Abstract
We describe a case of leucocytoclastic vasculitis manifested as exanthematous rash in a 57-year-old woman on long-term therapy with clopidogrel. The diagnosis was confirmed by skin biopsy. The patient was managed symptomatically with oral antihistaminics and topical steroids in consultation with dermatologists. Clopidogrel therapy was discontinued on suspicion of drug-induced vasculitis. The rash resolved completely within 2 weeks of withdrawal of clopidogrel, satisfying criteria for a probable adverse drug reaction. Leucocytoclastic vasculitis is an unusual adverse effect of clopidogrel therapy and even rarer as a late complication.
Background
Clopidogrel is a thienopyridine derivative that irreversibly inhibits the ADP receptor P2Y12, resulting in inhibition of platelet aggregation.1 It is indicated for secondary prevention of acute coronary syndromes in patients on medical management as well as those receiving percutaneous intervention with drug-eluting or bare metal stents. In both the instances, it is recommended for a period of 12 months.2 3
Barring an increased risk of bleeding, clopidogrel is generally considered to be a well-tolerated antiplatelet agent. This is in sharp contrast to ticlopidine, another antiplatelet agent with a mechanism of action similar to clopidogrel. Ticlopidine has been associated with severe neutropaenia, thrombocytopaenia and drug-induced haemolytic-uremic syndrome–thrombotic thrombocytopaenic purpura (HUS-TTP);4 as a result, the drug has largely been replaced by other thienopyridines such as clopidogrel and prasugrel. Clopidogrel has also been linked, albeit infrequently with hypersensitivity reactions.5–7 Substitution with ticlopidine is one of the strategies employed in such cases although there is high risk of cross-reactivity.8 Other strategies include administration of oral steroids5 6 and employment of clopidogrel desensitisation protocols.9 Discontinuation of clopidogrel without substitution with another antiplatelet agent is discouraged because of the risk of developing another acute coronary syndrome. This is because most cases of reported hypersensitivity to clopidogrel therapy occurred soon after the initiation of clopidogrel therapy. For instance, in the study by Campbell et al,5 the mean period to development of hypersensitivity was only 6±2 days. In contrast, our patient developed a hypersensitivity reaction after 1 year of therapy. This, combined with the low-risk nature of her disease (see below), permitted a complete cessation of antiplatelet therapy without adverse cardiac events. The markedly late onset of hypersensitivity in our patient is a unique feature and, to the best of our knowledge, has not been reported previously in medical literature.
Case presentation
A 57-year-old woman presented with a pruritic rash over the trunk and extremities over the past 2 weeks. One year prior to this, she had been evaluated at our hospital for persistent angina. At that time, ECG and echocardiography were both normal. Coronary angiography had shown insignificant coronary artery disease and she had been initiated on dual therapy with aspirin and clopidogrel. Subsequently, aspirin had been discontinued because of gastrointestinal intolerance, and monotherapy with clopidogrel continued. There was no other significant medical history. Physical examination confirmed the presence of a diffuse maculopapular rash, but did not reveal any other abnormalities. The nature of the rash was strongly suggestive of an allergic exanthema and a skin biopsy was performed.
Investigations
Routine laboratory investigations were essentially normal. Complete haemogram ruled out eosinophilia. Platelet count (182×109/l) and renal function tests (serum creatinine: 0.8 mg/dl) were normal. Skin biopsy performed showed fibrinoid necrosis of the walls of small vessel features consistent with a leucocytoclastic vasculitis (figure 1). Serum assays for antinuclear antibodies and antineutrophil cytoplasmic antibodies were negative.
Figure 1.
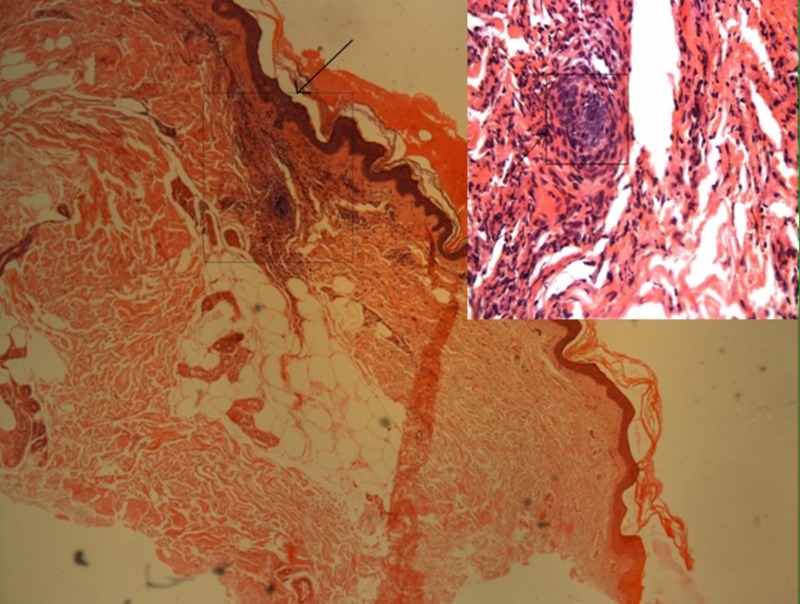
Photomicrograph of skin biopsy showing vasculitis in the superficial dermal vessels with fibrinoid necrosis of vessel wall and neutrophilic infiltration, consistent with leucocytoclastic vasculitis at a magnification of ×25, H&E stain. Area denoted in the box shown on the right-hand corner at higher magnification.
Differential diagnosis
Drug-induced leucocytoclastic vasculitis secondary to clopidogrel.
Idiopathic small vessel vasculitis.
Clopidogrel-induced thrombotic thrombocytopaenic purpura (this possibility was effectively ruled out by the absence of thrombocytopaenia and renal failure).
Treatment
A tentative diagnosis of clopidogrel-induced vasculitis was made. Keeping in mind the low-risk nature of her coronary artery disease and the fact that she had already received 12 months of clopidogrel therapy, a conscious decision was taken to immediately discontinue clopidogrel therapy. The patient was prescribed symptomatic treatment with a short course of oral antihistaminics and topical steroids.
Outcome and follow-up
Complete and durable resolution of the rash was noted within 2 weeks of stopping clopidogrel. Rechallenge with clopidogrel, or desensitisation protocols were not attempted. The patient remains on regular follow-up and cardiac event-free 6 months after cessation of clopidogrel therapy.
Discussion
A diagnosis of probable adverse drug reaction was made in this instance by the causality assessment criteria of the WHO Collaborating Centre for International Drug Monitoring, the Uppsala Monitoring Centre (WHO-UMC).10
Hypersensitivity and skin rash in response to clopidogrel have been described in a number of studies. A study by Cheema et al6 on 62 patients with hypersensitivity to clopidogrel, after percutaneous coronary intervention, described a generalised exanthema in 79% of cases, localised skin involvement in 16% of patients and angioedema in 5%.6 Lymphocyte-mediated delayed hypersensitivity was demonstrated by skin biopsy from the affected areas. These patients as well as those included in the study by Campbell et al5 showed excellent response to oral steroid administration, permitting continuation of therapy with clopidogrel.
The large CAPRIE trial which involved 9599 patients on therapy with clopidogrel reported skin rash as an adverse effect in 578 (6.02%) cases.11 Interestingly, the incidence of rash was four times greater than the incidence of the much feared complication of major bleeding (1.38%). Although the nature and/or histopathology of these hypersensitivity reactions were not described, the CAPRIE data suggest that hypersensitivity reactions with clopidogrel might be a relatively common occurrence.
The underlying mechanisms of hypersensitivity reactions to clopidogrel are yet to be precisely elucidated. Proposed mechanisms include direct mast cell activation, complement system activation, immune complex deposition, T-cell activation and IgE-mediated hypersensitivity.12 Hypersensitivity to metabolites of clopidogrel rather than clopidogrel itself has also been suggested as a possibility. In our case, immune complex deposition could readily explain the development of drug-induced leucocytoclastic vasculitis.
Learning points.
Hypersensitivity reactions are an under-recognised complication of clopidogrel therapy.
Leucocytoclastic vasculitis can develop as a delayed complication of clopidogrel therapy, as late as 1 year after the initiation of therapy.
Leucocytoclastic vasculitis can reverse completely with a discontinuation of clopidogrel therapy without recourse to systemic steroid therapy; this approach is reasonable in selected patients with low cardiac risk.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Angiolillo DJ. The evolution of antiplatelet therapy in the treatment of acute coronary syndromes: from aspirin to the present day. Drugs 2012;72:2087–116 [DOI] [PubMed] [Google Scholar]
- 2.Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2012;60:645–81 [DOI] [PubMed] [Google Scholar]
- 3.Kushner FG, Hand M, Smith SC Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update) a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2009;54:2205–41 [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Weinberg PD, Rozenberg-Ben-Dror K, et al. Thrombotic thrombocytopenic purpura associated with ticlopidine. A review of 60 cases. Ann Intern Med 1998;128:541–4 [DOI] [PubMed] [Google Scholar]
- 5.Campbell KL, Cohn JR, Fischman DLet al. Management of clopidogrel hypersensitivity without drug interruption. Am J Cardiol 2011;107:812–16 [DOI] [PubMed] [Google Scholar]
- 6.Cheema AN, Mohammad A, Hong Tet al. Characterization of clopidogrel hypersensitivity reactions and management with oral steroids without clopidogrel discontinuation. J Am Coll Cardiol 2011;58:1445–54 [DOI] [PubMed] [Google Scholar]
- 7.Lokhandwala JO, Best PJ, Butterfield JHet al. Frequency of allergic or hematologic adverse reactions to ticlopidine among patients with allergic or hematologic adverse reactions to clopidogrel. Circ Cardiovasc Interv 2009;2:348–51 [DOI] [PubMed] [Google Scholar]
- 8.Lokhandwala J, Best PJ, Henry Y, et al. Allergic reactions to clopidogrel and cross-reactivity to other agents. Curr Allergy Asthma Rep 2011;11:52–7 [DOI] [PubMed] [Google Scholar]
- 9.Owen P, Garner J, Hergott L, et al. 2nd Clopidogrel desensitization: case report and review of published protocols. Pharmacotherapy 2008;28:259–70 [DOI] [PubMed] [Google Scholar]
- 10.. The use of the WHO–UMC system for standardised case causality assessment. http://who-umc.org/Graphics/24734.pdf (accessed 31 Jul 2012).
- 11.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329–39 [DOI] [PubMed] [Google Scholar]
- 12.Gutiérrez-Fernández D, Fuentes-Vallejo MS, Rueda-Ygueravides MD, et al. Immediate hypersensitivity and delayed hypersensitivity to clopidogrel. Allergol Immunopathol (Madr) 2007;35:213–15 [DOI] [PubMed] [Google Scholar]


