Abstract
A 75-year-old woman presented 9 days post-total hip replacement with sudden onset of shortness of breath and fever. She had been discharged taking dabigatran. The patient was treated for sepsis with antibiotics and fluids. However, she deteriorated and was transferred to the intensive care unit. Following a 10 s asystolic episode the patient was thrombolysed with alteplase for presumed massive pulmonary embolism. Initially, her blood pressure and oxygen saturation improved. However, over the next few days, she remained persistently hypotensive. A CT scan of her chest, abdomen and pelvis demonstrated bilateral adrenal haemorrhages. A short synacthen test confirmed acute adrenal failure.
Background
Bilateral adrenal haemorrhages (BAH) following anticoagulation are very rare, difficult to diagnose and potentially fatal unless promptly recognised and treated. Many cases are only diagnosed post mortem. We report a case in which a patient failed to improve following thrombolysis for presumed massive pulmonary embolism due to adrenal failure.
Case presentation
A 75 -year-old woman presented to the emergency department with sudden onset of shortness of breath. This was associated with fever, and had been worsening over several hours. She had been discharged from the hospital, earlier that day, 9 days post-total hip replacement and had been discharged with prophylactic dabigatran 150 mg one time a day.
The patient had a medical history of obliterative bronchiolitis, treated with inhalers and was on no other regular medications. She lived independently.
On examination, she had a fever of 39.4 centigrade and was tachycardic. Her blood pressure was 120/57. Respiratory rate was 30 and oxygen saturations were 93% on 2 litres of oxygen.
Initial investigations showed haemoglobin 7 g/dl, white cell count 16.4×109/l, platelets 73×109/l, international normalised ratio 1.7, activated partial thromboplastin time ratio 3.5 (0.8–1.4), d-dimer >1000 μg/l, creatinine 268 μmol/l, urea 15 mmol/l and C reactive protein 296 mg/l. An ECG showed right bundle branch block. Arterial blood gases, on 2 litres of oxygen, showed type 1 respiratory failure (pO2 9.4 kPa and pCO2 5.0 kPa). Chest x-ray did not show any focal consolidation.
A diagnosis of pulmonary embolism associated with sepsis and acute renal failure was made. The patient was treated with broad spectrum antibiotics, low-molecular-weight heparin and intravenous fluids. However, she deteriorated rapidly and was transferred to the intensive care unit (ICU), where she was intubated and started on inotropes. An echocardiogram showed a dilated right heart with estimated pulmonary artery pressure of 50 mm Hg. A CT pulmonary angiogram was avoided due to unwillingness to administer contrast to a patient with acute renal failure. A further chest x-ray, performed 48 h after intubation, showed bilateral consolidation in keeping with pneumonia.
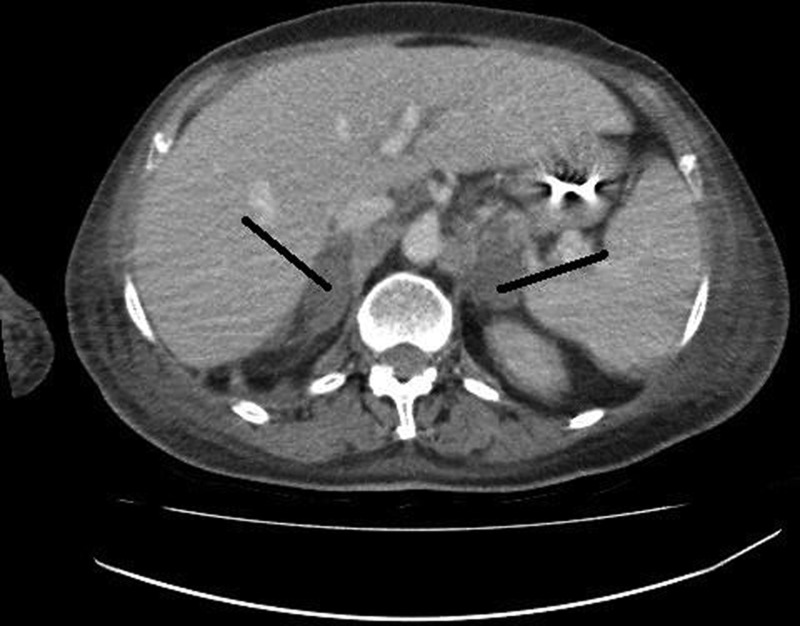
Over the next week, the patient was managed in the ICU with ventilatory support, inotropes, antibiotics and low-molecular-weight heparin. She did not require haemofiltration and was not anticoagulated with warfarin. After 7 days the patient's haemodynamic status deteriorated and she had a 10 s asystolic episode, during which she received chest compressions. A clinical diagnosis of massive pulmonary embolus was made and she was treated with thrombolysis (100 mg of alteplase over 2 h). A rapid improvement in her haemodynamic status followed – supporting the clinical diagnosis of massive pulmonary embolism. However, over the next few days, the patient remained hypotensive and required inotropes. She had no fever and her inflammatory markers had improved. Low-molecular-weight heparin was continued. A contrast CT scan of her chest, abdomen and pelvis was performed 8 days post-thrombolysis to look for a source of sepsis. The CT scan showed BAH (figure 1). No remaining pulmonary emboli were seen (note that this was not a CT pulmonary angiogram). The patient's sodium and potassium levels were normal. However, a short synacthen test confirmed adrenal failure. The patient was started on steroid replacement therapy and eventually made a good recovery.
Figure 1.
CT scan showing bilateral oval adrenal enlargement due to haemorrhage.
Investigations
Short synacthen test
| Time (min) | Cortisol (nmol/l) |
|---|---|
| 0 | 0 |
| 30 | 3 |
| 60 | 4 |
Treatment
The patient was treated with low-molecular-weight heparin, tissue plasminogen activator and then warfarin. Adrenal failure was treated with fludrocortisone and hydrocortisone.
Outcome and follow-up
After a stormy period in the ICU, the patient made a good recovery and was discharged. She was given warfarin for 3 months and steroid replacement therapy and was followed up in an endocrinology clinic.
Three months later a further short synacthen test confirmed persistent adrenal insufficiency. The patient was advised that she will require lifelong steroid replacement.
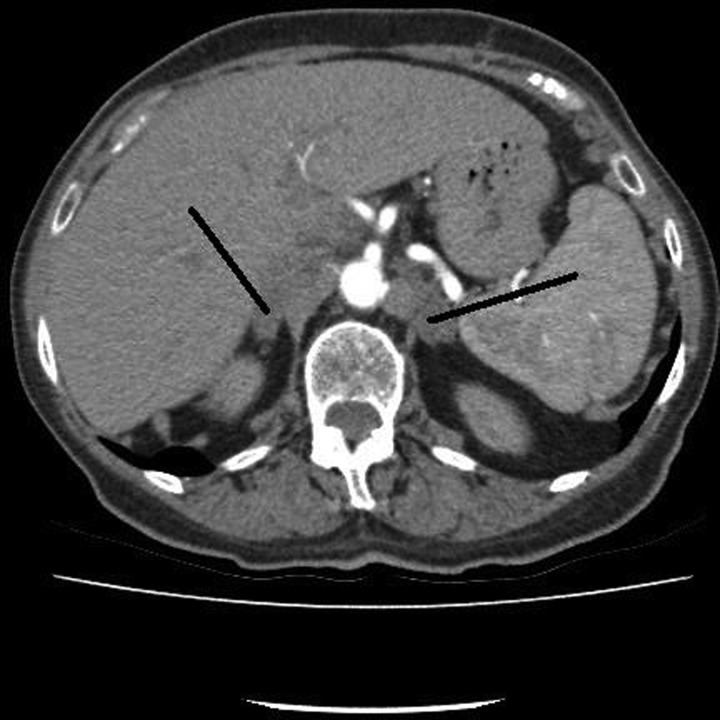
A CT pulmonary angiogram (performed to investigate breathlessness after cessation of warfarin) showed reduction in the size of adrenals consistent with resolving BAH (figure 2).
Figure 2.
CT pulmonary angiogram 4 months later showing reduction in the size of adrenal enlargement consistent with resolving haemorrhage. A short synacthen test confirmed persistent adrenal insufficiency.
Discussion
BAH is an uncommon condition that is difficult to diagnose and may lead to adrenal failure and death unless promptly treated.1 2 Many cases only become apparent at post mortem. BAH is normally associated with an acute illness such as overwhelming sepsis—often meningococcal (Waterhouse-Friderichsen syndrome), major surgery, trauma, cardiac failure, myocardial infarction, cirrhosis and complications of pregnancy. Anticoagulation and clotting disorders such as thrombocytopaenia—particularly in the context of an acute severe illness—are also strongly associated with BAH. In a case controlled study thrombocytopaenia, anticoagulation with heparin and sepsis were identified as the three most common risk factors for BAH.3
Our patient was predisposed to BAH by a critical illness involving massive pulmonary embolism, sepsis, renal failure, thrombocytopaenia and anticoagulation with dabigatran. She was then given heparin and finally thrombolysed. In this context, adrenal haemorrhage is not surprising. It is not possible to say exactly when it occurred. However, the patient's deterioration several days after thrombolysis with hypotension and increasing inotrope requirement without an evidence of sepsis suggests that it did occur following thrombolysis. However, anticoagulation with dabigatran, which accumulates in renal failure, and subsequent heparin use, cannot be excluded as the cause.
BAH due to anticoagulation with heparin is well described.3 4 However, to our knowledge this is the only report of BAH following thrombolysis. Unilateral adrenal haemorrhage post thrombolysis has been described.5 6 We speculate that the risk of BAH from thrombolysis is increased in critically ill patients who are septic.
Clinical features of adrenal failure due to BAH are non specific and may be easily mistaken for sepsis. In our patient, the diagnosis was made as a result of an incidental finding on CT scan undertaken to look for a septic source. This is a commonly reported scenario.4 7
An abdominal CT scan is key to making the diagnosis. In the right clinical context bilateral oval adrenal ‘masses’ of variable attenuation seen on CT scan may represent haemorrhage (the attenuation depends on the age of the haemorrhage). Follow-up CT scan may confirm adrenal haemorrhage when there is diminution or resolution of the ‘masses’ and density changes, consistent with resolving haematomas.7–9 Adrenal failure is readily confirmed by a short synacthen test and is likely to be persistent—as in our patient.10 Urgent steroid replacement therapy is needed.
Learning points.
Causes of bilateral adrenal haemorrhage (BAH) associated with adrenal insufficiency include burns, trauma, severe haemorrhage, Waterhouse-Friderichsen syndrome (adrenal haemorrhage due to sepsis—often meningococcal) as well as clotting disorders and anticoagulation.
Acute adrenal failure due to BAH is a rare life-threatening complication of anticoagulation and thrombolysis that is easily missed and should be considered in the differential diagnosis of persistent hypotension post-thrombolysis.
The key diagnostic test is an abdominal CT scan showing apparent oval adrenal ‘masses’ which are actually haematomas. Adrenal failure can be confirmed with a short synacthen test.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rao RH. Bilateral massive adrenal hemorrhage. Med Clin North Am 1995;79:107–29 [DOI] [PubMed] [Google Scholar]
- 2.Vella A, Nippoldt TB, Morris JC., III Adrenal hemorrhage: a 25-year experience at the Mayo clinic. Mayo Clin Proc 2001;76:161–8 [DOI] [PubMed] [Google Scholar]
- 3.Kovacs KA, Lam YM, Pater JL. Bilateral massive adrenal hemorrhage. Assessment of putative risk factors by the case-control method. Medicine (Baltimore) 2001;80:45–53 [DOI] [PubMed] [Google Scholar]
- 4.Echaniz A, Diz-Lois F. Bilateral adrenal haemorrhage secondary to heparin treatment. Ann Med Internal 1992;9:189–91 [PubMed] [Google Scholar]
- 5.Marty B, Maurer R. Unilateral adrenal haemorrhage following thrombolysis. Schweiz Rundsch Med Prax 1992;81:524–8 [PubMed] [Google Scholar]
- 6.Streensrud T, Muller LS. Unilateral adrenal haemorrhage following systemic thrombolysis. Interact Cardiovasc Thorac Surg 2008;7:430–1 [DOI] [PubMed] [Google Scholar]
- 7.Wolverson M, Kannegiesser H. CT of bilateral adrenal haemorrhage with acute adrenal insufficiency in the adult. AJR 1984;142:311–31 [DOI] [PubMed] [Google Scholar]
- 8.Ling D, Korbokin M, Silverman PM, et al. CT demonstration of bilateral adrenal haemorrhage. AJR 1983;141:307–8 [DOI] [PubMed] [Google Scholar]
- 9.Sacerdote M, Johnson P, Fishman E. CT of the adrenal gland: the many faces of adrenal hemorrhage. Emerg Radiol 2012;19:53–60 [DOI] [PubMed] [Google Scholar]
- 10.Jahangir-Hekmat M, Taylor HC, Levin H, et al. Adrenal insufficiency attributable to adrenal hemorrhage: long-term follow-up with reference to glucocorticoid and mineralocorticoid function and replacement. Endocr Pract 2004; 10:55–61 [DOI] [PubMed] [Google Scholar]