Abstract
Malignant serous cystic neoplasms (SCN) of the pancreas are exceptionally rare, and only a few cases have been reported. As a result, SCN have been unanimously classified as benign tumours. Contrary to this conviction, in 1989, George et al published the very first case of a patient found to have a malignant pancreatic SCN. Up to the time of the submission of this paper, 27 cases of serous cystoadenocarcinomas have been published. In all the previously published cases of malignant SCN, the correct diagnosis was made postoperatively or at the time of autopsy. The authors present a case of a 68-year-old patient who was incidentally found to have a large liver mass on transthoracic echocardiogram ordered for suspected coronary artery insufficiency. Subsequent investigations revealed an additional large mass in the pancreas and percutaneous biopsies of both lesions revealed histological features consistent with malignant SCN metastasised to the left hepatic lobe.
Background
Cystic lesions of the pancreas are heterogeneous in morphology and prognosis. During the last decade, several classifications have been proposed.
Radiological classification consists of four groups: (1) unilocular, (2) microcystic, (3) macrocystic and (4) cystic lesions with a solid component (table 1).1 2
Table 1.
Morphological and anatomical classification of cystic lesion of the pancreas
| Morphology | Radiological characteristics | Differential diagnosis | Features |
|---|---|---|---|
| Unilocular cysts | No septations or solid component | Pancreatic pseudocyst | Associated with pancreatitis, located everywhere in the pancreas, occur at any age and equally in both sexes |
| Intraductal papillary mucinous neoplasm(IPMN) | Can involve the main pancreatic duct as well as the secondary ducts. Mean age at presentation: 40 years and men are more often affected than women | ||
| Mucinous cystoadenoma | Usually located in the body and tail of the pancreas, mean age at presentation: 50 years and women are more often affected than men | ||
| Microcystic lesions | Collection of small cysts | Serous cystoadenoma | Presence of a central scar with calcifications, commonly located in the pancreatic head, smooth lobulated contour. Mean age at presentation: 60 years and women are more often affected than men |
| Serous cystoadenocarcinoma | Extremely rare condition. Radiological features similar to serous cystoadenoma | ||
| Macrocystic lesions | Cysts with fewer compartments, measuring at least 2 cm in size | Mucynous cystadeonoma | Usually located in the body and tail of the pancreas, mean age at presentation: 50 years and women are more often affected than men |
| Cysts with solid components | Solid and cystic lesions | IPMN | Can involve the main pancreatic duct as well as the secondary ducts. Mean age at presentation: 40 years and men are more often affected than women |
| Mucinous cystadenoma | Usually located in the body and tail of the pancreas, mean age at presentation: 50 years and women are more often affected than men | ||
| Mucinous cystadenocarcinoma | Presence of nodular and irregular contour | ||
| Solid neoplasms with cystic degeneration such as adenocarcinomas or islet cell tumours | Neuroendocrine tumours: hypervascular lesions with cystic degeneration. Adenocarcinoma: hypointense lesions with cystic degeneration | ||
| Solid and papillary epithelial neoplasm | Presence of solid and cystic lesions of the pancreas with hypointense characteristics. Mean age at presentation: 35 years and women are more often affected than men |
The most common cystic lesions of the pancreas are pseudocysts due to pancreatitis. The other cysts listed by decreasing order of frequency are (1) serous cystoadenomas and (2) mucin-rich cysts (intraductal papillary mucinous neoplasms (IPMN), mucinous cystoadenomas or cystoadenocarcinomas and solid pseudopapillary carcinomas). Pancreatic adenocarcinomas and neuroendocrine tumours with cystic degeneration are quite rare.
Among all the pancreatic cystic lesions, neoplasms represent only 10– 15% of cases.3 The majority of these neoplastic lesions are benign.3
In 1978, Compagno and Oertel classified neoplastic cysts of the pancreas into two different groups, serous or mucinous, according to their pathological features and their biological behaviours.4 This classification is still largely used. Serous neoplastic cysts are considered to have extremely low malignant potential, whereas mucinous cysts have well recognised risk of malignant transformation. Malignant mucinous cysts originate from the transformation of MCNs or IPMN, and the World Health Organization classifies them under tumours of the exocrine pancreas.5 Malignant neoplastic cysts of the pancreas represent only 1% of the total cases of pancreatic carcinomas.6
On the other hand, malignant transformation of SCN is extremely rare and usually they are diagnosed only after resection. In fact, preoperative radiological characteristics of malignant SCN are indistinguishable from benign diseases unless there is presence of metastatic disease.1 Cytology does not play any role in the diagnosis of malignant SCN. On the other hand, histology from a core needle biopsy can be useful although traits supporting the diagnosis of malignancy can be missed.7
There are no previous published reports where the diagnosis of malignant SCN was obtained prior to surgical resection. We present a rare case of an asymptomatic stage IV pancreatic malignant SCN accidentally discovered during an echocardiogram. To our knowledge, this is the very first report of a patient diagnosed by percutaneous core biopsy.
Case presentation
A 68-year-old man presented in the emergency room at a tertiary university medical centre with chief complaint of a sudden and persistent retrosternal chest pain irradiated to the left arm. On admission, his vitals were within the normal limits except for sinus tachycardia. Owing to suspected acute coronary syndrome, he underwent an urgent ECG, upright chest x-ray, serum levels of troponin and then he was further investigated with a transthoracic echocardiography. His work-up resulted negative for coronary ischaemia, but during his echocardiogram, a large heterogeneous liver lesion occupying the left lateral hepatic segments was identified. The echogenic mass measured 8.0 cm in maximum diameter and it was adjacent to both the left hemidiaphragm and the lesser gastric curvature.
The patient's medical history was significant for several episodes of mild-to-severe abdominal discomfort localised in his left upper quadrant associated with dyspepsia. His surgical history was unremarkable except for a right inguinal hernia repair several years earlier.
His physical examination was within the normal limits.
Investigations
Routine haematological and biochemical investigations, including renal, liver and pancreatic function tests were all within the normal limits. Serum tumour markers CA-19.9 and CEA were both within the normal ranges (CA-19.9=17.6 U/ml; normal values 0–35 U/ml and CEA=0.2; normal value less than 2.5 ng/ml).
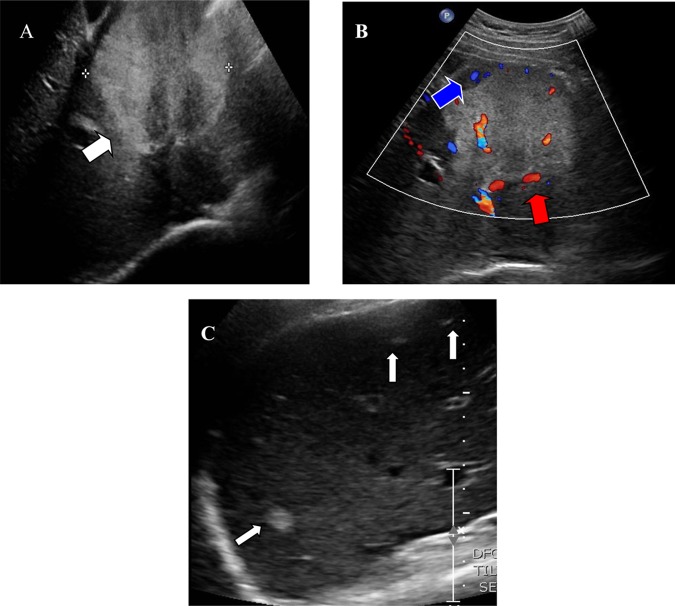
Ultrasonography of the abdomen showed a lobulated echogenic heterogeneous solid-cystic mass measuring 8.4×7.3×8.4 cm in the left hepatic lobe and a similar echogenic abnormality in the pancreas extending from the uncinate process to the tail of the gland (figure 1).
Figure 1.
(A) Ultrasound images demonstrate a large predominantly echogenic mass in the left lobe of the liver (white arrow). The mass has lobulated margins and contains hypoechoic areas which are wedge-shaped in appearance. (B) Arterial (red arrow) and venous (blue arrow) blood flow is demonstrated within the mass on Doppler imaging. (C) Small nodules suggestive for metastatic deposits are present in the surrounding left and right hepatic lobes (white arrows).
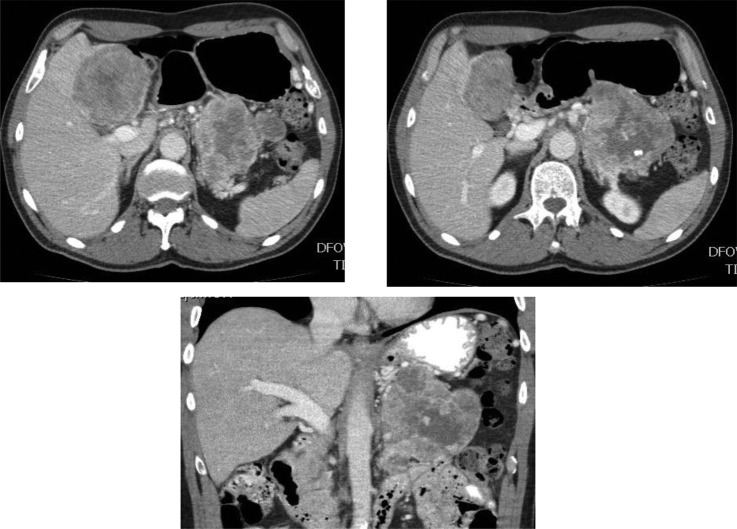
Following the abdominal ultrasound, a triple-phase contrast-enhanced CT scan demonstrated a heterogenous solid-cystic mass in the body and tail of the pancreas measuring 12×9×8 cm (figure 2). In addition, a solid mass with heterogenous pattern of enhancement measuring about 8.6 cm in diameter was seen in the left hepatic lobe and a smaller one in the right hepatic lobe. The pattern of enhancement in the liver mass closely resembled the pattern of enhancement in the pancreatic tumour. Encasement with occlusion of the distal splenic vein and infiltration of the retroperitoneum was noted and direct invasion of the posterior gastric wall could not been ruled out. There were no signs of intrahepatic biliary dilatation, ascites or lymphadenopathy in the abdomen and pelvis.
Figure 2.
(A) Intravenous contrast-enhanced CT image demonstrates a large lobulated solid and cystic mass in the pancreatic tail (white arrow) and in the left hepatic lobe (red arrow). (B) Example of several calcifications within the pancreatic mass (white arrow).
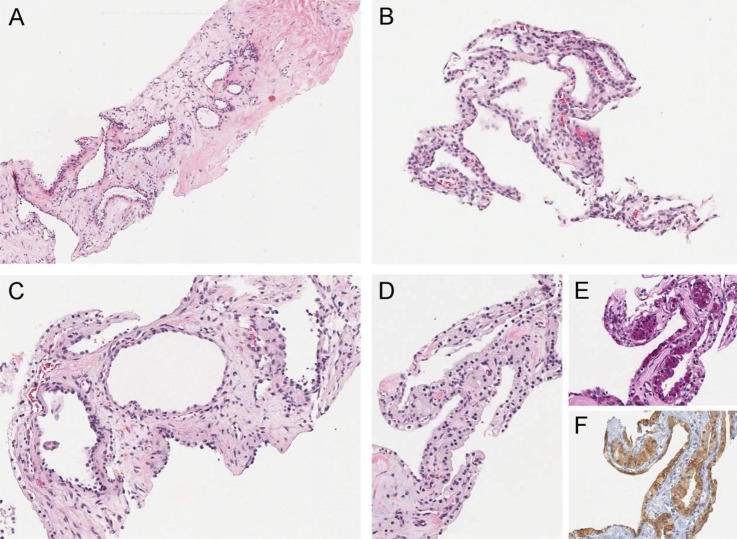
Ultrasonography guided core needle biopsy from both lesions was performed and the results were consistent with microcystic serous cystadenocarcinoma of pancreatic origin (figure 3).
Figure 3.
(A and B) Liver biopsy showing tumour with a microcystic growth pattern (H&E A mag ×, B mag ×2). The cysts are lined by a single layer of cuboidal cells with pale granular cytoplasm. The stroma between cysts is abundant in region (A) and scanty in region (B). Pancreas biopsy showing tumour with microcystic growth pattern identical to the liver tumour. Regions in (C) and (D) have abundant and scanty stroma between cysts, respectively. (E) PAS stain shows abundant glycogen in tumour epithelial cells. (F) Immunohistochemical stain shows the epithelial cells express inhibin (C–F magnification all ×2).
Differential diagnosis
Differential diagnosis of malignant SCN of the pancreas includes:
- Non-endocrine pancreatic tumours with cystic characteristics:
- – serous cystoadenomas
- – mucinous cystoadenomas
- – mucynous cystoadenocarcinomas
- – IPMN
- – pseudopapillary pancreatic cancers
Endocrine pancreatic tumours with cystic transformation
Pancreatic pseudocyst
Treatment
A multidisciplinary team was consulted and the patient underwent further investigations to rule out the presence of extrahepatic metastases. Medical and radiation oncologists as well as hepatobiliary surgeons and radiologists met together to provide their recommendations. There was agreement among all the specialists that malignant SCN involved the entire body and tail of the pancreas and part of the uncinate process with infiltration in the retroperitoneum. The primary tumour was adjacent to the left renal artery and vein and the metastatic deposits were located in segments 2 and 3 of the left hepatic lobe and segment 5 of the right hepatic lobe without involvement of the coeliac or superior mesenteric arteries. Surgical radical therapy would have required a total pancreatectomy with a left nephrectomy and a lateral left hepatectomy in addition to a right non-anatomical hepatic resection. Since the patient was completely asymptomatic, he refused upfront radical surgery and opted for a trial 4 months of neo-adjuvant chemotherapy with the hope of downstaging the tumour. An abdominal CT scan after the neo-adjuvant treatment showed that the pancreatic lesion was stable but there was progression of the degree of the pancreatic duct dilatation. The hepatic lesions were enlarged by a few millimeters without evidence of intrahepatic biliary dilatation or ascites and there was no evidence of abdominal or pelvic lymphadenopathy.
Outcome and follow-up
Owing to the indolent nature of this tumour, the patient and his family opted for a conservative approach and he continues to be asymptomatic 1 year after his diagnosis.
Discussion
Based on the histopathological classification by Compagno and Oertel,4 SCN of the pancreas fall in the category of benign cysts with virtually no risk of malignant transformation. This notion is still unchallenged by the majority of specialists of pancreatic diseases and continues to be widely reported in the scientific literature. MCN of the pancreas, on the other hand, have been well recognised as benign cysts with malignant potential.4 8 Owing to these differences, once patients are diagnosed with SCN, their management is usually conservative and surgical treatment recommended only when patients are symptomatic or when tumour growth develops.
Instead, MCN, are usually resected even when small and asymptomatic for fear of malignant transformation.9 10
In vision of the increasing number of cases where SCN transformed into malignant tumours, this paradigm should undergo some revision, as it is not entirely correct. Since 1989, when the first case of a malignant SCN was described, 26 new cases of patients diagnosed with malignant transformation of SCN have been reported in the literature and are summarised in table 2. As a result, SCN of the pancreas should not be considered totally benign and serial imaging tests for follow-up should be recommended. Unfortunately, despite all the recent advances in cross-sectional imaging modalities, it is still impossible to distinguish benign from malignant SCN unless there is evidence of metastatic disease or rapid growth.
Table 2.
Characteristics of pancreatic serous cystadenocarcinoma reported in the literature
| Case | Author | Year | Age | Sex | Signs or symptoms | Tumour size (maximum diameter) in cm | Metastases or local invasion | Surgical therapy | Outcome | Note |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | George et al1 | 1989 | 70 | M | Haemorrhage from gastric varices | 11 | Liver metastases. Stomach and spleen invasion. Infiltration and occlusion of the splenic vein | DP | Death during operation due to hemorrhage | |
| 2 | Friedman et al20 | 1990 | 74 | F | Right flank pain, weight loss. After 6 months palpable abdominal mass, enlarged supraclavicular lymph nodes and pleural effusions | 19 | Metastases in the liver, both lungs, bone marrow, both adrenal glands, lymph nodes in the porta hepatic and along the abdominal and thoracic aorta | None | Death due to advanced neoplasm | |
| 3 | Kamei et al21 | 1991 | 72 | F | Jaundice | 10 | Neural invasion | TP | NA | |
| 4 | Okada et al19 | 1991 | 63 | F | Abdominal pain | 12 | Liver metastases 4 years after operation | DP | Alive 5 years after initial operation | |
| 5 | Yoshimi et al18 | 1992 | 63 | F | Epigastric pain, palpable mass | 12 | Liver metastases 3 years after operation | DP | Alive 6 years after initial operation | |
| 6 | Ohta et al22 | 1993 | 64 | M | Unrelated incidental detection of the tumour on abdominal CT | 2.5 | Perivascular and vascular invasion | Enucleation | Alive 9 months after initial operation | |
| 7 | Widmaier et al23 | 1996 | 71 | M | Elevated liver function tests | 4 | Focal invasion of peripancreatic and pancreatic tissue. One lymph node metastasis | PPPD | Alive 1 year later | |
| 8 | Ishikawa et al24 | 1998 | 63 | F | Abdominal pain | 12 | Liver metastasis 3 years after operation | DP | NA | |
| 9 | Siech et al6 | 1998 | NA | NA | NA | NA | Local infiltration of neighbouring structures | NA | NA | Two cases reported |
| 10 | Eriguchi et al16 | 1998 | 65 | F | Palpable abdominal mass | 16 | Liver metastases at the time of initial operation. Liver metastases 9 years after operation | DP, microwave coagulo-necrotic therapy | Alive 10 years after initial operation | |
| 11 | Abe et al25 | 1998 | 71 | F | Palpable abdominal mass, general fatigue, weight loss | 12 | Invasion of a lymph node and adipose tissue | DP, splenectomy | Alive 2 years later | |
| 12 | Schmidt-Rohlfing et al26 | 1998 | 52–74 | 2 M, 2 F | NA | NA | In one case: local invasion of the adrenal gland. Other three cases: NA | DP, TP, PD, DP | NA | Four cases reported |
| 13 | Kimura et al14 | 1999 | 53, 66 | F, M | NA | 5.3 | In both cases: neural and stromal invasion | DP | In both cases alive 6 years after operation | 2 cases reported |
| 14 | Horvath et al17 | 1999 | 81 | F | NA | 6 | NA | DP | NA | |
| 15 | Wu et al27 | 1999 | 57 | F | Unrelated, incidental detection of the tumour on abdominal CT | 5.5 (largest cyst) | Diffuse infiltrative disease of the liver. Stomach invasion. Peritoneal metastases | NA | Recurrence 10 years after initial tumour resection | |
| 16 | Strobel et al12 | 2001 | 56 | F | Recurrent abdominal pain, diarrhoea, weight loss | 14 | Metachronous metastatic liver metastases | PPPD | Alive 3 years after initial operation | |
| 17 | Shintaku et al28 | 2005 | 85 | F | Fatigue, intermittent diarrhoea | 12 | Spleen invasion | DP, distal gastrectomy | Alive 10 months later | |
| 18 | Friebe et al15 | 2005 | 80 | F | Abdominal pain, anorexia, weight loss | 8 | Spleen invasion | DP, splenectomy | Alive 1 year later | |
| 19 | Galanis et al29 | 2007 | NA | NA | NA | NA | Synchronous and metachronous liver metastases | NA | NA | Two cases reported |
| 20 | Gupta et al30 | 2008 | 42 | F | Abdominal pain, palpable abdominal mass, diarrhoea, weight loss | 10 | No | DP, splenectomy | Alive 2 years later | |
| 21 | Franko et al31 | 2008 | NA | NA | NA | NA | NA | NA | NA | |
| 22 | King et al 32 | 2009 | 70 | M | Abdominal pain, haematemesis | 9 | Duodenum invasion | PPPD | Alive 7 years later | |
| 23 | Vadala et al33 | 2010 | 74 | M | NA | NA | Portal vein infiltration | PD, portal vein thrombectomy | NA | |
| 24 | Bano et al34 | 2011 | 62 | M | Abdominal pain, vomiting, weight loss, jaundice | 7 | Duodenum invasion, liver metastases | PD, microwave coagulo-necrotic therapy | Alive 1 year later | |
| 25 | Cho et al35 | 2011 | 64 | F | Dizziness, haematochezia | 12 | Colon and spleen invasion | DP,segmental resection of the colon, splenectomy | NA | |
| 26 | Bramis et al2 | 2012 | 86 | F | Abdominal pain | 17 | Stomach invasion, liver metastases | Inoperable, biopsies taken | Died 1 month later |
DP, distal pancreatectomy; PD, pancreaticoduodenectomy; PPPD, pylorus preserving pancreaticoduodenectomy, NA=not available; TP, total pancreatectomy.
One of the limitations of the current knowledge of this disease are the unknown rate and the predisposing factors of malignant transformation of SCN. This has a considerable impact on the selection of high-risk patients, the frequency of test that should be used for surveillance and what radiological modalities should be used. Owing to its rarity, there is no good evidence to guide physicians in their decision-making and formulate a consensus on the most cost-effective strategy to manage patients with SCN. Some authors suggest that if the tumours show signs of enlargement or if the patients become symptomatic, there should be a low threshold for surgical resection in vision of the fact that malignant transformation is rare but possible.11
Nevertheless, it should be kept in mind that an increasing number of malignant SCN has been reported in the past decade.6
The low prevalence of malignancy among SCN does not justify surgical resection unless it is symptomatic or showing interval growth. In fact, suspicion for malignant transformation should arise when new symptoms, worsening of symptoms or rapid enlargement of the mass occur.12 In these cases, resection is indicated, despite the lack of tissue diagnosis of malignancy.12–15 For the vast majority of SCN, the current practice remains essentially conservative to avoid potential morbidity and mortality associated with a major operation for very low-risk lesions.6 13 16 17
Patients, who underwent surgical resections for malignant SCN of the pancreas, seemed to have excellent prognosis even in the presence of metastatic disease.16 18 19 It is uncertain, though, if radical resection of the primary tumour and metastases is associated with better survival than observation. No comparative studies are available and the decision to proceed with resection should be carefully evaluated when dealing with stage IV tumours, especially in asymptomatic patients, because of the risks of perioperative mortality.1
In conclusion, SCN of the pancreas have a low probability of malignant transformation. Owing to this low probability, in the past, they were classified as benign cystic lesions without the need for any intervention or follow-up. In recent years, this notion has changed because of an increasing number of reported cases of malignant SCN. It is still uncertain what is the most cost-effective strategy to use for follow-up of patients with SCN and their predisposing factors for malignant transformation. Radical resection of symptomatic or expanding serous cystic lesions of the pancreas should be indicated in patients who are good surgical candidates. Several case reports have described long-term survival of patients undergoing complete excision of metastatic SCN. However, it is still unclear if this observation is secondary to the benefit of surgical treatment or if it is because of the indolent natural history of the disease.
Learning points.
Serous cystic neoplasms (SCN) were considered benign neoplastic cysts without malignant potential. Despite the rarity of the event, during the last few decades, several cases have been reported of patients malignant transformation of SCN of the pancreas.
When symptomatic, patient with SCN of the pancreas should be considered for surgical resection.
When asymptomatic, patients with radiological findings suggesting growth of SCN should be considered for resection.
It is unclear what are the best radiological tests and the frequency needed for the assessment of patients with SCN of the pancreas. Predisposing factors for malignant transformation are also uncertain.
There is no evidence to support the use of neo-adjuvant or adjuvant chemotherapy for serous cystoadenocarcinomas of the pancreas.
Long-term survival of patients undergoing radical resections for malignant SCN of the pancreas is significantly better than for other pancreatic malignancies.
Evidence to support resections for patients with metastatic SCN is based only on case reports.
The excellent prognosis associated with malignant SCN might justify surgical excision even in the presence of metastatic disease if patients are willing to undergo surgery, if they are good surgical candidates and if surgery can be performed with low morbidity and mortality. However, it is still unclear whether this observation is secondary to the benefit of the surgical treatment or if it is because of the indolent natural history of the disease.
Prospective studies would be beneficial, but very unlikely as malignant SCN remains a very rare condition.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.George DH, Murphy F, Michalski R, et al. Serous cystadenocarcinoma of the pancreas: a new entity? Am J Surg Pathol 1989;13:61–6 [DOI] [PubMed] [Google Scholar]
- 2.Bramis K, Petrou A, Papalambros A, et al. Serous cystadenocarcinoma of the pancreas: report of a case and management reflections. World J Surg Oncol 2012;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandez-del Castillo C, Warshaw AL. Cystic neoplasms of the pancreas. Pancreatology 2001;1:641–7 [DOI] [PubMed] [Google Scholar]
- 4.Compagno J, Oertel JE. Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol 1978;69:289–98 [DOI] [PubMed] [Google Scholar]
- 5.Kloppel G. Histological typing of tumors of the exocrine pancreas. WHO international histological classification of tumors 2nd edn Berlin: Springer, 1996 [Google Scholar]
- 6.Siech M, Tripp K, Schmidt-Rohlfing B, et al. Cystic tumours of the pancreas: diagnostic accuracy, pathologic observations and surgical consequences. Langenbecks Arch Surg 1998;383:56–61 [DOI] [PubMed] [Google Scholar]
- 7.Kehagias D, Smyrniotis V, Kalovidouris A, et al. Cystic tumors of the pancreas: preoperative imaging, diagnosis, and treatment. Int Surg 2002;87:171–4 [PubMed] [Google Scholar]
- 8.Thompson LD, Becker RC, Przygodzki RM, et al. Mucinous cystic neoplasm (mucinous cystadenocarcinoma of low-grade malignant potential) of the pancreas: a clinicopathologic study of 130 cases. Am J Surg Pathol 1999;23:1–16 [DOI] [PubMed] [Google Scholar]
- 9.Sarr MG, Murr M, Smyrk TC, et al. Primary cystic neoplasms of the pancreas. Neoplastic disorders of emerging importance-current state-of-the-art and unanswered questions. J Gastrointest Surg 2003;7:417–28 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-del Castillo C. Surgery of cystic neoplasms. Gastrointest Endosc Clin N Am 2002;12:803–12, ix [DOI] [PubMed] [Google Scholar]
- 11.Bosman F. WHO Classification of Tumors of the Digestive System. 4. Vol. 3. IARC WHO Classification of Tumors; No3.
- 12.Eriguchi N, Aoyagi S, Nakayama T, et al. Serous cystadenocarcinoma of the pancreas with liver metastases. J Hepatobiliary Pancreat Surg 1998;5:467–70 [DOI] [PubMed] [Google Scholar]
- 13.Horvath KD, Chabot JA. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J Surg 1999;178:269–74 [DOI] [PubMed] [Google Scholar]
- 14.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg 2005;242:413–9; discussion 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strobel O, Z'Graggen K, Schmitz-Winnenthal FH, et al. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion 2003;68:24–33 [DOI] [PubMed] [Google Scholar]
- 16.Kimura W, Makuuchi M. Operative indications for cystic lesions of the pancreas with malignant potential—our experience. Hepatogastroenterology 1999;46:483–91 [PubMed] [Google Scholar]
- 17.Friebe V, Keck T, Mattern D, et al. Serous cystadenocarcinoma of the pancreas: management of a rare entity. Pancreas 2005;31:182–7 [DOI] [PubMed] [Google Scholar]
- 18.Yoshimi N, Sugie S, Tanaka T, et al. A rare case of serous cystadenocarcinoma of the pancreas. Cancer 1992;69:2449–53 [DOI] [PubMed] [Google Scholar]
- 19.Okada T, Nonami T, Miwa T, et al. Hepatic metastasis of serous cystadenocarcinoma resected 4 years after operation of primary tumors—a case report. Nihon Shokakibyo Gakkai Zasshi 1991;88:2719–23 [PubMed] [Google Scholar]
- 20.Friedman HD. Nonmucinous, glycogen-poor cystadenocarcinoma of the pancreas. Arch Pathol Lab Med 1990;114:888–91 [PubMed] [Google Scholar]
- 21.Kamei K, Funabiki T, Ochiai M, et al. Multifocal pancreatic serous cystadenoma with atypical cells and focal perineural invasion. Int J Pancreatol 1991;10:161–72 [DOI] [PubMed] [Google Scholar]
- 22.Ohta T, Nagakawa T, Itoh H, et al. A case of serous cystadenoma of the pancreas with focal malignant changes. Int J Pancreatol 1993;14:283–9 [DOI] [PubMed] [Google Scholar]
- 23.Widmaier U, Mattfeldt T, Siech M, et al. Serous cystadenocarcinoma of the pancreas. Int J Pancreatol 1996;20:135–9 [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa T, Nakao A, Nomoto S, et al. Immunohistochemical and molecular biological studies of serous cystadenoma of the pancreas. Pancreas 1998;16:40–4 [DOI] [PubMed] [Google Scholar]
- 25.Abe H, Kubota K, Mori M, et al. Serous cystadenoma of the pancreas with invasive growth: benign or malignant? Am J Gastroenterol 1998;93:1963–6 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt-Rohlfing B, Siech M, Mattfeldt T, et al. Cystic neoplasms of the pancreas: surgical therapy and chances for cure. Z Gastroenterol 1998;36:939–45 [PubMed] [Google Scholar]
- 27.Wu CM, Fishman EK, Hruban RK, et al. Serous cystic neoplasm involving the pancreas and liver: an unusual clinical entity. Abdom Imaging 1999;24:75–7 [DOI] [PubMed] [Google Scholar]
- 28.Shintaku M, Arimoto A, Sakita N. Serous cystadenocarcinoma of the pancreas. Pathol Int 2005;55:436–9 [DOI] [PubMed] [Google Scholar]
- 29.Galanis C, Zamani A, Cameron JL, et al. Resected serous cystic neoplasms of the pancreas: a review of 158 patients with recommendations for treatment. J Gastrointest Surg 2007;11:820–6 [DOI] [PubMed] [Google Scholar]
- 30.Gupta R, Dinda AK, Singh MK, et al. Macrocystic serous cystadenocarcinoma of the pancreas: the first report of a new pattern of pancreatic carcinoma. J Clin Pathol 2008;61:396–8 [DOI] [PubMed] [Google Scholar]
- 31.Franko J, Cole K, Pezzi CM, et al. Serous cystadenocarcinoma of the pancreas with metachronous hepatic metastasis. Am J Clin Oncol 2008;31:624–5 [DOI] [PubMed] [Google Scholar]
- 32.King JC, Ng TT, White SC, et al. Pancreatic serous cystadenocarcinoma: a case report and review of the literature. J Gastrointest Surg 2009;13:1864–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vadala S, Calderera G, Cinardi N, et al. Serous cystadenocarcinoma of the pancreas with portal thrombosis. Clin Ter 2010;161:149–52 [PubMed] [Google Scholar]
- 34.Bano S, Upreti L, Puri SK, et al. Imaging of pancreatic serous cystadenocarcinoma. Jpn J Radiol 2011;29:730–4 [DOI] [PubMed] [Google Scholar]
- 35.Cho W, Cho YB, Jang KT, et al. Pancreatic serous cystadenocarcinoma with invasive growth into the colon and spleen. J Korean Surg Soc 2011;81:221–4 [DOI] [PMC free article] [PubMed] [Google Scholar]