Abstract
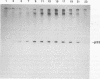
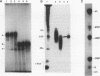
We report here the isolation and identification of the RNA specifically immunoprecipitated and covalently linked to the tumor suppressor gene product p53. After treatment with proteinase K, the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) band of p53 yields a single, discrete 157-nucleotide RNA, which was cloned, sequenced, and identified as 5.8S rRNA. 5.8S rRNA was obtained only after proteolysis of the p53 SDS-PAGE band. Free 5.8S rRNA did not comigrate with p53 in SDS-PAGE. This RNA was only immunoprecipitated from cells containing p53. Protein-free RNA obtained by proteolysis of the p53 band hybridized to the single-stranded DNA vector containing the antisense sequence of 5.8S rRNA. The covalence of the p53-5.8S rRNA linkage was demonstrated by the following findings: (i) p53 and the linked 5.8S rRNA comigrated in SDS-PAGE; (ii) only after treatment of the p53-RNA complex with proteinase K did the 5.8S rRNA migrate differently from p53-linked 5.8S rRNA; and (iii) this isolated RNA was found linked to phosphoserine, presumably at the 5' end. Covalent linkage to the single, specific RNA suggests that p53 may be involved in regulating the expression or function of 5.8S rRNA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bargonetti J., Friedman P. N., Kern S. E., Vogelstein B., Prives C. Wild-type but not mutant p53 immunopurified proteins bind to sequences adjacent to the SV40 origin of replication. Cell. 1991 Jun 14;65(6):1083–1091. doi: 10.1016/0092-8674(91)90560-l. [DOI] [PubMed] [Google Scholar]
- Bhat G. J., Lodes M. J., Myler P. J., Stuart K. D. A simple method for cloning blunt ended DNA fragments. Nucleic Acids Res. 1991 Jan 25;19(2):398–398. doi: 10.1093/nar/19.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J. DNA is linked to the rat liver DNA nicking-closing enzyme by a phosphodiester bond to tyrosine. J Biol Chem. 1981 May 25;256(10):4805–4809. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Eliyahu S., Pinhasi-Kimhi O., Oren M. Wild-type p53 can inhibit oncogene-mediated focus formation. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8763–8767. doi: 10.1073/pnas.86.22.8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer G., Bargonetti J., Zhu H., Friedman P., Prywes R., Prives C. Wild-type p53 activates transcription in vitro. Nature. 1992 Jul 2;358(6381):83–86. doi: 10.1038/358083a0. [DOI] [PubMed] [Google Scholar]
- Fields S., Jang S. K. Presence of a potent transcription activating sequence in the p53 protein. Science. 1990 Aug 31;249(4972):1046–1049. doi: 10.1126/science.2144363. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon J. V., Greaves R., Iggo R., Lane D. P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990 May;9(5):1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg D., Mechta F., Yaniv M., Oren M. Wild-type p53 can down-modulate the activity of various promoters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9979–9983. doi: 10.1073/pnas.88.22.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guddat U., Bakken A. H., Pieler T. Protein-mediated nuclear export of RNA: 5S rRNA containing small RNPs in xenopus oocytes. Cell. 1990 Feb 23;60(4):619–628. doi: 10.1016/0092-8674(90)90665-2. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Kinzler K. W., Bruskin A., Jarosz D., Friedman P., Prives C., Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708–1711. doi: 10.1126/science.2047879. [DOI] [PubMed] [Google Scholar]
- Kraiss S., Quaiser A., Oren M., Montenarh M. Oligomerization of oncoprotein p53. J Virol. 1988 Dec;62(12):4737–4744. doi: 10.1128/jvi.62.12.4737-4744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S. L., Cook D. M., Morris J. F., Gashler A., Sukhatme V. P., Rauscher F. J., 3rd Transcriptional repression mediated by the WT1 Wilms tumor gene product. Science. 1991 Sep 27;253(5027):1550–1553. doi: 10.1126/science.1654597. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Eckhart W. Mutation of the serine 312 phosphorylation site does not alter the ability of mouse p53 to inhibit simian virus 40 DNA replication in vivo. J Virol. 1990 Apr;64(4):1734–1744. doi: 10.1128/jvi.64.4.1734-1744.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek D. W., Eckhart W. Phosphorylation of p53 in normal and simian virus 40-transformed NIH 3T3 cells. Mol Cell Biol. 1988 Jan;8(1):461–465. doi: 10.1128/mcb.8.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990 Aug 24;62(4):671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Milner J., Medcalf E. A. Cotranslation of activated mutant p53 with wild type drives the wild-type p53 protein into the mutant conformation. Cell. 1991 May 31;65(5):765–774. doi: 10.1016/0092-8674(91)90384-b. [DOI] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Pene J. J., Knight E., Jr, Darnell J. E., Jr Characterization of a new low molecular weight RNA in HeLa cell ribosomes. J Mol Biol. 1968 May 14;33(3):609–623. doi: 10.1016/0022-2836(68)90309-4. [DOI] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Raycroft L., Wu H. Y., Lozano G. Transcriptional activation by wild-type but not transforming mutants of the p53 anti-oncogene. Science. 1990 Aug 31;249(4972):1049–1051. doi: 10.1126/science.2144364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Roth M. J., Brown D. R., Hurwitz J. Analysis of bacteriophage phi X174 gene A protein-mediated termination and reinitiation of phi X DNA synthesis. II. Structural characterization of the covalent phi X A protein-DNA complex. J Biol Chem. 1984 Aug 25;259(16):10556–10568. [PubMed] [Google Scholar]
- Samad A., Anderson C. W., Carroll R. B. Mapping of phosphomonoester and apparent phosphodiester bonds of the oncogene product p53 from simian virus 40-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):897–901. doi: 10.1073/pnas.83.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad A., Carroll R. B. The tumor suppressor p53 is bound to RNA by a stable covalent linkage. Mol Cell Biol. 1991 Mar;11(3):1598–1606. doi: 10.1128/mcb.11.3.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino R., Gerbi S. A. In vivo disruption of Xenopus U3 snRNA affects ribosomal RNA processing. EMBO J. 1990 Jul;9(7):2299–2308. doi: 10.1002/j.1460-2075.1990.tb07401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieg F. I., Simmons D. T. Characterization of the in vitro interaction between SV40 T antigen and p53: mapping the p53 binding site. Virology. 1988 May;164(1):132–140. doi: 10.1016/0042-6822(88)90628-9. [DOI] [PubMed] [Google Scholar]
- Shaulsky G., Goldfinger N., Ben-Ze'ev A., Rotter V. Nuclear accumulation of p53 protein is mediated by several nuclear localization signals and plays a role in tumorigenesis. Mol Cell Biol. 1990 Dec;10(12):6565–6577. doi: 10.1128/mcb.10.12.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soussi T., Caron de Fromentel C., Stürzbecher H. W., Ullrich S., Jenkins J., May P. Evolutionary conservation of the biochemical properties of p53: specific interaction of Xenopus laevis p53 with simian virus 40 large T antigen and mammalian heat shock proteins 70. J Virol. 1989 Sep;63(9):3894–3901. doi: 10.1128/jvi.63.9.3894-3901.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbrich N., Lin A., Wool I. G. Identification by affinity chromatography of the eukaryotic ribosomal proteins that bind to 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1979 Sep 10;254(17):8641–8645. [PubMed] [Google Scholar]
- Wade-Evans A., Jenkins J. R. Precise epitope mapping of the murine transformation-associated protein, p53. EMBO J. 1985 Mar;4(3):699–706. doi: 10.1002/j.1460-2075.1985.tb03686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K., Elela S. A., Nazar R. N. Inhibition of protein synthesis by anti-5.8 S rRNA oligodeoxyribonucleotides. J Biol Chem. 1990 Feb 15;265(5):2428–2430. [PubMed] [Google Scholar]
- Wolf D., Rotter V. Major deletions in the gene encoding the p53 tumor antigen cause lack of p53 expression in HL-60 cells. Proc Natl Acad Sci U S A. 1985 Feb;82(3):790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Gannon J. V., Lane D. P. Monoclonal antibody analysis of p53 expression in normal and transformed cells. J Virol. 1986 Aug;59(2):444–452. doi: 10.1128/jvi.59.2.444-452.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonish-Rouach E., Resnitzky D., Lotem J., Sachs L., Kimchi A., Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature. 1991 Jul 25;352(6333):345–347. doi: 10.1038/352345a0. [DOI] [PubMed] [Google Scholar]