% of infections and is associated with a high risk of mortality.12 It is differentiated from severe acute colitis by rate of onset and rapid clinical deterioration. Our patient had immunosuppression (independent risk factor of fulminant colitis).13–15 Leukocytosis, immunosuppression and the existence of shock are predictive of death during surgery.16 This is why we did not recommend surgery for our patient. In addition, when surgery is indicated, it must be achieved as early as possible.13 16 Surgery is indicated in patients with a history of inflammatory bowel disease, treated with intravenous immunoglobulins or recent surgery and in patients with colitis associated with signs of organ dysfunction (use of vasopressors, increased lactate, leukocytosis above 16×109/l).17 The intervention of choice is subtotal colectomy with terminal ileostomy which has lower morbidity and mortality compared with conservative procedures. The current level of evidence for surgical treatment of C difficile colitis remains low due to a lack of prospective randomised trials. In our case report surgery was differed because of immunosuppression. However, progression to severe refractory colitis should have led us to reconsider surgical management.
We chose to treat C difficile colitis with vancomycin because of the seriousness of the clinical presentation and the emergence of resistant strains of C difficile to metronidazole. Since 2000 failure of treatment with metronidazole has been reported with increasing frequency (18.2%),18 19 due to the presence in hospitals of more and more elderly patients with multiple diseases, increased use of wide-spectrum antibiotics and the possible emergence of more virulent or resistant strains of C difficile. We considered the possibility of metronidazole resistance in our patient who had been frequently hospitalised (for repeated transfusions). In severe infections, vancomycin was significantly more effective than metronidazole.20 Current recommendations favour the use of vancomycin as first-line treatment of severe C difficile infections.21 22 An earlier resolution of symptoms and a significantly lower risk of treatment failure have been observed with vancomycin.23 The combination of intravenous metronidazole and oral vancomycin seems justified because the existence of an ileus may hinder the action of oral antibiotics.24–27 After an initial improvement in the patient's condition, including the cessation of vasopressors, we had to resort to metronidazole in combination with oral vancomycin and enema because of the persistence of pancolitis observed on CT scan and before feeding. Treatment with piperacillin/tazobactam may also explain aggravation of this pseudomembranous colitis. Our patient was hospitalised before possible access to fidaxomicin, a new macrocyclic antibiotic that has shown efficacy in recurrent C difficile infections. This antibiotic will be available soon, questioning the antibiotic therapy that we used.
Finally, we discussed the use of treatment with immunoglobulin concentrates. This treatment was not administered because of the favourable evolution with initial resolution of septic shock, extubation and recovery of normal renal function. It is thought that C difficile colitis, severe and refractory to conventional treatments, may benefit from the injection of concentrated immunoglobulins.3 4 25–30 This additional treatment remains controversial up to now. Immunoglobulin therapy can be responsible for potential side effects: kidney failure and poor glycemic control in general; and headache, hypertension, digestive disorders specifically for human monoclonal antibody to C difficile toxin A.31 Lowy et al29 showed a significant reduction in recurrence of infections with C difficile treated with monoclonal antibodies against toxins A and B versus placebo.29 However, this treatment did not reduce the time to resolution of diarrhoea, the duration of initial hospitalisation or the severity of the initial episode. The data from this study seem difficult to extrapolate to our case, because our patient had two exclusion criteria (surgery in the last 24 h, and existence of a life-threatening underlying disease).
Conclusion
In a context of immunosuppression, pseudomembranous colitis may be responsible for necrotising fasciitis not as a C difficile infection but by weakening the intestinal barrier. This condition must be recognised quickly because the surgery is unlikely to be the same. Similarly, choice of antibiotic treatment should also be discussed because of the role of penicillin in the resistance to treatment of pseudomembranous colitis.
Learning points.
Immunosuppression severely complicates pseudomembranous colitis in perineal necrotising fasciitis.
Pseudomembranous colitis as origin of perineal necrotising fasciitis has to be identified because surgery is unlikely to be the same as usual.
Antibiotic treatment of perineal necrotising fasciitis have to be discussed because the potential role of penicillin in the resistance to treatment of pseudomembranous colitis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Libby DB, Bearman G. Bacteremia due to Clostridium difficile—review of the literature. Int J Infect Dis 2009;13:e305–9 [DOI] [PubMed] [Google Scholar]
- 2.Ray P, Das A, Singh K, et al. Clostridium tertium in necrotizing fasciitis and gangrene. Emerg Infect Dis 2003;9:1347–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyne L, Warny M, Qamar A, et al. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N Engl J Med 2000;342:390–7 [DOI] [PubMed] [Google Scholar]
- 4.Warny M, Vaerman JP, Avesani V, et al. Human antibody response to Clostridium difficile toxin A in relation to clinical course of infection. Infect Immun 1994;62:384–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green RJ, Dafoe DC, Raffin TA. Necrotizing fasciitis. Chest 1996;110:219–29 [DOI] [PubMed] [Google Scholar]
- 6.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis 2007;44:705–10 [DOI] [PubMed] [Google Scholar]
- 7.Edlich RF, Cross CL, Dahlstrom JJ, et al. Modern concepts of the diagnosis and treatment of necrotizing fasciitis. J Emerg Med 2010;39:261–5 [DOI] [PubMed] [Google Scholar]
- 8.Mathieu D. Hyperbaric oxygen for the treatment of necrotizing fasciitis. Ann Dermatol Venereol 2001;128:411–18 [PubMed] [Google Scholar]
- 9.Mathieu D, Nevière R, Lefebvre-Lebleu N, et al. Anaerobic infections of the soft tissues. Ann Chir 1997;51:272–87 [PubMed] [Google Scholar]
- 10.Kelly CP, LaMont JT. Clostridium difficile—more difficult than ever. N Engl J Med 2008;359:1932–40 [DOI] [PubMed] [Google Scholar]
- 11.Stange EF, Travis SPL, Vermeire S, et al. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis 2008;2:1–23 [DOI] [PubMed] [Google Scholar]
- 12.Adams SD, Mercer DW. Fulminant Clostridium difficile colitis. Curr Opin Crit Care 2007;13:450–5 [DOI] [PubMed] [Google Scholar]
- 13.Ali SO, Welch JP, Dring RJ. Early surgical intervention for fulminant pseudomembranous colitis. Am Surg 2008;74:20–6 [DOI] [PubMed] [Google Scholar]
- 14.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg 2002;235:363–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greenstein AJ, Byrn JC, Zhang LP, et al. Risk factors for the development of fulminant Clostridium difficile colitis. Surgery 2008;143:623–9 [DOI] [PubMed] [Google Scholar]
- 16.Lamontagne F, Labbé A-C, Haeck O, et al. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg 2007;245:267–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Butala P, Divino CM. Surgical aspects of fulminant Clostridium difficile colitis. Am J Surg 2010;200:131–5 [DOI] [PubMed] [Google Scholar]
- 18.Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 2005;40:1586–90 [DOI] [PubMed] [Google Scholar]
- 19.Pepin J, Alary M-E, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis 2005;40:1591–7 [DOI] [PubMed] [Google Scholar]
- 20.Zar FA, Bakkanagari SR, Moorthi KMLST, et al. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile—associated diarrhea, stratified by disease severity. Clin Infect Dis 2007;45:302–7 [DOI] [PubMed] [Google Scholar]
- 21.Gerding DN, Johnson S, Peterson LR, et al. Clostridium difficile—associated diarrhea and colitis. Infect Control Hosp Epidemiol 1995;16:459–77 [DOI] [PubMed] [Google Scholar]
- 22.Cocanour CS. Best strategies in recurrent or persistent Clostridium difficile infection. Surg Infect (Larchmt) 2011;12:235–9 [DOI] [PubMed] [Google Scholar]
- 23.Cianci P. Consensus development conference on diabetic foot wound care: a randomized controlled trial does exist supporting use of adjunctive hyperbaric oxygen therapy. Diabetes care 2000;23:873–4 [DOI] [PubMed] [Google Scholar]
- 24.Apisarnthanarak A, Razavi B, Mundy LM. Adjunctive intracolonic vancomycin for severe Clostridium difficile colitis: case series and review of the literature. Clin Infect Dis 2002;35:690–6 [DOI] [PubMed] [Google Scholar]
- 25.Kelly CP, Pothoulakis C, LaMont JT. Clostridium difficile colitis. N Engl J Med 1994;330:257–62 [DOI] [PubMed] [Google Scholar]
- 26.Levecq H, Cerf M. Antibiotic associated diarrhea. Ann Gastroenterol Hepatol (Paris) 1990;26:147–56 [PubMed] [Google Scholar]
- 27.Mohammedi I, Gachot B, Souweine B, et al. Severe forms of pseudomembranous colitis caused by Clostridium difficile. Ann Fr Anesth Reanim 1995;14:362–5 [DOI] [PubMed] [Google Scholar]
- 28.Babcock GJ, Broering TJ, Hernandez HJ, et al. Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile—induced mortality in hamsters. Infect Immun 2006;74:6339–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowy I, Molrine DC, Leav BA, et al. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010;362:197–205 [DOI] [PubMed] [Google Scholar]
- 30.Salcedo J, Keates S, Pothoulakis C, et al. Intravenous immunoglobulin therapy for severe Clostridium difficile colitis. Gut 1997;41:366–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor CP, Tummala S, Molrine D, et al. Open-label, dose escalation phase I study in healthy volunteers to evaluate the safety and pharmacokinetics of a human monoclonal antibody to Clostridium difficile toxin A. Vaccine 2008;26:3404–9 [DOI] [PMC free article] [PubMed] [Google Scholar]


