Abstract
Sclerosing haemangioma (SH) is a rare benign lung tumour with distinctive variety of histological patterns. SH typically presents as asymptomatic peripheral, solitary well-circumscribed lesion in women with median age at diagnosis in the fifth decade. Preoperative diagnosis of this tumour is difficult, and sometimes even intraoperative frozen sections cannot differentiate it from malignant tumours. Here, we present our experiences in investigating its characteristics. We report a case of a 19-year-old girl who presented with chest pain, cough and sputum and off and on haemoptysis for 6 months. Anti-tubercular treatment was given but provided no relief. CT chest showed a well-defined hypodense solid mass lesion with a soft tissue alternation. Lobectomy was performed. Microscopy revealed a tumour comprising of two distinct populations of cells surface and stromal cells which disposed in papillary, solid, sclerotic and haemorrhagic growth patterns. Histology and immunohistochemistry confirmed the diagnosis of SH of the lung.
Background
Sclerosing haemangioma (SH) of the lung is a rare benign solitary lesion with generally asymptomatic clinical presentation. It was first described by Liebow R Hubbell in 1956.1 It is a rare benign neoplasm with a distinctive variety of patterns lined by and filled with two distinct type of epithelial cells. The name is a misnomer because it is not considered as a vascular lesion now or does not express endothelial markers.2 Ultra structural and immunohistochemical studies showed that pulmonary SH is likely to have originated from epithelial cells probably type-II pneumocytes, hence the alternative designation is sclerosing pneumocytoma or pneumocytoma.3 4
Case presentation
A 19-year-old girl presented to the chest clinic with complaints of chest pain, cough and sputum for 1 year, followed by off and on haemoptysis for 6 months. There was no history of fever, tobacco addiction or other chronic illness. She had already started antitubercular treatment prescribed by a general practitioner before coming to our hospital.
Investigations
On examination she was mildly anaemic (haemoglobin 9.5 mg/dl). Other biochemical parameters were within normal limits. Staining of sputum smear for acid fast bacilli/culture for Mycobacterium tuberculosis was negative. CT chest showed a well-defined hypodense solid mass lesion with soft tissue attenuation measuring 3×2.9×3.2 cm present in left upper lobe of the lung (figure 1A). Bronchoscopic bronchial brush smears and BAL fluid smears were inconclusive and also negative for fungal elements. Finally lobectomy was performed. Gross examination showed lobectomy specimen of the lung with a well-circumscribed mass lesion measuring 2.5×2.2 cm. Cut surface of mass was solid gray white with a focal area of haemorrhage (figure 1B). Rest of the lung parenchyma was normal spongy in appearance.
Figure 1.
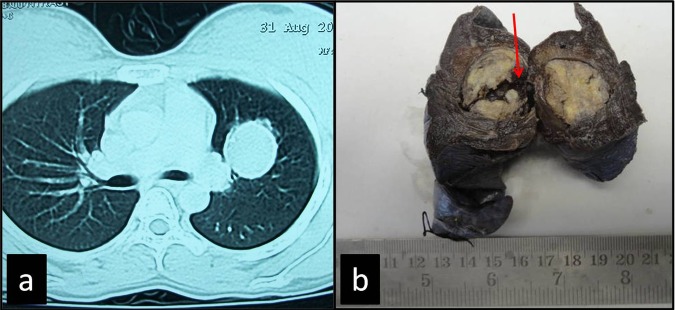
(A) Contrast-enhanced CT showed a well-defined hypodense solid mass lesion with soft tissue attenuation. (B) Gross specimen showing a well circumscribed parenchymal mass with focal area of haemorrhage (arrow).
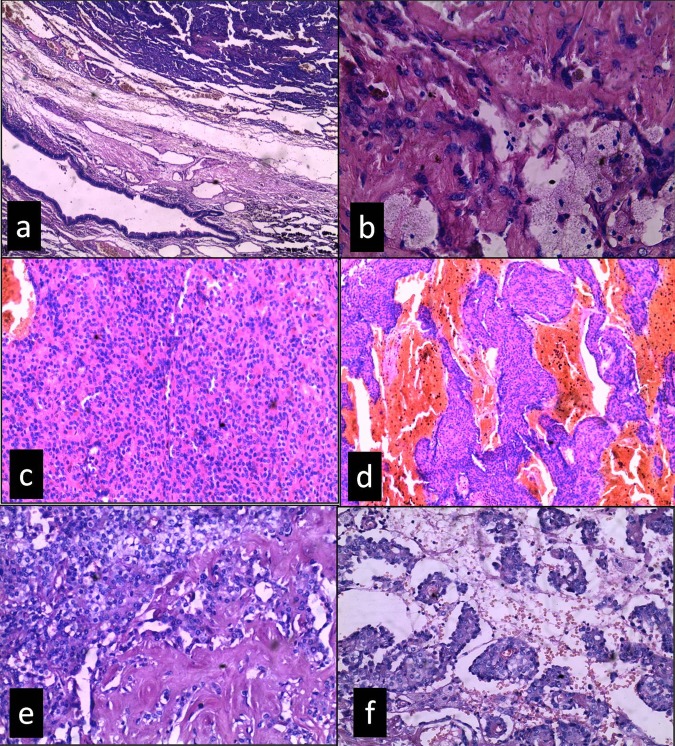
Histological examination of specimen showed a well-circumscribed tumour disposed in the papillary solid, sclerotic and haemorrhagic growth patterns (figure 2C–F) and surrounded by peripheral compressed lung parenchyma (figure 2A). Papillary area showed papillae lined with two types of cells first the oval to cuboidal cells (surface cells) having large vesicular nuclei, prominent nucleoli with eosinophilic cytoplasm and the second type of cells; stromal cells were small round cells showing well-defined cell borders with fine dispersed chromatin, inconspicuous nucleoli and eosinophilic cytoplasm (figure 3A). These stromal cells were filling the papillary cores and also forming sheets in solid areas. Other areas showed blood filled spaces or cavities (haemorrhagic). Papillae with hyaline cores as well as focal hyalinisation (sclerotic) area were present. Xanthoma cells were also present as small clusters or lying singly in sclerotic and papillary areas (figure 2B). Focal areas showed clear cell change in stromal cells. Cholestrol cleft and hemosiderin deposition was also evident. Overall, tumour cells were bland and mitoses were minimal.
Figure 2.
Sclerosing haemangioma showing different architectural patterns, (A) compressed lung tissue separating the tumour from normal lung parenchyma (H&E, ×100), (B) sclerotic area with foamy histiocytes (H&E, ×400), (C) solid, (D) haemorrhagic, (E) sclerotic area merging with clear cell change and (F) papillary (C–F (H&E, ×200).
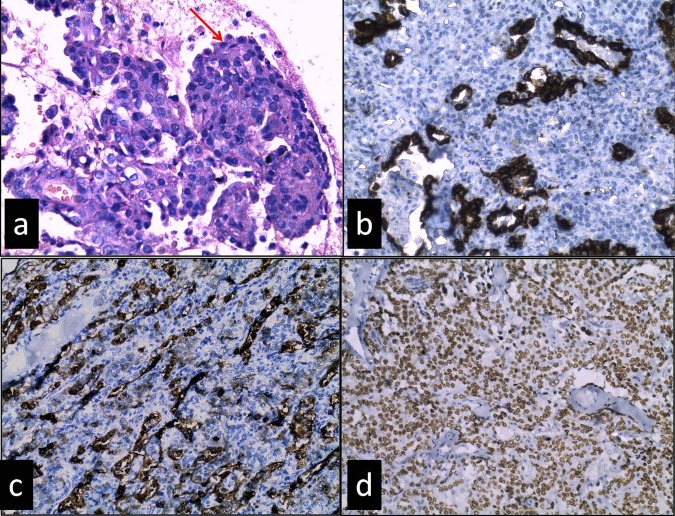
Figure 3.
(A) Papillae lined by surface cells (arrow) are cuboidal and filled with stromal cells are round, having bland morphology (H&E, ×400), (B) cytokeratin (CK) staining positive only in surface cells not in stromal cells (C) epithelial membrane antigen (EMA) positive in surface cells and a few stromal cells (D) thyroid transcription factor (TTF-1) showing strong positivity in both surface and stromal cells (B–D, immunohistochemistry; ×200).
Differential diagnosis
Initially, first clinical differential diagnosis was pulmonary tuberculosis before coming to this hospital based on clinical presentation and plain chest x-ray. The patient was put on anti-tubercular treatment (ATT) for 2 months but did not respond. However, after hospitalisation the CT showed a well circumscribed solitary nodule in the left upper lobe. This was subsequently excised and submitted for histopathological examination.
Preoperative clinical diagnosis by the operating surgeon was pulmonary hamartoma, peripheral lung adenoma and cyst.
Histological differential diagnoses of this case were sclerosing haemangima, carcinoid tumour, haemagioma, papillary adenocarcinoma of the lung and metastasis from thyroid and other gland. Immunohistrochemistry for pancytokeratin (CK) was positive only in surface cells. Epithelial membrane antigen (EMA) showed strong positivity in surface cells and variable positivity in stromal cells. Thyroid transcription factor-1 (TTF-1) showed strong nuclear positivity in both surface and stromal cells (figure 3B–D). Chromagranin and thyroglobulin were negative. CD34 was only positive in vessels, not in tumour cells. On the basis of distinct histomorphology and typical immunohistological staining patterns final diagnosis of sclerosing haemangioma of the lung was performed.
Treatment
Lobectomy was performed. Histology revealed diagnosis of sclerosing haemangioma; a benign tumour needs no further treatment and the patient was kept under follow-up.
Outcome and follow-up
Postoperative 6 months follow-up of the patient was uneventful.
Discussion
Pulmonary SH is a rare and benign neoplasm of the lung. It is quite rare in Western countries, but more common in Japan.2 Although it has been reported in all age groups including children, it typically occurs in middle aged women (80%) and most are asymptomatic.2 4 Symptoms such as atypical thoracic pain, cough, haemoptysis and dyspnoea might occur due to tumour enlargement and compromising of surrounding tissue.4–6 Few cases (20%) can present with respiratory complaints such as cough, haemoptysis or chest pain.2 Our case was a young lady who presented with chest pain, cough and haemoptysis. Tuberculosis is rampant in India and whenever a patient comes with complaint of cough with expectoration and haemoptysis, the first differential diagnosis in the Indian context is considered to be tuberculosis irrespective of sputum culture and smear positive or negative. Considering the age, symptoms and oval opacity in the lung, in the plane x-ray the physician must have started ATT without investigating the patient further. She was referred to our institution after 2 months when she did not respond to treatment. Although SH is thought to be benign, cases of lymph node metastases, local recurrence and multiple lesions have been reported. Lymph node metastases, however, do not seem to have an impact on long-term survival.5–7 Most lesions are solitary and often affect the lower lobe. However, 4% are multifocal and may involve any lobe.2 8 Chest x-ray shows a well-circumscribed lesion. Characteristically on CT. SH demonstrate a round to oval nodule or mass with smooth margins that enhances contrast media. Calcification might be detected in the minority of cases and there is no infiltration of the surrounding tissue.9 10
Grossly, SH is a well-circumscribed solid and firm mass size ranging from 0.3 to 11 cm in greatest dimension with a mean of approximately 3 cm. Cut surfaces are solid gray to tan with focal or extensive haemorrhage, cystic spaces or small foci of calcifications.6 9 10
SH show distinctive variety of growth patterns as described in the microscopic picture of this case. The majority of tumours show a combination of different growth patterns like papillary sclerotic, solid and haemorrhagic.2 4 8 9 One of these growth patterns may predominate. In our case all the histological patterns were observed.
The papillary growth pattern is most common and may merge with sclerotic or solid areas. Predominant papillary growth patterns may be confused with a carcinoma that also exhibits a papillary pattern like metastatic papillary thyroid carcinoma, mesothelioma and bronchioloalveolar carcinoma. In this respect, however, decreased Ki-67 labelling (mitosis), absence of atypical nuclear features and negative expression of thyroglobulin could help to differentiate them from SH. Sheets of round cells or typical papillary area can mimick the carcinoid. Presence of only one cell type along with strong expression of neuroendocrine markers in carcinoids helps to differentiate it from SH. Area of clear cell change can be confused with metastasis from renal cell carcinoma. Nuclear atypia and typical vascular pattern of renal cell carcinoma is not identified in SH.2 8 9 Benign lung tumour in differential diagnosis includes clear cell tumour, pulmonary hamartoma and haemangioma. In contrast to SH, clear cell tumours show abundant clear cells with scant stroma, thin-walled vessels and strong human melanoma black (HMB)-45 expression. Pulmonary hamartomas show a mixture of various tissues like cartilage, myxoid stroma, muscles, adipose tissue and trapped respiratory epithelium. True haemangiomas of the lung are very rare, have either a cavernous or capillary morphology, express endothelial markers and lack epithelial cells.2 9
Immunohistochemical staining pattern of sclerosing haemangiomais has been described by several workers. ‘Surface cells’ express pancytokeratin (CK), TTF-1, epithelial membrane antigen (EMA) and surfactant proteins A and B. Stromal cells (round cells) express TTF-1 and EMA. However, stromal cells lack CK and surfactant protein A and B expression. CD34/CD31 is not expressed by both types of epithelial cells.2–4 11
Learning points.
Sclerosing haemangioma (SH) in a majority of the instances is asymptomatic and may be a diagnostic challenge for the general practitioner and the pathologist because of its low incidence.
Preoperative diagnosis of this tumour is difficult, and sometimes even intraoperative frozen sections cannot differentiate it from malignant tumours.
SH when symptomatic (as in this case), may be misdiagnosed as tuberculosis specially in high prevalence areas.
Empirical treatment for tuberculosis should not be started without a definite diagnosis.
Footnotes
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Liebow AA, Hubbell DS. Sclerosing haemangioma (histiocytoma, xanthoma) of the lung. Cancer 1956;9:53–75 [DOI] [PubMed] [Google Scholar]
- 2.Flieder DB. Benign neoplasm of the lungs . In Goldblum JR, Zander DS, Farver CF. Pulmonary pathology: a volume in the series foundation in diagnostic pathology. 1st edn Churchill Livingstone: Elsevier, 2008:683–6 [Google Scholar]
- 3.Chan ACL, Chan JKC. Pulmonary sclerosing haemangioma consistently expresses thyroid transcription factor-1 (TTF-1):a new clue to its histogenesis. Am J Surg Pathol 2000;24:1531–6 [DOI] [PubMed] [Google Scholar]
- 4.Iyoda A, Hiroshima K, Shiba M, et al. Clinicopathological analysis of pulmonary sclerosing haemangioma. Ann Thorac Surg 2004;78:1928–31 [DOI] [PubMed] [Google Scholar]
- 5.Yano M, Yamakawa Y, Kiriyama M, et al. Sclerosing haemangioma with metastases to multiple nodal stations. Ann Thorac Surg 2002;73:981–3 [DOI] [PubMed] [Google Scholar]
- 6.Jungraithmayr W, Eggeling S, Ludwig C, et al. Sclerosing haemangioma of the lung: a benign tumour with potential for malignancy?. Ann Thorac Cardiovasc Surg 2006;12:352–4 [PubMed] [Google Scholar]
- 7.Miyagawa HA, Tazelaar HD, Langel DJ, et al. Pulmonary sclerosing haemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med 2003;127:321–5 [DOI] [PubMed] [Google Scholar]
- 8.Katzenstein AL, Gmelich JT, Carrington CB. Sclerosing haemangioma of the lung: a clinicopathologic study of 51 cases. Am J Surg Pathol 1980;4:343–56 [DOI] [PubMed] [Google Scholar]
- 9.Keylock CPTJB, Galvin JR, Franks TJ. Sclerosing haemangioma of the lung. Arch Pathol Lab Med 2009;133:820–5 [DOI] [PubMed] [Google Scholar]
- 10.Goto T, Maeshima A, Kato R. Microscopic sclerosing haemangioma diagnosed by histopathological examination after lung cancer surgery. Ann Thorac Cardiovasc Surg 2011;17:507–10 [DOI] [PubMed] [Google Scholar]
- 11.Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor-1 and other markers in sclerosing haemangioma of the lung. Arch Pathol Lab Med 2001;125:1335–9 [DOI] [PubMed] [Google Scholar]